Chapter 5
Children and adolescents
Principles of prescribing practice in childhood and adolescence1
- Target symptoms, not diagnoses. Diagnosis can be difficult in children and comorbidity is very common. Treatment should target key symptoms. While a working diagnosis is beneficial to frame expectations and help communication with patients and parents, it should be kept in mind that it may take some time for the illness to evolve.
- Technical aspects of paediatric prescribing. The Medicines Act 1968 and European legislation make provision for doctors to use medicines in an 'off-label' or out-oflicence capacity or to use unlicensed medicines. However, individual prescribers are always responsible for ensuring that there is adequate information to support the quality, efficacy, safety and intended use of a drug before prescribing it. It is recognised that the informed use of unlicensed medicines, or of licensed medicines for unlicensed applications, ('off-label' use) is often necessary in paediatric practice.
- Prescription writing: inclusion of age is a legal requirement in the case of prescription-only medicines for children under 12 years of age, but it is preferable to state the age for all prescriptions for children.
- Begin with less, go slow and be prepared to end with more. In out-patient care, dosage will usually commence lower in mg/kg per day terms than adults and finish higher in mg/kg per day terms, if titrated to a point of maximal response.
- Multiple medications are often required in the severely ill. Monotherapy is ideal. However, childhood-onset illness can be severe and may require treatment with psychosocial approaches in combination with more than one medication.2
- Allow time for an adequate trial of treatment. Children are generally more ill than their adult counterparts and will often require longer periods of treatment before responding. An adequate trial of treatment for those who have required in-patient care may well take 8 weeks for depression or schizophrenia.
- Where possible, change one drug at a time.
- Monitor outcome in more than one setting. For symptomatic treatments (such as stimulants for attention deficit hyperactivity disorder [ADHD]), bear in mind that the expression of problems may be different across settings (e.g. home and school); a dose titrated against parent reports may be too high for the daytime at school
- Patient and family medication education is essential. For some child and adolescent psychiatric patients the need for medication will be life-long. The first experiences with medications are therefore crucial to long-term outcomes and adherence.
References
- Nunn K, Dey C. The Clinician's Guide to Psychotropic Prescribing in Children and Adolescents. Sydney: Glade Publishing; 2003.
- Luk E, Reed E. Polypharmacy or Pharmacologically Rich? In: Nunn KP, Dey C, eds. The Clinician's Guide to Psychotropic Prescribing in Children and Adolescents, 2nd edn. Sydney: Glade Publishing; 2003, pp. 8–11.
Further reading
For detailed adverse effects of CNS drugs in children and adolescents, see:
Paediatric Formulary Committee. BNF for Children 2013–2014. London: Pharmaceutical Press; 2013.
Martin A, Scahill L, Charney DS, Leckman JF. Pediatric Psychopharmacology: Principles and Practice. New York: Oxford University Press; 2002.
Riddle MA et al. Introduction: Issues and viewpoints in pediatric psychopharmacology. Int Rev Psychiatry 2008; 20:119–120.
Depression in children and adolescents
Psychological intervention
The National Institute for Health and Clinical Excellence (NICE) guidelines1 recommend that psychological intervention should be considered as the first-line treatment for child and adolescent depression. Psycho-educational programmes, non-directive supportive therapy, group cognitive behavioural therapy (CBT) and self-help are indicated for mild-to-moderate depression. More specific and intensive psychological interventions including CBT, interpersonal psychotherapy and short-term family therapy are recommended for moderate-to-severe depression.1 The NICE guideline recommends the introduction of medication in conjunction with psychological treatments if there is failure to respond to psychological treatment.1 In the light of changing evidence this advice has recently been questioned with recommendations for the use of medication at a much earlier stage of treatment in cases of moderate-to-severe depression.2
Pharmacotherapy
The NICE guideline CG281 supports the use of selective serotonin reuptake inhibitors (SSRIs) but only in combination with psychological forms of therapy. Two US studies, Treatment of Adolescents with Depression Study (TADS)3 and Treatment of Resistant Depression in Adolescence (TORDIA)4 found that CBT confers benefit when used in combination with medication. A large UK study did not establish the benefits of combined therapy (fluoxetine plus CBT) and demonstrated that the use of fluoxetine on its own in addition to routine clinical care is effective in treating moderate-to-severe depression.5,6 Whether CBT provides added value to treatment and outcomes remains a controversial area, but in view of the recent research it is recommended that medication is started at a much earlier stage in treatment, especially if the depression is severe.2 Evidence7 now supports the administration of fluoxetine for moderate-to-severe depression sooner than the 12 weeks currently recommended in the original NICE guideline.2 The NICE Surveillance Group also suggests that the additional benefit of combining CBT and antidepressant treatment compared with the administration of antidepressants alone may not be as significant as previously thought.2
The more severe the depressive episode the more likely it is that medication, in combination with psychological treatment or on its own, will be efficacious in the early stages of treatment.8,9 Good initial response is a sign of improved rates of recovery and outcomes.3,4
Fluoxetine is the first-line pharmacological treatment.10 In the UK it is licensed for use for children and young people from 8–18 to treat moderate-to-severe major depression which is unresponsive to psychological therapy after 4–6 sessions. It is recommended that pharmacotherapy should be administered in combination with a concurrent psychological therapy.1,10–13 Cochrane agree that fluoxetine is the drug of choice in this patient group.8 A recent multiple-treatments meta-analysis14 confirmed fluoxetine's superiority over CBT and other drugs, but concluded that sertraline and mirtazapine might offer the optimal balance of efficacy and tolerability.
Fluoxetine and escitalopram are the only antidepressants approved by the US Food and Drug Administration (FDA) for adolescents and fluoxetine is the only FDAapproved medication for pre-pubertal children. Generally speaking, adolescents can be expected to respond better to antidepressants than younger children, particularly those under the age of 12.15
Studies in adults have shown that the elimination half-life of fluoxetine is 1–4 days and 7–15 days for its primary metabolite, norfluoxetine, making it a preferable SSRI for adolescents who are less likely to experience withdrawal effects when omitting a dose or stopping the medication abruptly.16,17 Body weight influences fluoxetine concentrations and starting doses of medication have to be lowered in children. However, during treatment the half-lives of most antidepressants are much lower in children than in adolescents and higher doses may have to be administered in order to achieve adequate blood concentration and therapeutic effects.15,17
Fluoxetine should be started at a low dose of 10 mg daily1 and increased weekly until a minimum effective dosage of 20 mg daily is achieved.15 Patients and their parents/carers should be informed about the potential side-effects associated with SSRI treatment and know how to seek help in an emergency. Any pre-existing symptoms that might be interpreted as side-effects (e.g. agitation, anxiety, suicidality) should be noted.
Alternative SSRIs and other antidepressants
If there is no response to fluoxetine and pharmacotherapy is still considered to be the most favourable option, an alternative SSRI such as sertraline and citalopram1 may be used cautiously by specialists. Evidence suggests some efficacy for sertraline1,18,19 but one randomised controlled trial (RCT) showed it to be inferior to CBT.20 Citalopram, also recommended by NICE,1 may be less effective10,21,22 and is probably more toxic in overdose.23
Escitalopram is the therapeutically active isomer of racemic citalopram.24 It has been shown to be efficacious in two RCTs25,26 and is approved by the FDA for use in 12–18 year olds.
Sertraline, citalopram and escitalopram are quickly metabolised by children and twice daily dosing should be considered.27,28 Sertraline, citalopram and escitalopram should also be started at low doses and titrated weekly up to minimum effective doses; sertraline 50–100 mg; citalopram 20 mg and escitalopram 10 mg.29
Paroxetine is considered to be an unsuitable option.1,10
The placebo response rate is high in young people with depression.8,29 On average drug and placebo response rates in children and adolescents differ by only 10%12 and the benefits of active treatment are likely to be modest. It is estimated that 1 in 6–10 may benefit from the active treatment (although 60% or more show improvement).1,12,30 There is some evidence to suggest dose increases can improve response.31
Tricyclic antidepressants (TCAs) are not effective in pre-pubertal children but may have marginal efficacy in adolescents.12,32 Amitriptyline (up to 200 mg/day), imipramine (up to 300 mg/day) and nortriptyline have all been studied in RCTs. Note that due to more extensive metabolism, young people require higher mg/kg doses than adults. The side-effect burden associated with TCAs is considerable. Vertigo, orthostatic hypotension, tremor and dry mouth limit tolerability.32 Tricyclics are also more cardiotoxic in young people than in adults. Baseline and on-treatment electrocardiograms (ECGs) should be performed. Co-prescribing with other drugs known to prolong the QTc interval should be avoided. There is no evidence that adolescents who fail to respond to SSRIs will respond to tricyclics.
There is little evidence for the use of mirtazapine33 but it is sometimes used in clinical practice where sleep is a problem.
Omega-3 fatty acids may be effective in childhood depression but evidence is minimal.34
St John's wort should be avoided because of the risk of interaction (see Chapter 7).
Severe depression that is life-threatening or unresponsive to other treatments may respond to electroconvulsive therapy (ECT).35 ECT should not be used in children under 12.1 The effects of ECT on the developing brain are unknown.
Safety of antidepressants
When prescribing SSRIs it is important that the dose is increased slowly to minimise the risk of treatment-emergent agitation and that patients are monitored closely for the development of treatment-emergent suicidal thoughts and acts. Patients should be seen at least weekly in the early stages of treatment. Side-effects linked to SSRIs include sedation, insomnia and gastrointestinal symptoms and, rarely, can induce bleeding, serotonin syndrome, activation and mania. More detailed reviews of these problems in adults can be found in Chapter 4.
There is evidence from meta-analyses of pooled trials that antidepressants increase the risk of suicidal behaviours in the short term although no completed suicides were reported in any of the trials.3,30,36–42 The risk of spontaneously reported suicidal ideation and suicidal behaviour in adolescents treated with antidepressant medication is 1–3 out of every 100 children.41 Conversely, some studies point to the risk of suicide associated with untreated depression.43 Reduced prescribing of SSRIs in the USA44 and The Netherlands45 has been linked to an increase in the rate of suicide.
The TADS study, which compared CBT with fluoxetine, placebo and combined CBT and fluoxetine, showed that all treatment arms were effective in reducing suicidal ideation, but that the combined treatment of fluoxetine and CBT reduced the risk of suicidal events to the greatest extent.3 Overall, the potential benefits of treatment with antidepressants outweigh the risks in relation to suicidal behaviours.
Starting and titrating the dose of SSRIs and alternative medication
The administration of all SSRIs should be monitored against the emergence of sideeffects and the dose should be reduced if side-effects persist beyond one week. In this case the dose of the medication should be lowered to the highest tolerable dose. SSRI medication should be administered for a minimum of 4–6 weeks and if the child or young person fails to respond and remains symptomatic a dose increase should be considered. A switch to another medication should be made if there is insufficient improvement after approximately 10–12 weeks (switch earlier if there are no signs of improvement). Medication effectiveness should be initially monitored at weekly intervals and its effectiveness re-evaluated every 4–6 weeks.28
Duration of treatment and discontinuation of SSRIs
There is little evidence regarding optimum duration of treatment.46 Adding CBT to fluoxetine during continuation treatment has shown sustained remission and lower rates of relapse in comparison to medication on its own.47 To consolidate the response to the acute treatment and avoid relapse, treatment with fluoxetine should continue for at least 6 months and up to 12 months.48,49 There is a significant reduction of the risk of relapse with a continuation of treatment for 6 months.28,48
At the end of treatment, the antidepressant dose should be tapered slowly to minimise discontinuation symptoms. Ideally this should be done over 6–12 weeks.1,28 Because of fluoxetine's long duration of action it can probably be safely tapered over 2 weeks.
Refractory depression
There are no clear clinical guidelines for the management of treatment-resistant depression in adolescents1,50 but there is evidence from the TORDIA published studies4 that adolescents who failed to respond to treatment with one SSRI may improve when switched to another SSRI or venlafaxine when the pharmacotherapy was combined with concurrent CBT. A switch to an SSRI was just as efficacious as a switch to venlafaxine with less severe side-effects. Recent TORDIA results demonstrate that with continued treatment of depression among treatment-resistant adolescents approximately one third remit.51 However, the venlafaxine group had more side-effects and there was an association with higher rates of suicidal events in those who entered the study with high suicidal ideation. Venlafaxine should be used with caution and under specialist guidance.1,4,52 Note that a recent large study suggested no increased risk of suicidality for venlafaxine.53
Augmentation with a second medication has not been studied in RCTs in depressed children and adolescents who have either not responded to treatment or have only shown a partial improvement. Case studies and post hoc TORDIA studies have demonstrated some benefits from the addition of antipsychotics.54–56
Risk of bipolar disorder
Some young people, and especially children, will develop behavioural activation in response to the administration of SSRIs. It is estimated that 3–8% of young people prescribed SSRIs present with heightened mood, restlessness and silliness which is transitory in nature. This disinhibitory response to starting SSRI medication or being prescribed increasing doses of medication needs to be differentiated from hypomania or mania.57 Early bipolar illness should be suspected when the presentation is one of severe depression, associated with psychosis or rapid mood shifts and the condition worsens on treatment with antidepressants. Early studies suggested that between 20% and 40% of children and young people presenting with depression will develop bipolar affective disorder (BAD)58 when treated with antidepressants (the antidepressants acting so as to reveal the disorder, not cause it). In some studies in bipolar patients treatment with antidepressants is associated with new or worsening rapid cycling in as many as 23% of patients.59 It seems that the younger the child, the greater the risk.60 In the case of emergent mania early treatment with atypical antipsychotics and mood stabilisers should be considered.61 More recently it has been advocated that cautiously administered SSRIs should not be withheld in cases of severe depression and BAD.62 There is limited evidence from open label studies that lamotrigine is effective in treating depression in the context of BAD.63,64 There is evidence from TORDIA that sub-syndromal manic symptoms at baseline and over time are predictors of poor outcome in adolescent depression.65 Adult studies suggest that olanzapine, quetiapine and lurasidone are superior to antidepressants in bipolar depression (see Chapter 3).
|
Box 5.1 Summary of treatment of depression in children and adolescents
|
|
First line
Second line
Third line
Fourth line
|
Fluoxetine + CBT
Escitalopram + CBT
Sertraline, citalopram (more toxic in overdose)
Venlafaxine (less well tolerated)
Mirtazapine (where sedation required)
Consider adding quetiapine/aripiprazole to SSRI treatment
|
|
References
- National Institute for Health and Clinical Excellence. Depression in children and young people: identification and management in primary, community and secondary care. Clinical Guideline 28, 2005. http://www.nice.org.uk
- National Institute for Health and Care Excellence. Depression in children and young people: review decision - Oct 13. Clinical Guideline 28, 2013. http://guidance.nice.org.uk/CG28/ReviewDecision/pdf/English
- The TADS Team. The Treatment for Adolescents With Depression Study (TADS): long-term effectiveness and safety outcomes. Arch Gen Psychiatry 2007; 64:1132–1143.
- Brent D et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: The TORDIA Randomized Controlled Trial. JAMA 2008; 299:901–913.
- Goodyer I et al. Selective serotonin reuptake inhibitors (SSRIs) and routine specialist care with and without cognitive behaviour therapy in adolescents with major depression: randomised controlled trial. BMJ 2007; 335:142.
- Dubicka B, March J, Wilkinson P, Kelvin R, Vitiello B, Goodyer I. The treatment of adolescent major depression: A comparison of the ADAPT and TADS trials. In: Yule W, ed. Depression in Childhood and Adolescence: The Way Forward. London: Association for Child and Adolescent Mental Health; 2009.
- Dubicka B et al. Combined treatment with cognitive-behavioural therapy in adolescent depression: meta-analysis. Br J Psychiatry 2010; 197:433–440.
- Hetrick SE et al. Newer generation antidepressants for depressive disorders in children and adolescents. Cochrane Database Syst Rev 2012; 11:CD004851.
- March J et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA 2004; 292:807–820.
- Medicines and Healthcare Products Regulatory Agency. Selective serotonin reuptake inhibitors (SSRIs): Overview of regulatory status and CSM advice relating to major depressive disorder (MDD) in children and adolescents including a summary of available safety and efficacy data. London: MHRA; 2005. http://www.mhra.gov.uk
- Kratochvil CJ et al. Selective serotonin reuptake inhibitors in pediatric depression: is the balance between benefits and risks favorable? J Child Adolesc Psychopharmacol 2006; 16:11–24.
- Tsapakis EM et al. Efficacy of antidepressants in juvenile depression: meta-analysis. Br J Psychiatry 2008; 193:10–17.
- Whittington CJ et al. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet 2004; 363:1341–1345.
- Ma D et al. Comparative efficacy, acceptability, and safety of medicinal, cognitive-behavioral therapy, and placebo treatments for acute major depressive disorder in children and adolescents: a multiple-treatments meta-analysis. Curr Med Res Opin 2014.
- Sakolsky DJ et al. Antidepressant exposure as a predictor of clinical outcomes in the Treatment of Resistant Depression in Adolescents (TORDIA) study. J Clin Psychopharmacol 2011; 31:92–97.
- Wilens TE et al. Fluoxetine pharmacokinetics in pediatric patients. J Clin Psychopharmacol 2002; 22:568–575.
- Findling RL et al. The relevance of pharmacokinetic studies in designing efficacy trials in juvenile major depression. J Child Adolesc Psychopharmacol 2006; 16:131–145.
- Donnelly CL et al. Sertraline in children and adolescents with major depressive disorder. J Am Acad Child Adolesc Psychiatry 2006; 45:1162–1170.
- Rynn M et al. Long-term sertraline treatment of children and adolescents with major depressive disorder. J Child Adolesc Psychopharmacol 2006; 16:103–116.
- Melvin GA et al. A comparison of cognitive-behavioral therapy, sertraline, and their combination for adolescent depression. J Am Acad Child Adolesc Psychiatry 2006; 45:1151–1161.
- Wagner KD et al. A randomized, placebo-controlled trial of citalopram for the treatment of major depression in children and adolescents. Am J Psychiatry 2004; 161:1079–1083.
- von Knorring AL et al. A randomized, double-blind, placebo-controlled study of citalopram in adolescents with major depressive disorder. J Clin Psychopharmacol 2006; 26:311–315.
- Klein-Schwartz W et al. Comparison of citalopram and other selective serotonin reuptake inhibitor ingestions in children. Clin Toxicol (Phila) 2012; 50:418–423.
- Hyttel J et al. The pharmacological effect of citalopram residues in the (S)-(+)-enantiomer. J Neural Transm Gen Sect 1992; 88:157–160.
- Wagner KD et al. A double-blind, randomized, placebo-controlled trial of escitalopram in the treatment of pediatric depression. J Am Acad Child Adolesc Psychiatry 2006; 45:280–288.
- Emslie GJ et al. Escitalopram in the treatment of adolescent depression: a randomized placebo-controlled multisite trial. J Am Acad Child Adolesc Psychiatry 2009; 48:721–729.
- Axelson DA et al. Sertraline pharmacokinetics and dynamics in adolescents. J Am Acad Child Adolesc Psychiatry 2002; 41:1037–1044.
- Sakolsky D et al. Developmentally informed pharmacotherapy for child and adolescent depressive disorders. Child Adolesc Psychiatr Clin N Am 2012; 21:313–25, viii.
- Jureidini JN et al. Efficacy and safety of antidepressants for children and adolescents. BMJ 2004; 328:879–883.
- Bridge JA et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA 2007; 297:1683–1696.
- Heiligenstein JH et al. Fluoxetine 40-60 mg versus fluoxetine 20 mg in the treatment of children and adolescents with a less-than-complete response to nine-week treatment with fluoxetine 10-20 mg: a pilot study. J Child Adolesc Psychopharmacol 2006; 16:207–217.
- Hazell P et al. Tricyclic drugs for depression in children and adolescents. Cochrane Database Syst Rev 2013; 6:CD002317.
- Haapasalo-Pesu KM et al. Mirtazapine in the treatment of adolescents with major depression: an open-label, multicenter pilot study. J Child Adolesc Psychopharmacol 2004; 14:175–184.
- Nemets H et al. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry 2006; 163:1098–1100.
- McKeough G. Electroconvulsive therapy. In: Nunn KP, Dey C, eds. The Clinician's Guide to Psychotropic Prescribing in Children and Adolescents. Sydney: Glade Publishing; 2003, pp. 358–365.
- Martinez C et al. Antidepressant treatment and the risk of fatal and non-fatal self harm in first episode depression: nested case-control study. BMJ 2005; 330:389.
- Kaizar EE et al. Do antidepressants cause suicidality in children? A Bayesian meta-analysis. Clin Trials 2006; 3:73–90.
- Mosholder AD et al. Suicidal adverse events in pediatric randomized, controlled clinical trials of antidepressant drugs are associated with active drug treatment: a meta-analysis. J Child Adolesc Psychopharmacol 2006; 16:25–32.
- Simon GE et al. Suicide risk during antidepressant treatment. Am J Psychiatry 2006; 163:41–47.
- Olfson M et al. Antidepressant drug therapy and suicide in severely depressed children and adults: A case-control study. Arch Gen Psychiatry 2006; 63:865–872.
- Hammad TA et al. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry 2006; 63:332–339.
- Dubicka B et al. Suicidal behaviour in youths with depression treated with new-generation antidepressants: meta-analysis. Br J Psychiatry 2006; 189:393–398.
- Gibbons RD et al. Relationship between antidepressants and suicide attempts: an analysis of the Veterans Health Administration data sets. Am J Psychiatry 2007; 164:1044–1049.
- Libby AM et al. Decline in treatment of pediatric depression after FDA advisory on risk of suicidality with SSRIs. Am J Psychiatry 2007; 164:884–891.
- Gibbons RD et al. Early evidence on the effects of regulators' suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry 2007; 164:1356–1363.
- Kennard BD et al. Relapse and recurrence in pediatric depression. Child Adolesc Psychiatr Clin N Am 2006; 15:1057–79, xi.
- Kennard BD et al. Cognitive-behavioral therapy to prevent relapse in pediatric responders to pharmacotherapy for major depressive disorder. J Am Acad Child Adolesc Psychiatry 2008; 47:1395–1404.
- Emslie GJ et al. Fluoxetine treatment for prevention of relapse of depression in children and adolescents: a double-blind, placebo-controlled study. J Am Acad Child Adolesc Psychiatry 2004; 43:1397–1405.
- Emslie GJ et al. Fluoxetine versus placebo in preventing relapse of major depression in children and adolescents. Am J Psychiatry 2008; 165:459–467.
- Birmaher B et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry 2007; 46:1503–1526.
- Emslie GJ et al. Treatment of Resistant Depression in Adolescents (TORDIA): week 24 outcomes. Am J Psychiatry 2010; 167:782–791.
- Brent DA et al. Predictors of spontaneous and systematically assessed suicidal adverse events in the treatment of SSRI-resistant depression in adolescents (TORDIA) study. Am J Psychiatry 2009; 166:418–426.
- Cooper WO et al. Antidepressants and suicide attempts in children. Pediatrics 2014; 133:204–210.
- Vitiello B et al. Long-term outcome of adolescent depression initially resistant to selective serotonin reuptake inhibitor treatment: a follow-up study of the TORDIA sample. J Clin Psychiatry 2011; 72:388–396.
- Pathak S et al. Adjunctive quetiapine for treatment-resistant adolescent major depressive disorder: a case series. J Child Adolesc Psychopharmacol 2005; 15:696–702.
- Wagner KD et al. Out of the black box: treatment of resistant depression in adolescents and the antidepressant controversy. J Child Adolesc Psychopharmacol 2012; 22:5–10.
- Wilens TE et al. Disentangling disinhibition. J Am Acad Child Adolesc Psychiatry 1998; 37:1225–1227.
- Geller B et al. Rate and predictors of prepubertal bipolarity during follow-up of 6- to 12-year-old depressed children. J Am Acad Child Adolesc Psychiatry 1994; 33:461–468.
- Ghaemi SN et al. Diagnosing bipolar disorder and the effect of antidepressants: a naturalistic study. J Clin Psychiatry 2000; 61:804–808.
- Martin A et al. Age effects on antidepressant-induced manic conversion. Arch Pediatr Adolesc Med 2004; 158:773–780.
- Dubicka B et al. Pharmacological treatment of depression and bipolar disorder in children and adolescents. Adv Psychiatr Treat 2010; 16:402–412.
- Joseph MF et al. Antidepressant-coincident mania in children and adolescents treated with selective serotonin reuptake inhibitors. Future Neurol 2009; 4:87–102.
- Chang K et al. An open-label study of lamotrigine adjunct or monotherapy for the treatment of adolescents with bipolar depression. J Am Acad Child Adolesc Psychiatry 2006; 45:298–304.
- Pavuluri MN et al. Effectiveness of lamotrigine in maintaining symptom control in pediatric bipolar disorder. J Child Adolesc Psychopharmacol 2009; 19:75–82.
- Maalouf FT et al. Do sub-syndromal manic symptoms influence outcome in treatment resistant depression in adolescents? A latent class analysis from the TORDIA study. J Affect Disord 2012; 138:86––95.
Bipolar illness in children and adolescents
Diagnostic issues
Bipolar affective disorder (BAD) in children has become an area of intense research interest and controversy in recent years.1,2 While classical manic presentations fulfilling DSM-IV or ICD-10 criteria are well known to clinicians treating adolescents, they are rare in younger children.3,4 Claims that mania in pre-puberty may present as chronic (non-episodic) irritability or with extremely short (few hours) episodes should be treated with great caution.2 Short-lived episodes of exuberance are normative in children, while temper outbursts and mood lability can present in children with a wide range of other primary diagnoses (such as conduct, anxiety, depressive, and autism spectrum disorders).5 A detailed developmental assessment should therefore be the basis of any treatment decisions.
Clinical guidance
Before prescribing
- Establish a clinical diagnosis informed by a structured instrument assessment if possible. Try to monitor symptom patterns prospectively with mood or sleep diaries. If in doubt, seek specialist advice early on.
- Explain the diagnosis to the patient and family and invest time and effort in psychoeducation. This is likely to improve adherence and there is evidence that it reduces relapse rates at least in adults.6
- Measure baseline symptoms of mania (e.g. Young Mania Rating Scale7[YMRS]), depression (e.g. Children's Depression Rating Scale8[CDRS]), and impairment (e.g. Clinical Global Impression - BAD version9). Use these to set a clear and realistic treatment goal.
- Measure baseline height, weight, blood pressure and baseline bloods (including fasting glucose, lipids and prolactin levels).
What to prescribe
- Either second-generation antipsychotics (SGA) or mood stabilisers (MS) may be used as first-line treatment for youth with BAD, according to existing guidelines.10,11 Most of the evidence is for the treatment of acute episodes.
- SGAs seem to show greater short-term efficacy (effect size (ES) = 0.65 compared with placebo) than MS (ES = 0.20 compared with placebo) in youth, according to a recent meta-analysis.12
- SGA seem to produce significantly greater weight gain and somnolence in youth compared with adults.12
- Polycystic ovary syndrome and associated infertility are particular concerns when valproate is used in adolescent girls and NICE11 recommends avoiding its use in women of child-bearing age. Beware of teratogenicity.
- Adherence to lithium and blood-level testing may be difficult in adolescents. Beware of teratogenicity.
- Combinations of SGAs with MS are common but NICE guidelines11 should be noted.
- Overall, we recommend the use of SGAs as first line for the acute treatment of mania in children and adolescents (see Table 5.1, Table 5.2, Table 5.3), similar to recommendations in adults.
After prescribing
- Assess and measure symptoms on a regular basis to establish effectiveness.
- Monitor weight and height at each visit and repeat bloods at 3 months (then every 6 months). Offer advice on healthy lifestyle and exercise.
- The duration of most medication trials is between 3–5 weeks. This should guide decisions about how long to try a single drug in a patient. A complete absence of response at 1–2 weeks should prompt a switch to another SGA.
- If non-response, check compliance, measure levels (where possible), and consider increasing dose. Consider concurrent use of SGA and MS.
- Judicious extrapolation of the evidence from adults13 is required because of the very limited evidence base in youth with BAD. This includes treatment duration and prophylaxis.11,12
- We recommend that a successful acute treatment of a mood episode should be continued as long-term prophylaxis.
Specific issues
- Bipolar depression is a common clinical challenge, the treatment of which has been understudied in youth. In adults, there is considerably better evidence about efficacious treatments (see section on 'Bipolar depression' in Chapter 3), such as quetiapine;14,15 surprisingly, however, a small study in 32 adolescents,16 followed by a larger RCT17 (n = 193) failed to show effectiveness. This study had a high placebo response and this will need to be reviewed once the data have been published in an academic journal. Lurasidone was recently shown to be effective in bipolar depression in adults18 with a benign metabolic profile, which makes it a good candidate for trials in youth. Note that lamotrigine has only modest, if any, effects in adult bipolar depression;19 it has not been studied in RCTs in children and adolescents and is, therefore, not recommended. Antidepressants should be used with care and only in presence of an antimanic agent.11 There is very little evidence for the benefit of antidepressants in bipolar depression in adults.20 Due to the dearth of trials in youth, we would recommend careful extrapolation from adult studies and use of quetiapine in older adolescents as first-line treatment.
- The exact relationship between ADHD and BAD is still debated. Some evidence suggests that stimulants in children with ADHD and manic symptoms may be well tolerated21 and that they may be safe and effective to use after mood stabilisation.21 Caution and experience with prescribing these drugs are required.
Table 5.1 Summary of RCT evidence on medication used in youth with bipolar mania
|
Medication
|
Comment
|
|
Lithium
|
One double-blind placebo-controlled randomised trial23 showed significant reductions in substance use and clinical ratings after 6 weeks, in 25 adolescents with BAD and co-morbid substance abuse. In a double blind placebo-controlled discontinuation trial (n = 40) over 2 weeks, no significant difference in relapse rates were found between lithium and placebo24
Lithium and divalproex did not differ in an 18-months maintenance trial in youths (n = 60) who initially stabilised on combination pharmacotherapy of lithium and divalproex25
|
|
Valproate
|
In an RCT (n = 150)26 divalproex ER (titrated to clinical response or 80-125 mg/L) did not lead to significant differences in mean YMRS compared with placebo at 4 weeks
|
|
Oxcarbazepine
|
A double-blind placebo-controlled study (n = 116) did not show significant differences between placebo and oxcarbazepine (mean dose 15 mg/day) in reducing mania rating at 7 weeks27
|
|
Olanzapine
|
A double blind, placebo-controlled study (n = 161)28 showed olanzapine (5-20 mg/day) to be significantly more effective than placebo in YMRS mean score reduction over a period of 3 weeks. Note the higher weight gain in the treatment group (weight gain was 3.7 kg for olanzapine versus 0.3 kg for placebo) and the associated significantly increased fasting glucose, total cholesterol, AST, ALT, and uric acid
|
|
Risperidone
|
A double blind, placebo-controlled study (n = 169) showed risperidone (at doses 0.5-2.5 or 3-6 mg) to be significantly more effective than placebo in YMRS mean score reduction in a 3-week follow up.29 The lower dose seems to lead to same benefits at a lower risk of side-effects. Sleepiness and fatigue common in the treatment arms. Note, mean weight increase in treatment groups (0.7 kg versus 1.7 kg for the low and 1.4 kg for the high dose arm)
In the Treatment of Early Age Mania (TEAM) study, higher response rates (and metabolic side-effects) occurred with risperidone (mean dose of 2.57 mg) versus lithium (mean level of 1.09 mmol/L) and divalproex sodium (mean level of 113.6 mg/L).30 However, the results need to be interpreted with caution as the definition of mania was broad
|
|
Quetiapine
|
A double blind, placebo-controlled study (n = 277)31 showed quetiapine (at doses of 400 mg/day or 600 mg/day) to be significantly better than placebo in reducing mean YMRS scores at 3 weeks. The most common side-effects included somnolence and sedation. Weight gain was 1.7 kg in the quetiapine group versus 0.4 kg for placebo
Quetiapine is effective as an adjunct to valproate compared with valproate alone (n = 30, 6 weeks)32 and was equally effective as valproate in a double blind trial (n = 50, 4 weeks)33
|
|
Aripiprazole
|
A double blind placebo controlled study34,35 showed aripiprazole (at doses 10 mg/day or 30 mg/day) to be significantly better than placebo in reducing mean YMRS scores at both 4 weeks (n = 296)34 and 30 weeks (n = 210)35. Note, the significantly higher incidence of extrapyramidal side-effects in the treatment groups (especially the higher dose). Weight gain was significantly higher in the treatment groups compared to placebo (3.0 kg versus 6.5 kg for the low and 6.6 kg for the high dose arm) at week 30 but not at week 4
|
|
Ziprasidone
|
A double blind, placebo-controlled trial (n = 237)36 showed ziprasidone (at flexible doses 40-160 mg) to be significantly more effective than placebo in reducing mean YMRS scores at 4 weeks. Sedation and somnolence were the most common side-effects, while it demonstrated a neutral metabolic profile and no QTc prolongation
Ziprasidone is not marketed in the UK and some other countries
|
|
ALT, alanine transaminase; AST, aspartate aminotransferase; BAD, bipolar affective disorder; ER, extended release; NICE, National Institute for Health and Clinical Excellence; RCT, randomised controlled trial; YMRS, Young Mania Rating Scale.
|
Table 5.2 Recommended first-line treatments for acute mania*
|
Drug
|
Dose
|
|
Aripiprazole
|
10 mg daily
|
|
Olanzapine
|
5-20 mg daily
|
|
Quetiapine
|
Up to 400 mg daily
|
|
Risperidone
|
0.5-2.5 mg daily
|
|
*Continue acutely effective dosing regimen as prophylaxis.
|
Table 5.3 Recommended first-line treatments for bipolar depression*
|
Drug
|
Dose
|
|
Lurasidone
|
18.5-111 (20-120) mg daily
|
|
Olanzapine
|
5-2 0 mg daily
|
|
Quetiapine
|
Up to 300 mg daily
|
|
*Continue acutely effective dosing regimen as prophylaxis.
|
- The DSM-5 has introduced the new category of Disruptive Mood Dysregulation Disorder (DMDD) to capture severely irritable children (who were commonly misdiagnosed as having BAD in parts of the USA). There is no established treatment for DMDD yet. Lithium is ineffective,22 but SSRIs and psychological treatment options may be considered.5
Other treatments
There is evidence for adults and children that adjunct treatments including psychoeducation, CBT and especially family-focused interventions, can enhance treatment and reduce depression relapse rates in bipolar disorder.13
References
- Carlson GA et al. Phenomenology and diagnosis of bipolar disorder in children, adolescents, and adults: complexities and developmental issues. Dev Psychopathol 2006; 18:939–969.
- Leibenluft E. Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry 2011; 168:129–142.
- Costello EJ et al. The Great Smoky Mountains Study of Youth. Goals, design, methods, and the prevalence of DSM-III-R disorders. Arch Gen Psychiatry 1996; 53:1129–1136.
- Stringaris A et al. Youth meeting symptom and impairment criteria for mania-like episodes lasting less than four days: an epidemiological enquiry. J Child Psychol Psychiatry 2010; 51:31–38.
- Krieger FV et al. Bipolar disorder and disruptive mood dysregulation in children and adolescents: assessment, diagnosis and treatment. Evid Based Ment Health 2013; 16:93–94.
- Colom F et al. A randomized trial on the efficacy of group psychoeducation in the prophylaxis of recurrences in bipolar patients whose disease is in remission. Arch Gen Psychiatry 2003; 60:402–407.
- Young RC et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133:429–435.
- Poznanski EO et al. Preliminary studies of the reliability and validity of the children's depression rating scale. J Am Acad Child Psychiatry 1984; 23:191–197.
- Spearing MK et al. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res 1997; 73:159–171.
- Kowatch RA et al. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2005; 44:213–235.
- National Institute for Clinical Excellence. Bipolar disorder. The management of bipolar disorder in adults, children and adolescents, in primary and secondary care. Clinical Guideline 38, 2006. http://www.nice.org.uk
- Correll CU et al. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord 2010; 12:116–141.
- Geddes JR et al. Treatment of bipolar disorder. Lancet 2013; 381:1672–1682.
- Calabrese JR et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry 2005; 162:1351–1360.
- Thase ME et al. Efficacy of quetiapine monotherapy in bipolar I and II depression: a double-blind, placebo-controlled study (the BOLDER II study). J Clin Psychopharmacol 2006; 26:600–609.
- Delbello MP et al. A double-blind, placebo-controlled pilot study of quetiapine for depressed adolescents with bipolar disorder. Bipolar Disord 2009; 11:483–493.
- AstraZeneca. An 8-week, multicenter, double-blind, randomized, parallel-group, placebo-controlled study of the efficacy and safety of quetiapine fumarate (SEROQUEL) extended-release in children and adolescent subjects with bipolar depression (Clinical Trial NCT00811473), 2012. http://clinicaltrials.gov/ct2/show/study/NCT00811473
- Loebel A et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry 2014; 171:160–168.
- Calabrese JR et al. Lamotrigine in the acute treatment of bipolar depression: results of five double-blind, placebo-controlled clinical trials. Bipolar Disord 2008; 10:323–333.
- Pacchiarotti I et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry 2013; 170:1249–1262.
- Goldsmith M et al. Antidepressants and psychostimulants in pediatric populations: is there an association with mania? Paediatr Drugs 2011; 13:225–243.
- Dickstein DP et al. Randomized double-blind placebo-controlled trial of lithium in youths with severe mood dysregulation. J Child Adolesc Psychopharmacol 2009; 19:61–73.
- Geller B et al. Double-blind and placebo-controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry 1998; 37:171–178.
- Kafantaris V et al. Lithium treatment of acute mania in adolescents: a placebo-controlled discontinuation study. J Am Acad Child Adolesc Psychiatry 2004; 43:984–993.
- Findling RL et al. Double-blind 18-month trial of lithium versus divalproex maintenance treatment in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry 2005; 44:409–417.
- Wagner KD et al. A double-blind, randomized, placebo-controlled trial of divalproex extended-release in the treatment of bipolar disorder in children and adolescents. J Am Acad Child Adolesc Psychiatry 2009; 48:519–532.
- Wagner KD et al. A double-blind, randomized, placebo-controlled trial of oxcarbazepine in the treatment of bipolar disorder in children and adolescents. Am J Psychiatry 2006; 163:1179–1186.
- Tohen M et al. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry 2007; 164:1547–1556.
- Haas M et al. Risperidone for the treatment of acute mania in children and adolescents with bipolar disorder: a randomized, double-blind, placebo-controlled study. Bipolar Disord 2009; 11:687–700.
- Geller B et al. A randomized controlled trial of risperidone, lithium, or divalproex sodium for initial treatment of bipolar I disorder, manic or mixed phase, in children and adolescents. Arch Gen Psychiatry 2012; 69:515–528.
- Pathak S et al. Efficacy and safety of quetiapine in children and adolescents with mania associated with bipolar I disorder: a 3-week, doubleblind, placebo-controlled trial. J Clin Psychiatry 2013; 74:e100–e109.
- Delbello MP et al. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry 2002; 41:1216–1223.
- Delbello MP et al. A double-blind randomized pilot study comparing quetiapine and divalproex for adolescent mania. J Am Acad Child Adolesc Psychiatry 2006; 45:305–313.
- Findling RL et al. Acute treatment of pediatric bipolar I disorder, manic or mixed episode, with aripiprazole: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2009; 70:1441–1451.
- Findling RL et al. Aripiprazole for the treatment of pediatric bipolar I disorder: a 30-week, randomized, placebo-controlled study. Bipolar Disord 2013; 15:138–149.
- Findling RL et al. Ziprasidone in adolescents with schizophrenia: results from a placebo-controlled efficacy and long-term open-extension study. J Child Adolesc Psychopharmacol 2013; 23:531–544.
Psychosis in children and adolescents
Schizophrenia is rare in children but the incidence increases rapidly in adolescence. Early-onset schizophrenia-spectrum (EOSS) disorder is often chronic and in the majority of cases requires long-term treatment with antipsychotic medication.1
There have been three major RCTs of first-generation antipsychotics (FGAs), all of them showing high rates of extrapyramidal side-effects (EPSs) and significant sedation.1 Treatment-emergent dyskinesias can also be problematic.2 First-generation antipsychotics should generally be avoided in children.
There have been a number of randomised controlled trials of second-generation antipsychotics in EOSS disorder.3–8 Olanzapine, risperidone and aripiprazole have all been shown to be effective in the treatment of psychosis but there is no information to support the superiority of any one agent over another. There is also some evidence from uncontrolled trials for quetiapine9–11 and for ziprasidone,12 but concerns have been raised about the safety of ziprasidone.13,14
Children and adolescents are at greater risk than adults for side-effects such as extrapyramidal symptoms, raised prolactin, sedation, weight gain and metabolic effects.15
There is evidence that clozapine is effective in treatment-resistant psychosis in adolescents, although this population may be more prone to neutropenia and seizures than adults.16–18
Overall, algorithms for treating psychosis in young people are the same as those for adult patients (see Chapter 2). NICE19 recommends oral antipsychotics in conjunction with family interventions and individual CBT. Doses should be at the lower end of the adult range if licensed for children and adolescents; below the lower range if not. See Box 5.2.
Box 5.2 Summary of drug treatment of psychosis in children and adolescents
|
Box 5.2 Summary of drug treatment of psychosis in children and adolescents
|
|
First choice
|
Allow patient to choose from:
aripiprazole (to 10 mg),
olanzapine (to 10 mg)
risperidone (to 3 mg)
|
|
Second choice
|
Switch to alternative from list above*
|
|
Third choice
|
Clozapine
|
|
* Based on data obtained from the treatment of younger adults, olanzapine should be tried before moving to clozapine.20
|
|
References
- Kumra S et al. Efficacy and tolerability of second-generation antipsychotics in children and adolescents with schizophrenia. Schizophr Bull 2008; 34:60–71.
- Connor DF et al. Neuroleptic-related dyskinesias in children and adolescents. J Clin Psychiatry 2001; 62:967–974.
- Sikich L et al. A pilot study of risperidone, olanzapine, and haloperidol in psychotic youth: a double-blind, randomized, 8-week trial. Neuropsychopharmacology 2004; 29:133–145.
- Findling RL et al. A multiple-center, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry 2008; 165:1432–1441.
- Haas M et al. A 6-week, randomized, double-blind, placebo-controlled study of the efficacy and safety of risperidone in adolescents with schizophrenia. J Child Adolesc Psychopharmacol 2009; 19:611–621.
- Haas M et al. Efficacy, safety and tolerability of two dosing regimens in adolescent schizophrenia: double-blind study. Br J Psychiatry 2009; 194:158–164.
- Sikich L et al. Double-blind comparison of firstand second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: findings from the treatment of early-onset schizophrenia spectrum disorders (TEOSS) study. Am J Psychiatry 2008; 165:1420–1431.
- Kryzhanovskaya L et al. Olanzapine versus placebo in adolescents with schizophrenia: a 6-week, randomized, double-blind, placebocontrolled trial. J Am Acad Child Adolesc Psychiatry 2009; 48:60–70.
- McConville B et al. Long-term safety, tolerability, and clinical efficacy of quetiapine in adolescents: an open-label extension trial. J Child Adolesc Psychopharmacol 2003; 13:75–82.
- Schimmelmann BG et al. A prospective 12-week study of quetiapine in adolescents with schizophrenia spectrum disorders. J Child Adolesc Psychopharmacol 2007; 17:768–778.
- McConville BJ et al. Pharmacokinetics, tolerability, and clinical effectiveness of quetiapine fumarate: an open-label trial in adolescents with psychotic disorders. J Clin Psychiatry 2000; 61:252–260.
- Patel NC et al. Experience with ziprasidone. J Am Acad Child Adolesc Psychiatry 2002; 41:495.
- Scahill L et al. Sudden death in a patient with Tourette syndrome during a clinical trial of ziprasidone. J Psychopharmacol 2005; 19:205–206.
- Blair J et al. Electrocardiographic changes in children and adolescents treated with ziprasidone: a prospective study. J Am Acad Child Adolesc Psychiatry 2005; 44:73–79.
- Correll CU. Addressing adverse effects of antipsychotic treatment in young patients with schizophrenia. J Clin Psychiatry 2011; 72:e01.
- Kumra S et al. Childhood-onset schizophrenia. A double-blind clozapine-haloperidol comparison. Arch Gen Psychiatry 1996; 53:1090–1097.
- Shaw P et al. Childhood-onset schizophrenia: A double-blind, randomized clozapine-olanzapine comparison. Arch Gen Psychiatry 2006; 63:721–730.
- Kumra S et al. Clozapine and "high-dose" olanzapine in refractory early-onset schizophrenia: a 12-week randomized and double-blind comparison. Biol Psychiatry 2008; 63:524–529.
- National Institute for Health and Care Excellence. Psychosis and schizophrenia in children and young people: Recognition and management. Clinical Guideline 155, 2013. http://www.nice.org/
- Agid O et al. An algorithm-based approach to first-episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry 2011; 72:1439–1444.
Further reading
Masi G et al. Management of schizophrenia in children and adolescents: focus on pharmacotherapy. Drugs 2011; 71:179–208.
Anxiety disorders in children and adolescents
Diagnostic issues
Fear and worry are common in children and they are part of normal development. At the same time, anxiety disorders often begin in childhood and adolescence1 and they are the most common psychiatric disorders in this age group, with overall prevalence between 8% and 30% depending on the impairment cut-offs used.2 Anxiety disorders may be even more common in children with neurodevelopment disorders.3
In children, the more obvious clinical presentation with distress and avoidance may be masked by prominent behavioural symptoms (e.g. irritability and angry outbursts linked to avoidance). Therefore, the assessment and treatment of anxiety disorders in children needs to be undertaken by clinicians who can discriminate normal, developmentally appropriate worries, fears and shyness from anxiety disorders that significantly impair a child's functioning, and who can appreciate developmental variations in the presentation of symptoms.
Clinical guidance
Anxiety symptoms in children and adolescents often improve with age, presumably in parallel to the development of the prefrontal cortex and, in particular, executive functions. However, anxiety disorders are distressing and impairing conditions that need to be treated promptly. Chronic stress mediators may have significant impact on brain development4 and functional impairment linked to anxiety symptoms may prevent young people from accessing normative experiences that are critical for social, emotional, and cognitive development. Consistent with these detrimental effects, young people with anxiety disorders are, for example, three times more likely to have anxiety and depression in adult life compared to nonanxious youths.5
Guidelines for the treatment of anxiety disorders in children and adolescents have been made available in the UK and the US. NICE guidelines focus on the treatment of social anxiety disorder in children and adolescents, suggesting the use of cognitive behavioural therapy and cautioning against the routine use of pharmacological treatment for social anxiety in this age group.6 Guidelines from the American Academy of Child and Adolescent Psychiatry (AACAP) cover the treatment of all non-obsessive compulsive disorder (OCD), non-post-traumatic stress disorder (PTSD) anxiety disorders7. AACAP guidelines suggest multimodal treatment including psycho-education, psychotherapy (e.g. a 12-session course of exposure-based CBT), and pharmacotherapy. Drug treatment is endorsed for moderate-to-severe anxiety symptoms, when impairment makes participation in psychotherapy difficult, or when psychotherapy leads to only partial response.
Prescribing for anxiety disorders in children and adolescents
Before prescribing
- Exclude other diagnoses. Anxiety symptoms can be mimicked by a range of psychiatric disorders including depression (inattention, sleep problems), bipolar disorder (irritability, sleep problems, restlessness), oppositional-defiant disorder (irritability, oppositional behaviour), psychotic disorders (social withdrawal, restlessness), ADHD (inattention, restlessness), Asperger syndrome (social withdrawal, poor social skills, repetitive behaviours and routines), and learning disabilities. They may also be mimicked by a range of endocrine (hyperthyroidism, hypoglycaemia, pheochromocytoma), neurological (migraine, seizures, delirium, brain tumours), cardiovascular (cardiac arrhythmias), and respiratory (asthma) conditions and lead intoxication. Anxiety-like symptoms can be observed in response to several drugs and substances including anti-asthma medications, sympathomimetics, steroids, SSRIs, antipsychotics (akathisia), diet pills, cold medicines, caffeine and energy drinks.
- Beware contraindications to SSRIs and potential interactions.
- Measure baseline severity. Structured interviews including the Anxiety Disorders Interview Schedule (ADIS) and the Kiddie-Schedule for Affective Disorders and Schizophrenia (Kiddie-SADS). Questionnaires including the Revised Children's Anxiety and Depression Scale (RCADS), Screen for Child Anxiety and Related Emotional Disorders (SCARED), or the Multidimensional Anxiety Scale for Children (MASC). Measures of functional impairment including the Children's Global Assessment Scale (CGAS) and the Clinical Global Impression scales (CGI)
- Obtain consent. Discuss treatment with the young person and the family (e.g. name of medication, starting/estimated ending dose, titration timeline, possible side-effects and strategies to monitor/minimise them, strategies to monitor progress, interventions for treatment-resistant cases). Document consent in writing.
What to prescribe
- SSRIs are the medications of choice for the treatment of anxiety disorders in children and adolescents. A Cochrane systematic review8 shows that there are seven shortterm RCTs (< 16 weeks; n treatment = 453, n control = 389) testing the efficacy of SSRIs (fluoxetine, fluvoxamine, paroxetine, sertraline) on changes in impairment for anxiety disorders in young people (CGI-I), with an overall relative risk of response of 2.38 [95% CI= 2.01–2.83] over placebo, number needed to treat (NNT) of 2–3, and no significant difference among SSRIs. The Childhood Anxiety Multimodal Study (CAMS) showed that monotherapy with sertraline (55% response) is as effective as CBT for anxiety (60% response) compared with placebo (24% response), and that combined therapy with sertraline and CBT is most likely to be successful (81% response).9 Sertraline, fluoxetine and fluvoxamine have been approved by the US FDA for treatment of paediatric OCD, and fluoxetine and escitalopram have been approved for treatment of paediatric depression. In 2004, the US FDA issued a Black Box warning for concerns related to worsening of depression, agitation, and suicidal ideation linked to SSRIs. These concerns were based on a review of studies of adolescents with depression rather than young people with anxiety.
- Venlafaxine was tested in two short-term RCTs (n treatment = 295, n control = 311) with an overall relative risk of response of 1.46 [95% CI= 1.25–1.71] over placebo (which was significantly lower than the overall effect of SSRIs; see above). Because of the different pharmacodynamic actions, venlafaxine could be considered a secondline treatment when SSRIs are ineffective. The evidence base for this strategy in this group of patients is, however, non-existent.
- The efficacy and safety of buspirone and mirtazapine in young people with anxiety disorders is not known, although open-label studies10,11 suggest that they might be effective in relieving anxiety symptoms.
- Benzodiazepine use is not supported by controlled trials in children,12 and may lead to paradoxical disinhibition in some children. Nevertheless, benzodiazepine use is at times considered in clinical practice to 'potentiate' therapeutic effect during initial titration of SSRIs (or to mitigate adverse effects) and for rapid tranquillisation.
A summary of the medications and doses used in the treatment of anxiety disorders is shown in Table 5.4.
Table 5.4 Typical dosage of medications for treatment of anxiety disorders in children and adolescents
|
Medication
|
Starting dose (mg)
|
Dose range (mg)
|
|
SSRI
|
|
Sertraline
|
12.5-25
|
25-200 od
|
|
Fluoxetine
|
5-10
|
10-60 od
|
|
Fluvoxamine
|
12.5-25
|
50-200 (bd if > 50)
|
|
Paroxetine
|
5-10
|
10-40 od
|
|
Citalopram*
|
5-10
|
10-40 od
|
|
SNRI
|
|
Venlafaxine ER
|
37.5
|
37.5-225 od
|
|
5-HT1A partial agonist
|
|
Buspirone*
|
5 tds
|
15-60 od
|
|
Tetracyclic
|
|
Mirtazapine*
|
7.5-15
|
7.5-30 at night
|
|
Benzodiazepine* (prn)
|
|
Clonazepam
|
0.25-0.5
|
-
|
|
Lorazepam
|
0.5-1
|
-
|
|
Always check dose with latest formal guidance, e.g. BNF for Children.
*Treatments not supported by RCT evidence.
bd, bis die (twice a day); ER, extended release; od, omni die (once a day); prn, pro re nata (as required); SNRI, selective noradrenaline reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; tds, ter die sumendus (three times a day).
|
After prescribing
- Acute phase
- Start at low dose and titrate at regular (e.g. weekly) intervals.
- Monitor response (e.g. RCADS, SCARED, MASC, CGAS, CGI-I) frequently and systematically.
- Monitor side-effects. SSRIs are generally well tolerated during treatment for anxiety disorders in young people. However, side-effects including gastrointestinal symptoms (e.g. nausea, vomiting, dyspepsia, abdominal pain, diarrhoea, constipation), headache, increased motor activity, and insomnia may occur, often in mild and transient form.
- Therapeutic effect should start after 3–6 weeks of treatment but maximum effect can take up to 12–16 weeks. It is important to communicate this to families.
- If partial or non-response, consider accuracy of diagnosis, adequacy of medication trial, and compliance of patient.
- To improve response, consider: adding CBT, changing medication (e.g. switch SSRIs, other classes), or combining medications (e.g. for co-morbidities, to treat side-effects, to potentiate action).
- Maintenance phase
- Continue maintenance treatment for at least 1 year of stable improvement.
- Monitor response and side-effects regularly.
- Discontinuation phase
- Because of lack of information on long-term safety and possible improvement in symptoms with age and learning, consider discontinuing treatment after a period of stable improvement. A trial off-medication should be started at a period of low stress/demands. Discontinuation should also be considered if the medication is no longer working or the side-effects are too severe. Taper SSRIs slowly to minimise risk of withdrawal symptoms. Monitor closely for recurrence of symptoms/relapse and, if deterioration is noted, promptly restart medications.
Specific issues
Treatment of anxiety disorders in pre-school children must routinely focus on psychotherapy. In rare cases when a very young child has extreme ongoing symptoms and impairment, clinicians should reconsider diagnosis and case formulation, and reassess the adequacy of the psychotherapy trial. There are no RCTs of pharmacological interventions for anxiety in pre-school children but case reports suggest potential benefit of fluoxetine and buspirone.13
There has also been an interest in the role of pharmacological intervention to augment the effect of exposure therapy in PTSD.14 An RCT showed that administration of d-cycloserine, a partial agonist of the N-methyl-D-aspartate (NMDA) receptor involved in fear learning and extinction, potentiates the therapeutic effect of psychotherapy in adults with social anxiety.15 No study has tested this effect in young people.
References
- Kessler RC et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62:593–602.
- Merikangas KR et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry 2010; 49:980–989.
- Simonoff E et al. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 2008; 47:921–929.
- Danese A et al. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav 2012; 106:29–39.
- Pine DS et al. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry 1998; 55:56–64.
- National Institute for Health and Care Excellence. Social anxiety disorder: recognition, assessment and treatment. Clinical Guideline 159, 2013. http://www.nice.org.uk/
- Connolly SD et al. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry 2007; 46:267–283.
- Ipser JC et al. Pharmacotherapy for anxiety disorders in children and adolescents. Cochrane Database Syst Rev 2009; CD005170.
- Walkup JT et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med 2008; 359:2753–2766.
- Buitelaar JK et al. Buspirone in the management of anxiety and irritability in children with pervasive developmental disorders: results of an open-label study. J Clin Psychiatry 1998; 59:56–59.
- Mrakotsky C et al. Prospective open-label pilot trial of mirtazapine in children and adolescents with social phobia. J Anxiety Disord 2008; 22:88–97.
- Simeon JG et al. Clinical, cognitive, and neurophysiological effects of alprazolam in children and adolescents with overanxious and avoidant disorders. J Am Acad Child Adolesc Psychiatry 1992; 31:29–33.
- Gleason MM et al. Psychopharmacological treatment for very young children: contexts and guidelines. J Am Acad Child Adolesc Psychiatry 2007; 46:1532–1572.
- Parsons RG et al. Implications of memory modulation for post-traumatic stress and fear disorders. Nat Neurosci 2013; 16:146–153.
- Hofmann SG et al. Augmentation of exposure therapy with d-cycloserine for social anxiety disorder. Arch Gen Psychiatry 2006; 63:298–304.
Obsessive compulsive disorder in children and adolescents
The treatment of obsessive compulsive disorder (OCD) in children follows the same principles as in adults (see Chapter 4). Cognitive behavioural therapy is effective in this patient group and is the treatment of first choice1,2 although it may be combined with medication.3
Sertraline4–6 (from 6 years of age) and fluvoxamine (from 8 years of age) are the SSRIs licensed in the UK for the treatment of OCD in young people. Studies spanning 20 years have established the efficacy of SSRIs in the paediatric population in placebo-controlled trials. Fluoxetine, fluvoxamine, paroxetine, citalopram, escitalopram and sertraline have all been shown to be efficacious and safe in young people with OCD. Clomipramine is a tricyclic with strong serotonin reuptake inhibition activity, which has been shown to be consistently superior to SSRIs5 in the paediatric population (aged 6–18 years). Clomipramine therefore remains a useful drug for some individuals, although its sideeffect profile (sedation, dry mouth, potential for cardiac side-effects) tend to limit its use in this age group. As a consequence SSRIs generally remain the recommended first choice medication for children and young people with OCD. All SSRIs appear to be equally effective, although they have different pharmacokinetics and side-effects.5 A meta-analysis of 12 RCTs of pharmacotherapy against control, in young people (under 19 years of age) showed that medication is consistently significantly more effective than placebo, and that there is no evidence that there are any clinically relevant differences between SSRIs.5
Initiation of treatment with medication
Clomipramine and SSRIs show a similar slow and incremental effect on obsessions and compulsions from as early as 1–2 weeks after initiation and placebo-referenced improvements continue for at least 24 weeks. Symptoms of depression show improvements in parallel with the OCD. In some cases, the effects can take several weeks to appear. In addition, the earliest signs of improvement may be apparent to an informant before the patient. In some instances improvements may take some months to become apparent. In light of this response profile, it is important to inform patients and their families about this, in addition to not feeling rushed to change medication because of only modest initial changes in symptoms. The use of an observer-rated quantitative measure such as the Children's Yale-Brown Obsessive Compulsive Scale (CY-BOCS), may therefore be helpful to monitor progress in clinical settings.The British Association of Psychopharmacology suggest starting at the lowest dose known to be effective and waiting for up to 12 weeks before evaluating effectiveness.7 Upward dosage titration is recommended if there is insufficient clinical response. In clinical practice, a balance has to be struck between tolerability and the rate of dosage increase in busy clinical services.
Prescribing SSRIs in children
In 2004, the British Medicines and Healthcare product and Regulatory Authority agency (MHRA) cautioned against the use of SSRIs in children and young people, due to a possible increased risk of suicidal ideation.8 Subsequent reanalysis of SSRI use in depressed adolescents showed a modest two-fold rise in suicidal ideation or behaviours. There were no completed suicides in over 4400 children and adolescents. Careful reanalysis of treatment data highlights that SSRIs are clearly more efficacious in the OCD group of patients than they are in the treatment of moderate depressive episodes in children and young people. Investigators concluded that in the paediatric OCD group, the pooled risk for suicidal ideation and attempts was less than 1% across all studies. This of course is an important risk and should be explained and carefully monitored. Nonetheless, the naturalistic course of untreated OCD is that it tends not to spontaneously remit and has tremendous morbidity. Careful, judicious use of medication is therefore important in alleviating the considerable suffering caused by OCD in children and young people.
On occasion, medications (SSRIs) other than sertraline, fluvoxamine and clomipramine may be used as 'off-label' preparations with the appropriate and suitable caution. Indeed, NICE guidance9 for the treatment of OCD recommends the use of SSRIs before use of clomipramine, due to the latter drug's greater propensity for side-effects and need for cardiac monitoring. Factors guiding the choice of other medications may include issues such as the presence of other disorders (fluoxetine for OCD with comorbid depression); a good treatment response to a certain drug in other family members; the presence of other disorders, as well as cost and availability. Some children find tablets or capsules hard to swallow and there are no licensed OCD preparations available in liquid form, although 'off-label' efficacious alternatives would include fluoxetine and escitalopram.
NICE guidelines for the assessment and treatment of OCD
NICE published guidelines in 2005 on the evidence-based treatment options for OCD (and body dysmorphic disorder) for young people and adults. NICE recommends a 'stepped care' model, with increasing intensity of treatment according to clinical severity and complexity.9 The assessment of the severity and impact of OCD can be aided by the use of the CY-BOCS questionnaire, both at baseline, and as a helpful monitoring tool.10
The summary treatment algorithm from the NICE guideline is shown in Figure 5.1.
CBT and medication in the treatment of childhood OCD
Medication has occasionally been used as initial treatment where there is no CBT available, or if the child is unable or unwilling to engage in CBT. Studies now show convincingly that CBT is superior to placebo and that that efforts should be made to try and ensure access to a suitably experienced CBT practitioner. On occasion, medication may be commenced before starting CBT, for instance in the context of significant co-morbid anxiety or depressed mood. Medication may also be indicated in those whose capacity to access CBT is limited by learning disabilities, although every attempt should be made to modify CBT protocols for such children.
The principle study that directly compared the efficacy of CBT, sertraline, and their combination, in children and adolescents, concluded that children with OCD should begin treatment with CBT alone or CBT plus an SSRI.2
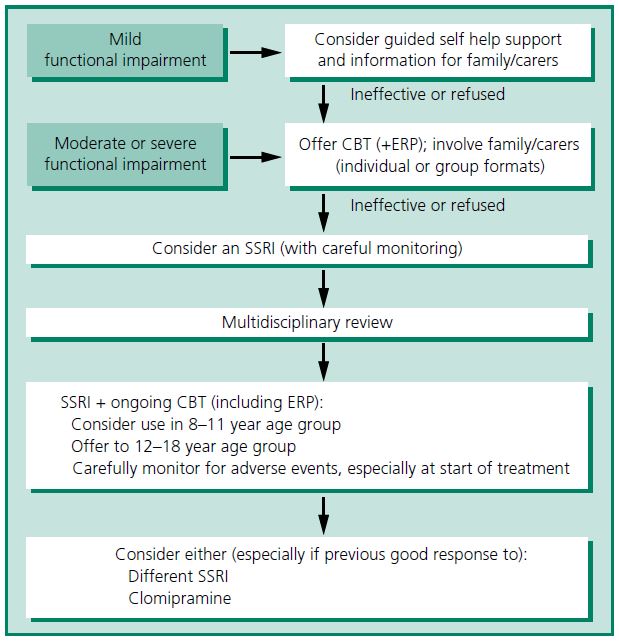
Figure 5.1 Treatment options for children and young people with OCD. CBT, cognitive behavioural therapy; ERP, exposure and response prevention; SSRI, selective serotonin reuptake inhibitor. Adapted from NICE guidance9 and reproduced from Heyman et al.11 with permission from BMJ Publishing Group Ltd.
Treatment of refractory OCD in children
Evidence from randomised trials suggests that up to three-quarters of medicated patients make an adequate response to treatment. Roughly one-quarter of children with OCD will therefore fail to respond to an initial SSRI, administered for at least 12 weeks at the maximum tolerated dose, in combination with an adequate trial of CBT and ERP. These children should be reassessed, clarifying compliance, and ensuring that co-morbidity is not being missed. These children should usually have additional trials of at least one other SSRI. Research suggests that approximately 40% respond to a second SSRI.12 Following this, if the response is limited, a child should usually be referred to a specialist centre. Trials of clomipramine may be considered and/or augmentation with a low dose of risperidone.11,13 Research hints at the fact that using a medication with a different method of action such as risperidone or clomipramine may benefit patients who have failed to respond to two adequate SSRI trials. There is evidence that antipsychotic augmentation, as an 'off-label' therapy, can benefit patients whose response to treatment has been inadequate despite at least 3 months of maximal tolerated SSRI. Unfortunately, only one-third of treatment-resistant adult cases showed a meaningful response to this augmentation strategy. The data would therefore suggest that caution should be exercised when augmenting treatment packages for OCD in children and young people. Often children whose OCD has been difficult to treat have co-morbidities such as autism spectrum disorder, ADHD, or tic disorders. The response to medication can be differentially affected by these co-morbidities. For instance, cases with tic disorders may benefit somewhat more from augmentation with second-generation antipsychotics. Careful clinical review and reformulation is important in OCD treatment resistance. The impact of co-morbidities and wider psychosocial factors need to be considered for their impact on the treatment response overall.
Neither ketamine14 nor riluzole15 are effective in refractory childhood OCD.
Duration of treatment and long-term follow-up
Untreated OCD tends to run a chronic course. A series of adult studies have shown that discontinuation of medication tends to result in symptomatic relapse. Some authors have suggested that those with co-morbidities are at the greatest risk of relapse. Given that studies frequently exclude cases with additional co-morbidities, it is likely that the relapse rates have been underestimated. NICE guidelines recommend that if a young person has responded to medication, treatment should continue for at least 6 months after remission. Clinical experience would suggest that when discontinuation of treatment is attempted it should be done slowly, cautiously and in a transparent manner with the patient and their family. Once again, the careful use of clinical outcome measures should be considered when stopping medication. The role of maintenance CBT and medication is under increasing scrutiny. Both appear to offer promise in maintaining gains made after initial treatment. It is important that throughout childhood, adolescence and into adult life, the individual with OCD should have access to health-care professionals, treatment opportunities and other support as needed, and NICE recommends that if relapse occurs, people with OCD should be seen as soon as possible rather than placed on a routine waiting list.
References
- O'Kearney RT et al. Behavioural and cognitive behavioural therapy for obsessive compulsive disorder in children and adolescents. Cochrane Database Syst Rev 2006; CD004856.
- Freeman JB et al. Cognitive behavioral treatment for young children with obsessive-compulsive disorder. Biol Psychiatry 2007; 61:337–343.
- Mancuso E et al. Treatment of pediatric obsessive-compulsive disorder: a review. J Child Adolesc Psychopharmacol 2010; 20:299–308.
- The Pediatric OCD Treatment Study Team (POTS). Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA 2004; 292:1969–1976.
- Geller DA et al. Which SSRI? A meta-analysis of pharmacotherapy trials in pediatric obsessive-compulsive disorder. Am J Psychiatry 2003; 160:1919–1928.
- March JS et al. Treatment benefit and the risk of suicidality in multicenter, randomized, controlled trials of sertraline in children and adolescents. J Child Adolesc Psychopharmacol 2006; 16:91–102.
- Baldwin DS et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: A revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol 2014; 28:403–439.
- Medicines and Healthcare Products Regulatory Agency. Report of the CSM expert working group on the safety of selective serotonin reuptake inhibitor antidepressants. London: MHRA; 2004. http://www.mhra.gov.uk
- National Institute for Health and Clinical Excellence. Obsessive-compulsive disorder: Core interventions in the treatment of obsessivecompulsive disorder and body dysmorphic disorder. Clinical Guideline 31, 2005. http://www.nice.org.uk.
- Scahill L et al. Children's Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry 1997; 36:844–852.
- Heyman I et al. Obsessive-compulsive disorder. BMJ 2006; 333:424–429.
- Grados M et al. Pharmacotherapy in children and adolescents with obsessive-compulsive disorder. Child Adolesc Psychiatr Clin N Am 1999; 8:617–34, x.
- Bloch MH et al. A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Mol Psychiatry 2006; 11:622–632.
- Bloch MH et al. Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biol Psychiatry 2012; 72:964–970.
- Grant PJ et al. 12-week, placebo-controlled trial of add-on riluzole in the treatment of childhood-onset obsessive-compulsive disorder. Neuropsychopharmacology 2014; 39:1453–1459.
Post-traumatic stress disorder in children and adolescents
Diagnostic issues
Traumatic events and post-traumatic stress disorder (PTSD) are common in young people. One in four children experiences traumatic events1 and nearly 1 in 10 children develops PTSD2 before the age of 18. The prevalence of PTSD in adolescents is 4% in males and 6% in females from the general population,3 and could be as high as 30% in young people attending emergency departments. Furthermore, young people with significant PTSD symptoms, but sub-threshold criteria for diagnosis, may show similar impairment and distress to children and adolescents with a diagnosis of PTSD and thus require treatment.4 Response to trauma may also involve other anxiety disorders, depression, self-harm, aggression, and substance abuse.
A diagnosis of PTSD is based on the triad of intrusive re-experiencing, avoidance of stimuli associated with the trauma, and hyper-arousal after trauma exposure. However, in children, re-experiencing may not be reported in the form of distressing visual flashbacks, but rather could be noted as compulsive repetition of aspects of trauma in play, drawings, or verbalisation, or as nightmares. Furthermore, certain types of avoidance (sense of a foreshortened future, inability to recall important aspects of the event) may not be detectable because of insufficient abilities with abstract cognition or verbal expression. In adolescents, PTSD symptoms are often associated with, and may be masked by, impulsive and aggressive behaviours.5,6 Because of the varied clinical manifestations, the assessment and treatment of PTSD in children and adolescents needs to be undertaken by clinicians who can appreciate developmental variations in the presentation of symptoms.
Clinical guidance
Guidelines for treatment of PTSD in children and adolescents are available in the UK and the US. NICE guidelines advise that treatment should be 12-sessions of traumafocused CBT for PTSD resulting from a single event (longer for chronic or recurrent events) and discourage routine prescription of medications.7 Guidelines by the American Academy of Child and Adolescent Psychiatry (AACAP) recommend trauma-focused CBT as first-line treatment for young people with PTSD and use of pharmacotherapy if the child's symptom severity, lack of response, or co-morbidity suggest need for additional interventions.8 The AACAP guidelines discuss SSRIs treatment, but also treatment with anti-adrenergic and second-generation antipsychotic medications.
Prescribing for anxiety disorders in young people
Before prescribing
- Exclude other diagnoses. (see section on 'Anxiety disorders in children and adolescents' earlier in this chapter.)
- Beware contraindication to SSRIs and potential interactions.
- Measure baseline severity. Structured interviews including the Anxiety Disorders Interview Schedule (ADIS) and the Kiddie-Schedule for Affective Disorders and Schizophrenia (Kiddie-SADS). Questionnaires, including the Child PTSD Symptom Scale (CPSS) and the UCLA Posttraumatic Stress Disorder Reaction Index. Measures of functional impairment including the Children's Global Assessment Scale (CGAS) and the Clinical Global Impression scales (CGI).
- Obtain consent. (see section on 'Anxiety disorders in children and adolescents' earlier in this chapter.)
What to prescribe
- SSRIs have shown only minimal evidence of clinical efficacy for the treatment of PTSD in children and adolescents, despite their efficacy in adults.9 A small 12-week RCT of add-on sertraline (n = 11) to routine TF-CBT treatment showed only marginal benefit of pharmacological treatment over TF-CBT and placebo (n = 11), which was not statistically significant.10 A larger 10-week RCT with flexibly dosed sertraline (n treated = 67; n placebo = 64) failed to detect a benefit over placebo.11 A small (n = 8) open-label study suggests potential efficacy of citalopram.12 It is possible that SSRIs may be more effective for the treatment of PTSD in young people in the presence of co-morbid major depressive episode, anxiety disorders, and OCD, although the evidence base for this is minimal.
- Anti-adrenergic medications have been studied for the treatment of PTSD in young people because of the evidence of noradrenergic hyperactivity in PTSD13,14 and the suggestive evidence of efficacy in adults.15 Clonidine is an α2-adrenergic agonist that reduces norepinephrine release. Clonidine is used 'off-label' in several paediatric conditions and an open-label trial (n = 7) in children showed that clonidine can improve PTSD symptoms, in particular re-living symptoms.16 Guanfacine is also an α2-adrenergic agonist. A case study suggested that guanfacine can improve PTSD symptoms, again particularly re-living symptoms, in young people.17 The most common side-effects of α2-adrenergic agonist are dry mouth and dizziness. Blood pressure should be monitored regularly and discontinuation should be slow to avoid rebound hypertension. Prazosin is an α1-adrenergic antagonist that reduces the post-synaptic effect of norepinephrine. Evidence in children and adolescents is limited to case reports which showed improvement of PTSD symptoms.18 Prazosin should be titrated slowly (e.g. 1 mg/week) and blood pressure (risk of orthostatic hypotension) should be carefully monitored, particularly early in treatment. Propranolol is a β-antagonist that reduces the post-synaptic effect of norepinephrine. In a on-off-on study, propranolol was shown to improve PTSD in children and adolescents.19 The most common side-effects include hypotension, bradycardia, dizziness, and bronchospasm. Blood pressure should be monitored regularly during titration.
- Second-generation antipsychotics have been studied for treatment of PTSD in children and adolescents based on the role of dopamine in various aspects of fear conditioning20 and on the efficacy of risperidone, olanzapine, aripiprazole (either as monotherapy or as adjunctive to SSRI therapy) on PTSD in adults.15,21 Evidence in children and adolescents is limited to case series and case studies with risperidone22 and quetiapine,23 which showed positive results.
Table 5.5 Typical dosage of medications for the treatment of PTSD in children and adolescents. These clinical guidelines are based on less than robust research evidence (e.g. case series) in children and adolescents and on extrapolation of data from adult trials
|
Medication
|
Starting dose (mg)
|
Dose range (mg)
|
|
SSRI
|
|
Sertraline
|
12.5-25
|
50-200 od
|
|
Citalopram
|
5-10
|
10-40 od
|
|
Anti-Adrenergic
|
|
Clonidine
|
0.05 nocte
|
0.1-0.2 nocte
|
|
Guanfacine
|
0.5 bd
|
1-3 nocte
|
|
Prazosin
|
1 nocte
|
2-4 nocte
|
|
Propranolol
|
10 tds
|
40-80/day
|
|
Second-generation antipsychotics
|
|
Risperidone
|
0.5
|
0.5-1 od
|
|
Quetiapine
|
25-50
|
50-200 od (at night)
|
|
Always check dose against latest formal guidance, e.g. BNF for Children.
bd, bis die (twice a day); nocte, at night; od, omni die (once a day); tds, ter die sumendus (three times a day).
|
- Mood stabilisers have been studied for the treatment of PTSD in adults, generally adjunctively in combination with SSRIs, and have been found to be effective.15 The literature in children and adolescents is limited to one open-label study (n = 28) with carbamazepine24 and one open-label study (n = 12) with valproate semisodium25 that showed positive results.
A summary of the medications and doses used in the treatment of PTSD is shown in Table 5.5.
After prescribing
- Acute phase
- Start at low dose and titrate at regular (e.g. weekly) intervals.
- Monitor response (e.g. CPSS, CGAS, CGI-I) frequently and systematically.
- Monitor side-effects.
- If partial or non-response, consider (1) accuracy of diagnosis, (2) adequacy of medication trial, and (3) compliance of patient.
- Maintenance phase
- Monitor response and side-effects regularly.
- Discontinuation phase
- Consider discontinuing treatment after a period of stable improvement.
- A trial off medication should be started at a period of low stress/demands.
- Discontinuation should also be considered if the medication is no longer working or the side-effects are too severe.
- Taper medications slowly to minimise risk of withdrawal symptoms.
- Monitor closely for recurrence of symptoms/relapse.
Specific issues
Treatment of PTSD in pre-school children must routinely focus on psychotherapy with either child–parent psychotherapy (CPP) or pre-school CBT. Pharmacological treatment of PTSD in pre-school children is not recommended.26
There has been an interest in preventive psychopharmacological interventions in the aftermath of trauma exposure, based on the findings that arousal and noradrenergic hyperactivity may promote consolidation of trauma memories.27 After initial positive results with the use of propranolol,28 subsequent larger studies and also studies in children and adolescents29 failed to detect significant protective effects. Morphine has a similar ability to inhibit noradrenergic activity, and studies in children and adolescents30 and adults31 suggest that morphine use after trauma might be effective in preventing development of PTSD. These findings require replication and morphine should not be used to prevent PTSD in routine clinical practice.
There has also been an interest in the role of pharmacological intervention to augment the effect of exposure therapy in PTSD.32 An RCT showed that administration of d-cycloserine, a partial agonist of the NMDA receptor involved in fear learning and extinction, potentiate the therapeutic effect of psychotherapy in adults with PTSD.33 No study has tested this effect in children and adolescents.
References
- Costello EJ et al. The prevalence of potentially traumatic events in childhood and adolescence. Trauma Stress 2002; 15:99–112.
- Breslau N et al. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry 1991; 48:216–222.
- Kilpatrick DG et al. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: results from the National Survey of Adolescents. J Consult Clin Psychol 2003; 71:692–700.
- Carrion VG et al. Toward an empirical definition of pediatric PTSD: the phenomenology of PTSD symptoms in youth. J Am Acad Child Adolesc Psychiatry 2002; 41:166–173.
- Scheeringa MS et al. PTSD in children and adolescents: toward an empirically based algorithma. Depress Anxiety 2011; 28:770–782.
- Meiser-Stedman R et al. The posttraumatic stress disorder diagnosis in preschooland elementary school-age children exposed to motor vehicle accidents. Am J Psychiatry 2008; 165:1326–1337.
- National Institute for Health and Clinical Excellence. Post-traumatic stress disorder (PTSD): The management of PTSD in adults and children in primary and secondary care. Clinical Guideline 26, 2005. http://www.nice.org.uk/guidance/CG26
- Cohen JA et al. Practice parameter for the assessment and treatment of children and adolescents with posttraumatic stress disorder. J Am Acad Child Adolesc Psychiatry 2010; 49:414–430.
- Stein DJ et al. Pharmacotherapy for post traumatic stress disorder (PTSD). Cochrane Database Syst Rev 2006; CD002795.
- Cohen JA et al. A pilot randomized controlled trial of combined trauma-focused CBT and sertraline for childhood PTSD symptoms. J Am Acad Child Adolesc Psychiatry 2007; 46:811–819.
- Robb AS et al. Sertraline treatment of children and adolescents with posttraumatic stress disorder: a double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol 2010; 20:463–471.
- Seedat S et al. An open trial of citalopram in adolescents with post-traumatic stress disorder. Int Clin Psychopharmacol 2001; 16:21–25.
- Geracioti TD, Jr. et al. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry 2001; 158:1227–1230.
- De B et al. Urinary catecholamine excretion in sexually abused girls. J Am Acad Child Adolesc Psychiatry 1994; 33:320–327.
- Strawn JR et al. Psychopharmacologic treatment of posttraumatic stress disorder in children and adolescents: a review. J Clin Psychiatry 2010; 71:932–941.
- Harmon RJ et al. Clonidine for posttraumatic stress disorder in preschool children. J Am Acad Child Adolesc Psychiatry 1996; 35:1247–1249.
- Horrigan JP. Guanfacine for PTSD nightmares. J Am Acad Child Adolesc Psychiatry 1996; 35:975–976.
- Strawn JR et al. Prazosin treatment of an adolescent with posttraumatic stress disorder. J Child Adolesc Psychopharmacol 2009; 19:599–600.
- Famularo R et al. Propranolol treatment for childhood posttraumatic stress disorder, acute type. A pilot study. Am J Dis Child 1988; 142:1244–1247.
- Pezze MA et al. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol 2004; 74:301–320.
- Pae CU et al. The atypical antipsychotics olanzapine and risperidone in the treatment of posttraumatic stress disorder: a meta-analysis of randomized, double-blind, placebo-controlled clinical trials. Int Clin Psychopharmacol 2008; 23:1–8.
- Keeshin BR et al. Risperidone treatment of an adolescent with severe posttraumatic stress disorder. Ann Pharmacother 2009; 43:1374.
- Stathis S et al. A preliminary case series on the use of quetiapine for posttraumatic stress disorder in juveniles within a youth detention center. J Clin Psychopharmacol 2005; 25:539–544.
- Looff D et al. Carbamazepine for PTSD. J Am Acad Child Adolesc Psychiatry 1995; 34:703–704.
- Steiner H et al. Divalproex sodium for the treatment of PTSD and conduct disordered youth: a pilot randomized controlled clinical trial. Child Psychiatry Hum Dev 2007; 38:183–193.
- Gleason MM et al. Psychopharmacological treatment for very young children: contexts and guidelines. J Am Acad Child Adolesc Psychiatry 2007; 46:1532–1572.
- Cahill L et al. Beta-adrenergic activation and memory for emotional events. Nature 1994; 371:702–704.
- Vaiva G et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry 2003; 54:947–949.
- Nugent NR et al. The efficacy of early propranolol administration at reducing PTSD symptoms in pediatric injury patients: a pilot study. J Trauma Stress 2010; 23:282–287.
- Saxe G et al. Relationship between acute morphine and the course of PTSD in children with burns. J Am Acad Child Adolesc Psychiatry 2001; 40:915–921.
- Holbrook TL et al. Morphine use after combat injury in Iraq and post-traumatic stress disorder. N Engl J Med 2010; 362:110–117.
- Parsons RG et al. Implications of memory modulation for post-traumatic stress and fear disorders. Nat Neurosci 2013; 16:146–153.
- de Kleine RA et al. A randomized placebo-controlled trial of d-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biol Psychiatry 2012; 71:962–968.
Attention deficit hyperactivity disorder
ADHD in Children
- A diagnosis of attention deficit hyperactivity disorder (ADHD) should be made only after a comprehensive assessment by a specialist—usually, a child psychiatrist or a paediatrician with expertise in ADHD.1 Appropriate psychological, psychosocial and behavioural interventions should be put in place. Drug treatments should be only a part of the overall treatment plan.
- The indication for drug treatment is the presence of impairment resulting from ADHD; in mild-to-moderate cases the first treatments are usually behaviour therapy and education; medication is indicated as first-line therapy only in severe cases (e.g. those diagnosed as hyperkinetic disorder), and as second-line when psychological approaches have not been successful within a reasonable time (e.g. 8 weeks) or are inappropriate.
- Methylphenidate is usually the first choice of drug when a drug is indicated. It is a central nervous stimulant with a large evidence base from trials. Adverse effects include insomnia, anorexia, raised blood pressure and growth deceleration, which can usually be managed by symptomatic management and/or dose reduction (see Box 5.3).
- Dexamfetamine is an alternative central nervous system (CNS) stimulant; effects and adverse reactions are broadly similar to methylphenidate, but there is much less evidence on efficacy and safety than exists for methylphenidate, and it plays a part in illegal drug taking. Both methylphenidate and dexamfetamine are Controlled Drugs; prescriptions should be written appropriately and for not more than 28 days.
|
Box 5.3 NICE guidance: summary of treatment for attention deficit hyperactivity disorder19
|
- Drug treatment should only be initiated by a specialist and only after comprehensive assessment of mental and physical health and social influences.
- For cases with moderate (or lesser) degrees of severity, psychological interventions are recommended as initial therapy, with medication subsequently if still required.
- For severe cases (i.e. those with pervasive impairment from their ADHD), medication will usually be the first-line treatment.
- Methylphenidate, dexamfetamine and atomoxetine are recommended within their licensed indications.
- Methylphenidate is usually first choice of medication, but decision should include consideration of:
- co-morbid conditions (tics, Tourette's syndrome, epilepsy)
- tolerability and adverse effects
- convenience of dosing
- potential for diversion
- patient/parent preference.
- If using methylphenidate, consider modified-release preparations (convenience of single-day dosage, improving adherence, reducing stigma, acceptability to schools); or multiple doses of immediate-release (greater flexibility in controlling time-course of action, closer initial titration).
- Where more than one agent is considered suitable, the product with the lowest cost should be prescribed.
- Monitoring should include measurement of height and weight (with entry on growth charts) and recording of blood pressure and heart rate.
|
|
- Lisdexamfetamine is a'prodrug'; the dexamfetamine is complexed with the aminoacid lysine and in this form is inactive. It is gradually broken down (in red blood cells) so that dexamfetamine is gradually made available. It therefore has a similar practical role to extended-release preparations of methylphenidate; and, like them, is unlikely to be abused for recreational or dependency-driven purposes. Several randomised controlled trials have established it as superior to placebo in children2,3 and adolescents.4 Effect size from preliminary research appears to be at least as great as that of Oros-methylphenidate3 and it seems to have a similar range of adverse effects.5 Long-term data suggest that it can be considered as an alternative to extended-release methylphenidate.6
- Atomoxetine7–10 is a suitable first-line alternative. It may be particularly useful for children who do not respond to stimulants or whose medication cannot be administered during the day. It may also be suitable where stimulant diversion is a problem or when 'dopaminergic' adverse effects (such as tics, anxiety and stereotypies) become problematic on stimulants. Parents should be warned of the possibilities of suicidal thinking and liver disease emerging and advised of the possible features that they might notice.
- Third-line drugs include clonidine11 and tricyclic antidepressants.12 Very few children should receive these drugs for ADHD alone. There is some evidence supporting the efficacy of carbamazepine13 and bupropion. There is no evidence to support the use of second-generation antipsychotics14 for ADHD symptoms, but risperidone may be helpful in reducing severe coexistent levels of aggression and agitation, especially in those with moderate learning disability.15 Modafanil appears to be effective16 but has not been compared with standard treatments and its safety is not established. Guanfacine is approved in the USA17 but at present in the UK only for Phase 3 trials.
- Co-morbid psychiatric illness is common in ADHD children. Stimulants are often helpful overall12 but are unlikely to be appropriate for children who have a psychotic illness and problems with substance misuse should be managed in their own right before considering ADHD treatment.18
- Once stimulant treatment has been established, it is appropriate for repeat prescriptions to be supplied through general practitioners.19
ADHD in adults
Adult ADHD is recognised by both ICD-10 and DSM-V, and NICE guidance regards the first-line treatment as medication, following the same principles as for drug treatment in children.
- At least 25% of ADHD children will still have symptoms at the age of 30. It is appropriate to continue treatment started in childhood in adults whose symptoms remain disabling.
- A new diagnosis of ADHD in an adult should only be made after a comprehensive assessment, including information from other informants and where possible from adults who knew the patient as a child.
- The prevalence of substance misuse and antisocial personality disorder are high in adults whose ADHD was not recognised in childhood.20 Methylphenidate can be effective in this population,21 but caution is appropriate in prescribing and monitoring.
- Methylphenidate is usually the first choice of medication. Atomoxetine is also effective22 and is the only medication licensed for use in adults—and then only when treatment was initiated before the age of 18 years. Monitoring for symptoms of liver dysfunction and suicidal thinking is advised.
References
- National Institute for Health and Clinical Excellence. Methylphenidate, atomoxetine and dexamfetamine for attention deficit hyperactivity disorder (ADHD) in children and adolescents - guidance. Technology Appraisal 98, 2006. http://www.nice.org.uk
- Biederman J et al. Lisdexamfetamine dimesylate and mixed amphetamine salts extended-release in children with ADHD: a double-blind, placebo-controlled, crossover analog classroom study. Biol Psychiatry 2007; 62:970–976.
- Coghill D et al. European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/ hyperactivity disorder. Eur Neuropsychopharmacol 2013; 23:1208–1218.
- Findling RL et al. Efficacy and safety of lisdexamfetamine dimesylate in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2011; 50:395–405.
- Heal DJ et al. Amphetamine, past and present–a pharmacological and clinical perspective. J Psychopharmacol 2013; 27:479–496.
- Findling RL et al. Long-term effectiveness and safety of lisdexamfetamine dimesylate in school-aged children with attention-deficit/ hyperactivity disorder. CNS Spectr 2008; 13:614–620.
- Michelson D et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 2002; 159:1896–1901.
- Kratochvil CJ et al. Atomoxetine and methylphenidate treatment in children with ADHD: a prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry 2002; 41:776–784.
- Weiss M et al. A randomized, placebo-controlled study of once-daily atomoxetine in the school setting in children with ADHD. J Am Acad Child Adolesc Psychiatry 2005; 44:647–655.
- Kratochvil CJ et al. A Double-Blind, Placebo-Controlled Study of Atomoxetine in Young Children With ADHD. Pediatrics 2011; 127:e862–e868.
- Connor DF et al. A meta-analysis of clonidine for symptoms of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1999; 38:1551–1559.
- Hazell P. Tricyclic antidepressants in children: is there a rationale for use? CNS Drugs 1996; 5:233–239.
- Silva RR et al. Carbamazepine use in children and adolescents with features of attention-deficit hyperactivity disorder: a meta-analysis. J Am Acad Child Adolesc Psychiatry 1996; 35:352–358.
- Einarson TR et al. Novel antipsychotics for patients with attention-deficit hyperactivity disorder: a systematic review. Ottawa: Canadian Coordinating Office for Health Technology Assessment (CCOHTA) 2001;Technology Report No 17.
- Correia Filho AG et al. Comparison of risperidone and methylphenidate for reducing ADHD symptoms in children and adolescents with moderate mental retardation. J Am Acad Child Adolesc Psychiatry 2005; 44:748–755.
- Biederman J et al. A comparison of once-daily and divided doses of modafinil in children with attention-deficit/hyperactivity disorder: a randomized, double-blind, and placebo-controlled study. J Clin Psychiatry 2006; 67:727–735.
- Biederman J et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics 2008; 121:e73–e84.
- Hutchins P, Hazell P, Nunn K. Attention deficit hyperactivity disorder (ADHD). In: Nunn KP, Dey C, eds. The Clinician's Guide to Psychotropic Prescribing in Children and Adolescents. Sydney: Glade Publishing; 2003, pp.162–171.
- National Institute for Health and Clinical Excellence. Attention Deficit Hyperactivity Disorder: diagnosis and management of ADHD in children, young people and adults. Clinical Guideline 72, 2008. http://guidance.nice.org.uk.
- Cosgrove PVF. Attention deficit hyperactivity disorder. Primary Care Psychiatry 1997; 3:101–114.
- Spencer T et al. A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit hyperactivity disorder. Arch Gen Psychiatry 1995; 52:434–443.
- Spencer T et al. Effectiveness and tolerability of atomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1998; 155:693–695.
Further reading
Kutcher S et al. International consensus statement on attention-deficit/hyperactivity disorder (ADHD) and disruptive behaviour disorders (DBDs): clinical implications and treatment practice suggestions. Eur Neuropsychopharmacol 2004;14:11–28.
Nutt DJ et al. Evidence-based guidelines for management of attention-deficit/hyperactivity disorder in adolescents in transition to adult services and in adults: recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2007;21:10–41.
Taylor E et al. European clinical guidelines for hyperkinetic disorder—first upgrade. Eur Child Adolesc Psychiatry 2004;13 Suppl 1:17–30.
Prescribing in attention deficit hyperactivity disorder
See Table 5.6 for prescribing in ADHD.
Table 5.6 Prescribing in attention deficit hyperactivity disorder
|
Medication
|
Onset and duration of action
|
Dose
|
Comment
|
Recommended monitoring
|
|
Methylphenidate immediate release (Ritalin, Equasym)1,2
|
Onset: 20-60 min
Duration: 2-4 hours
|
Initially 5-10 mg daily titrated up to a maximum of 2 mg/kg/day in divided doses using weekly increments of 5-10 mg (maximum 100 mg)
|
Usually first-line treatment.
Generally well tolerated3
Controlled Drug
|
Blood pressure
Pulse
Height and weight
Monitor for insomnia, mood and appetite change and the development of tics4
|
|
Methylphenidate sustained release (Concerta XL)1,2,5-7
|
Concerta Onset:
30 min-2 hours
Duration: 12 hours
|
Concerta: Initially 18 mg in the morning, titrated up to a maximum of
54 mg - or after review up to 108 mg in adults
18 mg Concerta = 15 mg Ritalin
|
An afternoon dose of Ritalin may be required in some children to optimise treatment
Controlled Drug
|
Discontinue if no benefits seen in 1 month
|
|
Also Equasym XL8-9
|
Equasym XL:
Onset: 20-60 min
Duration: 8 hours
|
Equasym XL:
Initially 10 mg increasing as necessary to 60 mg once daily (max 100 in adults)
|
Controlled Drug
|
|
|
Also Medikinet10
|
Onset: 20-60 min
Duration up to 8 hours
|
Dose as Equasym
Capsules can be opened and sprinkled11
|
A larger fraction of the drug is available immediately than in other modified-release forms
Controlled Drug
|
|
|
Dexamfetamine immediate release (Dexedrine)3,12
|
Onset: 20-60 min
Duration: 3-6 hours
|
2.5-10mg daily to start, titrated up to a maximum of 20 mg (occasionally 40 mg) in divided doses using weekly increments of 2.5 mg
|
Considered to be less well tolerated than methylphenidate3
Controlled Drug
|
|
|
Lisdexamfetamine (Elvanse)13-15
|
Onset: 20-60min
Duration: 13+ hours
|
Initially 30mg in the morning, titrated up to a maximum of 70mg
|
Prodrug gradually hydrolysed to dexamfetamine
Treat as Controlled Drug
|
As for methylphenidate
Full long-term efficacy and safety data awaited
|
|
Atomoxetine16,17
|
Approximately 4-6 weeks (atomoxetine is a NA reuptake inhibitor)
|
When switching from a stimulant, continue stimulant for first 4 weeks of therapy
For children < 70 kg: start with 0.5mg/kg/day and increase after a minimum of 7 days to 1.2 mg/kg (single or divided doses) and increase up to 1.8 mg/kg/day if necessary
For children > 70 kg: start with 40 mg and increase after a minimum of 7 days to 80 mg
|
Efficacy may be a little lower than found for methylphenidate.18 May be useful where stimulant diversion is a problem19
Once-daily dosing convenient in schoolchildren
Not a Controlled Drug
|
Pulse
Blood pressure
Height
Weight
|
|
|
References
- Wolraich ML et al. Pharmacokinetic considerations in the treatment of attention-deficit hyperactivity disorder with methylphenidate. CNS Drugs 2004; 18:243–250.
- Joint Formulary Committee. BNF 67 March-September 2014 (online). London: Pharmaceutical Press; 2014. http://www.medicinescomplete. com/mc/bnf/current/
- Efron D et al. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics 1997; 100:662–666.
- Gadow KD et al. Efficacy of methylphenidate for attention-deficit hyperactivity disorder in children with tic disorder. Arch Gen Psychiatry 1995; 52:444–455.
- Hoare P et al. 12–month efficacy and safety of OROS MPH in children and adolescents with attention-deficit/hyperactivity disorder switched from MPH. Eur Child Adolesc Psychiatry 2005; 14:305–309.
- Remschmidt H et al. Symptom control in children and adolescents with attention-deficit/hyperactivity disorder on switching from immediaterelease MPH to OROS MPH Results of a 3-week open-label study. Eur Child Adolesc Psychiatry 2005; 14:297–304.
- Wolraich ML et al. Randomized, controlled trial of OROS methylphenidate once a day in children with attention-deficit/hyperactivity disorder. Pediatrics 2001; 108:883–892.
- Findling RL et al. Comparison of the clinical efficacy of twice-daily Ritalin and once-daily Equasym XL with placebo in children with Attention Deficit/Hyperactivity Disorder. Eur Child Adolesc Psychiatry 2006; 15:450–459.
- Anderson VR et al. Spotlight on Methylphenidate Controlled-Delivery Capsules (Equasymtrade markXL, Metadate CDtrade mark) in the treatment of children and adolescents with Attention-Deficit Hyperactivity Disorder. CNS Drugs 2007; 21:173–175.
- Haessler F et al. A pharmacokinetic study of two modified-release methylphenidate formulations under different food conditions in healthy volunteers. Int J Clin Pharmacol Ther 2008; 46:466–476.
- Fischer R et al. Bioequivalence of a methylphenidate hydrochloride extended-release preparation: comparison of an intact capsule and an opened capsule sprinkled on applesauce. Int J Clin Pharmacol Ther 2006; 44:135–141.
- Cyr M et al. Current drug therapy recommendations for the treatment of attention deficit hyperactivity disorder. Drugs 1998; 56:215–223.
- Biederman J et al. Lisdexamfetamine dimesylate and mixed amphetamine salts extended-release in children with ADHD: a double-blind, placebo-controlled, crossover analog classroom study. Biol Psychiatry 2007; 62:970–976.
- Coghill D et al. European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/ hyperactivity disorder. Eur Neuropsychopharmacol 2013; 23:1208–1218.
- Findling RL et al. Efficacy and safety of lisdexamfetamine dimesylate in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2011; 50:395–405.
- Kelsey DK et al. Once-daily atomoxetine treatment for children with attention-deficit/hyperactivity disorder, including an assessment of evening and morning behavior: a double-blind, placebo-controlled trial. Pediatrics 2004; 114:e1–e8.
- Wernicke JF et al. Cardiovascular effects of atomoxetine in children, adolescents, and adults. Drug Saf 2003; 26:729–740.
- Kratochvil CJ et al. Atomoxetine and methylphenidate treatment in children with ADHD: a prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry 2002; 41:776–784.
- Heil SH et al. Comparison of the subjective, physiological, and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug Alcohol Depend 2002; 67:149–156.
Autism spectrum disorders
Autism spectrum disorders (ASD) are conditions characterised by core deficits in three areas of development (domains); language, social interaction and behaviour (stereotypies and/or restricted and unusual patterns of interests). The autism spectrum comprises autism, Asperger's syndrome and pervasive developmental disorders-not otherwise specified (PDD-NOS) and are categorised under pervasive developmental disorders (PDD) in ICD 10. Rett's syndrome and childhood disintegrative disorder are also categorised under PDD in the ICD, though they are aetiologically distinct, with different characteristics and outcomes from ASD. The focus of this section is on treatments for ASD.
Diagnosis of ASD is straightforward. There are a range of well validated instruments for history taking from parents/guardians and objective assessment of the individual in question. However, the heterogeneity of problems seen within ASD makes detailed clinical assessment essential. Often the greatest diagnostic difficulty occurs at the milder end of the spectrum. It is important to evaluate any co-morbid neurodevelopmental, medical and psychiatric disorders that may complicate the symptom profile. These include mental retardation, attention deficit hyperactivity disorder (ADHD), epilepsy, anxiety, obsessive-compulsive and mood disorders, sleep disturbance, self-harm, irritability and aggression towards others.
Pharmacotherapies are commonly used in individuals with ASD as adjuncts to psychological interventions and there are now several published reports describing controlled and open-label clinical trials. The bulk of the evidence to date is for the efficacy of risperidone, methylphenidate and some selective reuptake inhibitors in the treatment of problem behaviours or co-existing disorders in ASD. Preliminary controlled trials of sodium valproate, atomoxetine and aripiprazole are promising. There is a potential role for α2-agonists, cholinergic agents, glutamatergic agents and oxytocin and these require further investigation.1
Currently there is no single medication for ASD that alleviates symptoms in all three domains. Targeting pharmacological interventions at problem behaviours and the level of impairment these cause, is essential. Such interventions should always be considered to be individual treatment trials. The efficacy and adverse effects associated with pharmacotherapy should be systematically monitored, bearing in mind that individuals with ASD often have impaired communication. Standardised behaviour ratings scales and adverse effect checklists are an essential tool in monitoring progress.2 A very wide range of pharmacological interventions have been studied in autism (both allopathic and 'alternative') but few are well supported.3
Pharmacological treatment of core symptoms of ASD
Restricted repetitive behaviours and interests domain
Restricted repetitive behaviours and interests (RRBI) behaviours are distressing and disruptive to functioning and therefore an important treatment target to improve overall outcomes in ASD.4 Behavioural therapies should be used as a first line but some behaviours can be difficult to manage. When these are severe, with significant impact on educational and social performance, and/or pose risks to others and/or self, then pharmacological treatment should be considered.
SSRIs have become the most the most widely prescribed medications to treat RRBIs in paediatric ASD populations. The evidence supporting the effectiveness of SSRIs in ameliorating these symptoms remains limited with the bulk of reports being from single case-studies and open-label trials with only a few RCTs published to date.5–7 The SSRIs that have been studied include fluoxetine, fluvoxamine, sertraline, citalopram and escitalopram. While side-effects have generally been considered to be mild, increased activation and agitation occurred in some subjects. The current available literature reports inconsistent benefit from SSRIs and there remains uncertainty about the optimal dose regime, which may be lower than those used for treatment of depression in typically developing individuals.8,9 The mean dose of fluoxetine has been approximately 10 mg per day, starting with 2.5 mg (see Box 5.5). Note that a recent Cochrane review found 'no evidence of effect of SSRIs in children and emerging evidence of harm'.10
Other potential pharmacological treatments include second-generation antipsychotics,11 anticonvulsants12 and the neuropeptide, oxytocin.13 Research with respect to risperidone indicates that it is effective in reducing repetitive behaviours in children who have high levels of irritability or aggression.14 Reductions in core repetitive behaviours have also been reported.11,15,16
Social and communication impairment domain
Currently, no drug has been consistently shown to improve the core social and communication impairments in ASD. Risperidone may have a secondary effect through improvement in irritability.17 Glutamatergic drugs and oxytocin are currently the most promising.18
Pharmacological treatment of co-morbid problem behaviours in ASD
Inattention, over activity and impulsiveness in ASD (symptoms of ADHD)
Children with ASD have high rates of inattention, over activity and impulsiveness.19 Adequate numbers of controlled trials of pharmacotherapy to treat these symptoms in children with ASD are lacking.20 The largest controlled trial to date has been with methylphenidate and conducted by the Research Units on Paediatric Psychopharmacology (RUPP) Autism Network.21,22 In a previous retrospective and prospective study of children with ASD, Santosh and colleagues23 reported positive benefits of treatment with methylphenidate. In general, methylphenidate produces highly variable responses in children with ASD and ADHD symptoms. These responses range from a marked improvement with few side-effects through to poor response and/or problematic sideeffects. Although there has recently been a slight shift in reporting positive effects of methylphenidate on ADHD symptoms in children with ASD, it is widely accepted that the efficacy in this group is limited and that adverse side-effects are more commonly reported compared to children with ADHD alone.1,24,25 However, where ADHD symptoms are severe and/or disabling, it is reasonable to proceed with a treatment trial of methylphenidate. It is advisable to warn parents of the lower likelihood of response and the potential side-effects and to proceed with low initial does (~0.125 mg/kg three times daily) increasing with small increments. Treatment should be stopped immediately if behaviour deteriorates or there are unacceptable side-effects.
Atomoxetine is a noradrenergic reuptake inhibitor that has been licensed to treat ADHD. There is preliminary evidence from small open-label trials that it may be useful in children with ASD but large scale RCTs are awaited.26 A recent review has suggested that atomoxetine is more effective in individuals with milder ASD symptoms.27 Whilst the number of open label and RCTs is increasing, the evidence of benefit across the severity of ASD spectrum remains conflicting.1
There is some evidence from controlled studies for risperidone and α2-agonists (clonidine and guanfacine) however there is little or no evidence in favour of SSRIs, venlafaxine benzodiazepines or anticonvulsant mood stabilisers.28
Irritability (aggression, self-injurious behaviour, tantrums)
Aggression to others and self is a common problem behaviour in ASD. Although behavioural and environment approaches are recommended as first-line treatments, more severe and dangerous behaviours usually necessitate pharmacological intervention.29 Duration of recommended treatment is difficult to derive from published evidence but treatment appears to be beneficial for up to 6–12 months.30 Efforts to reduce and possibly discontinue such treatment at the end of this period should be strongly considered.29,30
Second-generation antipsychotics are the first-line pharmacological treatment for children and adolescents with ASD and associated irritability.30–32 The first licensed in children is risperidone.33,34 Treatment of irritability in adults with ASD is reported in a placebo-controlled trial to respond in a similar way.35 Though side-effects such as weight-gain, increased appetite and somnolence can be problematic,36–39 an adverse impact on cognitive performance has not been found after up to 8 weeks of treatment.40 See Box 5.4 for the Medicines and Healthcare Regulatory Agency (MHRA) recommended dosages for risperidone.
Aripiprazole is the other FDA-approved second-generation antipsychotic for use in children and adolescents with ASD.41 A recent review and meta-analysis of short-term (8 weeks) aripiprazole in the treatment of irritability in ASD children aged 6–17 years42 found there to be a significant reduction in irritability with a moderate effect size, when compared with placebo. A more recent Cochrane review43 concludes that whilst aripiprazole may be beneficial in managing irritability, hyperactivity and stereotypies in children with ASD, it is not without side-effects which include weight gain, sedation, sialorrhoea and EPS. The usual recommended clinical dose for maintenance is between 5 and 15 mg daily.30
The effectiveness of other SGAs such as olanzapine and ziprasidone has not been tested in adequately powered RCTs. Available data suggest that mood stabilisers and anticonvulsants may not be as effective as SGAs for the treatment of irritability in ASD.44 Limited data support the combination of risperidone and topiramate being better than risperidone alone.1,45
Sleep disturbance
Children with ASD have significant sleep problems46 and there are a range of behavioural and pharmacological treatments available for this group. It is essential to understand the aetiology of the sleep problem before embarking on a course of treatment. Typical sleep problems in this group are sleep-onset insomnia, sleep-maintenance insomnia, and irregularities of the sleep–wake cycle, including early morning awakening. Abnormalities in the melatonin system have recently received much attention.47
Melatonin, has been shown in 17 studies to be beneficial in children with ASD.48 More recent RCTs continue to show promising results, although larger RCTs are needed.1,30 Doses range from 1 mg to 10 mg. Melatonin is usually very well tolerated. General seizures did not recur in children who were seizure free nor increase in those with epilepsy.49 See section on 'Melatonin in the treatment of insomnia in children and adolescents' later in this chapter.
Risperidone may benefit sleep difficulties in those with extreme irritability. In the anxious or depressed child, antidepressants may be beneficial. Insomnia due to hyperarousal may benefit from clonidine or clonazepam.50
Pathologic aggression in children and adolescents with ASD
Children and adolescents with psychiatric illness, like adults, may display pathologic aggression (PA) that is destructive, severe, chronic, and unresponsive to psychosocial and psychopharmacological treatment of their underlying condition(s) and psychosocial interventions specifically targeting their aggression. For this subset of young people with persistent aggression, pharmacotherapy may be an appropriate treatment option to optimise their functioning. It is important to understand what drives the aggressive behaviour and to intervene appropriately. This topic is reviewed by Barzman and Findling.51 In general, the use of pharmacological intervention for pathologic aggression should only be considered when (1) the underlying condition is adequately treated, (2) any current treatments are not contributing and (3) all other psychological and behavioural treatment options fail to ensure the safety and optimal functioning of the child or young person. With respect to co-morbid psychiatric illness, the most common primary diagnoses include bipolar disorders and psychotic illness. Learning disability is also common.
There is most evidence supporting the use of risperidone in aggressive behaviour.52–54 There are fewer data for olanzapine, quetiapine, aripiprazole and clozapine. Risperidone can cause significant extrapyramidal side-effects in young people and like almost all SGAs can cause considerable weight gain, metabolic (hyperglycaemia) and hormonal (hyperprolactinaemia) imbalance. Weight gain is usually worse in children than in adults.55 A more recent systematic review56 highlights the importance of careful safety monitoring of SGAs used in children and adolescents and provides evidence based guidance on effectiveness and safety monitoring practice.
Controlled studies support the use of mood stabilisers such as lithium57,58 and sodium valproate59 as being effective in the treatment of persistent aggression in children and adolescents.
There are no controlled trials of pharmacological treatments in children younger than 5 years of age.
Use of risperidone in children and adolescents
|
Box 5.4 MHRA guidance for risperidone prescribing in children and adolescents60
|
|
Risperidone is indicated for the treatment of autism in children (aged 5 and over) and adolescents. The dosage of risperidone should be individualised according to the response of the patient.
|
|
Doses of risperidone in paediatric patients with ASD (by total mg/day)
|
|
Weight categories
|
Days 1-3
|
Days 4-14+
|
Increments if dose increases are needed
|
Dose range
|
|
< 20 kg
|
0.25 mg
|
0.5 mg
|
+0.25 mg
at ≥ 2 week intervals
|
0.5 mg-1.5 mg
|
|
≥ 20 kg
|
0.5 mg
|
1.0 mg
|
+0.5 mg
at ≥ 2 week intervals
|
1.0 mg-2.5 mg*
|
|
* Subjects weighing > 45 kg may require higher doses: maximum dose studied was 3.5 mg/day.
For prescribers preferring to dose on a mg/kg/day basis the following guidance is provided.
|
|
Doses of risperidone in paediatric patients with ASD (by mg/kg/day)
|
|
Weight categories
|
Days 1-3
|
Days 4-14+
|
Increments if dose increases are needed
|
Dose range
|
|
All
|
0.01 mg/kg/day
|
0.02 mg/kg/day
|
+0.01 mg/kg/day at ≥ 2 week intervals
|
0.2 mg/kg/day -0.06 mg/kg/day
|
General considerations
- Risperidone can be administered once daily or twice daily.
- Patients experiencing somnolence may benefit from taking the whole daily dose at bedtime.
- Once sufficient clinical response has been achieved and maintained, consideration may be given to gradually lowering the dose to achieve the optimal balance of efficacy and safety.
- There is insufficient evidence from controlled trials to indicate how long treatment should continue.
Adverse effects
Weight gain, somnolence and hyperglycaemia require monitoring, and the long-term safety of risperidone in children and adolescents with ASD remains to be fully determined.
|
|
Fluoxetine in children and adolescents
When using fluoxetine to treat repetitive behaviours in ASD patients, doses much lower than those used to treat depression are normally required. It is advisable to use a liquid preparation and begin at the lowest possible dose, monitoring for side-effects. A suitable regime is outlined in Box 5.5.
|
Box 5.5 Use of fluoxetine in children and adolescents
|
|
Liquid fluoxetine: (as hydrochloride) 20 mg/5 mL
2.5 mg/day a day for 1 week; note that 2.5 mg = 0.625 mL which is difficult to measure accurately.
Follow with flexible titration schedule based on weight, tolerability, and side-effects up to a maximum dose of 0.8 mg/kg/day (0.3 mg/kg for week 2, 0.5 mg/kg/day for week 3, and 0.8 mg/kg/day subsequently). Reduction may be indicated if side-effects are problematic.
Adverse effects
- Monitor for emergent suicidal behaviour, self-harm and hostility, particularly at the beginning of treatment.
- Hyponatraemia is also possible - see section on 'Antidepressant-induced hyponatraemia' in Chapter 4.
|
|
References
- Sung M et al. What's in the pipeline? Drugs in development for autism spectrum disorder. Neuropsychiatr Dis Treat 2014; 10:371–381.
- Greenhill LL. Assessment of safety in pediatric psychopharmacology. J Am Acad Child Adolesc Psychiatry 2003; 42:625–626.
- Rossignol DA. Novel and emerging treatments for autism spectrum disorders: a systematic review. Ann Clin Psychiatry 2009; 21:213–236.
- Leekam SR et al. Restricted and repetitive behaviors in autism spectrum disorders: a review of research in the last decade. Psychol Bull 2011; 137:562–593.
- McDougle CJ et al. A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry 1996; 53:1001–1008.
- Buchsbaum MS et al. Effect of fluoxetine on regional cerebral metabolism in autistic spectrum disorders: a pilot study. Int J Neuropsychopharmacol 2001; 4:119–125.
- Hollander E et al. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology 2005; 30:582–589.
- Aman MG et al. Medication patterns in patients with autism: temporal, regional, and demographic influences. J Child Adolesc Psychopharmacol 2005; 15:116–126.
- Soorya L et al. Psychopharmacologic interventions for repetitive behaviors in autism spectrum disorders. Child Adolesc Psychiatr Clin N Am 2008; 17:753–771.
- Williams K et al. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst Rev 2013; 8: CD004677.
- McDougle CJ et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry 2005; 162:1142–1148.
- Hollander E et al. A double-blind placebo-controlled pilot study of olanzapine in childhood/adolescent pervasive developmental disorder. J Child Adolesc Psychopharmacol 2006; 16:541–548.
- Hollander E et al. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger's disorders. Neuropsychopharmacology 2003; 28:193–198.
- McDougle CJ et al. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessivecompulsive disorder. Arch Gen Psychiatry 2000; 57:794–801.
- McCracken JT et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002; 347:314–321.
- Arnold LE et al. Parent-defined target symptoms respond to risperidone in RUPP autism study: customer approach to clinical trials. J Am Acad Child Adolesc Psychiatry 2003; 42:1443–1450.
- Canitano R et al. Risperidone in the treatment of behavioral disorders associated with autism in children and adolescents. Neuropsychiatr Dis Treat 2008; 4:723–730.
- Posey DJ et al. Developing drugs for core social and communication impairment in autism. Child Adolesc Psychiatr Clin N Am 2008; 17:787–801.
- Simonoff E et al. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 2008; 47:921–929.
- Mahajan R et al. Clinical practice pathways for evaluation and medication choice for attention-deficit/hyperactivity disorder symptoms in autism spectrum disorders. Pediatrics 2012; 130 Suppl 2:S125–S138.
- Research Units on Pediatric Psychopharmacology (RUPP) Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry 2005; 62:1266–1274.
- Posey DJ et al. Positive effects of methylphenidate on inattention and hyperactivity in pervasive developmental disorders: an analysis of secondary measures. Biol Psychiatry 2007; 61:538–544.
- Santosh PJ et al. Impact of comorbid autism spectrum disorders on stimulant response in children with attention deficit hyperactivity disorder: a retrospective and prospective effectiveness study. Child Care Health Dev 2006; 32:575–583.
- Siegel M et al. Psychotropic medications in children with autism spectrum disorders: a systematic review and synthesis for evidence-based practice. J Autism Dev Disord 2012; 42:1592–1605.
- Williamson ED et al. Psychotropic medications in autism: practical considerations for parents. J Autism Dev Disord 2012; 42:1249–1255.
- Arnold LE et al. Atomoxetine for hyperactivity in autism spectrum disorders: placebo-controlled crossover pilot trial. J Am Acad Child Adolesc Psychiatry 2006; 45:1196–1205.
- Ghanizadeh A. Atomoxetine for treating ADHD symptoms in autism: a systematic review. J Atten Disord 2013; 17:635–640.
- Aman MG et al. Treatment of inattention, overactivity, and impulsiveness in autism spectrum disorders. Child Adolesc Psychiatr Clin N Am 2008; 17:713–38, vii.
- National Institute for Health and Care Excellence. Autism: The management and support of children and young people on the autism spectrum. Clinical Guideline 170, 2013. http://publications.nice.org.uk/autism-cg170
- Kaplan G et al. Psychopharmacology of autism spectrum disorders. Pediatr Clin North Am 2012; 59:175-87, xii.
- McDougle CJ et al. Atypical antipsychotics in children and adolescents with autistic and other pervasive developmental disorders. J Clin Psychiatry 2008; 69 Suppl 4:15–20.
- Parikh MS et al. Psychopharmacology of aggression in children and adolescents with autism: a critical review of efficacy and tolerability. J Child Adolesc Psychopharmacol 2008; 18:157–178.
- Jesner OS et al. Risperidone for autism spectrum disorder. Cochrane Database Syst Rev 2007; CD005040.
- Scahill L et al. Risperidone approved for the treatment of serious behavioral problems in children with autism. J Child Adolesc Psychiatr Nurs 2007; 20:188–190.
- McDougle CJ et al. A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry 1998; 55:633–641.
- Caccia S. Safety and pharmacokinetics of atypical antipsychotics in children and adolescents. Paediatr Drugs 2013; 15:217–233.
- Sharma A et al. Efficacy of risperidone in managing maladaptive behaviors for children with autistic spectrum disorder: a meta-analysis. J Pediatr Health Care 2012; 26:291–299.
- Kent JM et al. Risperidone dosing in children and adolescents with autistic disorder: a double-blind, placebo-controlled study. J Autism Dev Disord 2013; 43:1773–1783.
- Maayan L et al. Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol 2011; 21:517–535.
- Aman MG et al. Acute and long-term safety and tolerability of risperidone in children with autism. J Child Adolesc Psychopharmacol 2005; 15:869–884.
- Curran MP. Aripiprazole: in the treatment of irritability associated with autistic disorder in pediatric patients. Paediatr Drugs 2011; 13:197–204.
- Douglas-Hall P et al. Aripiprazole: a review of its use in the treatment of irritability associated with autistic disorder patients aged 6-17. Journal of Central Nervous System Diseases 2011; 3:1–11.
- Ching H et al. Aripiprazole for autism spectrum disorders (ASD). Cochrane Database Syst Rev 2012; 5: CD009043.
- Stigler KA et al. Pharmacotherapy of irritability in pervasive developmental disorders. Child Adolesc Psychiatr Clin N Am 2008; 17:739–752.
- Rezaei V et al. Double-blind, placebo-controlled trial of risperidone plus topiramate in children with autistic disorder. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34:1269–1272.
- Krakowiak P et al. Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: a populationbased study. J Sleep Res 2008; 17:197–206.
- Sanchez-Barcelo EJ et al. Clinical uses of melatonin in pediatrics. Int J Pediatr 2011; 2011:892624.
- Doyen C et al. Melatonin in children with autistic spectrum disorders: recent and practical data. Eur Child Adolesc Psychiatry 2011; 20:231–239.
- Andersen IM et al. Melatonin for insomnia in children with autism spectrum disorders. J Child Neurol 2008; 23:482–485.
- Johnson KP et al. Sleep in children with autism spectrum disorders. Curr Treat Options Neurol 2008; 10:350–359.
- Barzman DH et al. Pharmacological treatment of pathologic aggression in children. Int Rev Psychiatry 2008; 20:151–157.
- Aman MG et al. Double-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviors in children with subaverage intelligence. Am J Psychiatry 2002; 159:1337–1346.
- Snyder R et al. Effects of risperidone on conduct and disruptive behavior disorders in children with subaverage IQs. J Am Acad Child Adolesc Psychiatry 2002; 41:1026–1036.
- Reyes M et al. A randomized, double-blind, placebo-controlled study of risperidone maintenance treatment in children and adolescents with disruptive behavior disorders. Am J Psychiatry 2006; 163:402–410.
- De Hert M et al. Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: A systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur Psychiatry 2011; 26:144–158.
- Pringsheim T et al. Evidence-based recommendations for monitoring safety of second-generation antipsychotics in children and youth. Paediatr Child Health 2011; 16:581–589.
- Campbell M et al. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry 1995; 34:445–453.
- Malone RP et al. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry 2000; 57:649–654.
- Donovan SJ et al. Divalproex treatment for youth with explosive temper and mood lability: a double-blind, placebo-controlled crossover design. Am J Psychiatry 2000; 157:818–820.
- Medicines and Healthcare Products Regulatory Agency. Variation Assessment Form. London: MHRA; 2005. http://www.mhra.gov.uk/home/ groups/pl-p/documents/websiteresources/con2025027.pdf
Tics and Tourette's syndrome
Transient tics occur in 5–20% of children. Tourette's syndrome (TS) occurs in about 1% of children and is defined by persistent motor and vocal tics. As many as 65% of individuals with TS will have no, or only very mild, tics in adult life. Tics wax and wane over time and are variably exacerbated by external factors such as stress, inactivity and fatigue, depending on the individual. Tics are about 2–3 times more common in boys than girls.1
Detection and treatment of co-morbidity
Co-morbid OCD, attention deficit hyperactivity disorder, depression, anxiety, and behavioural problems are more prevalent than would be expected by chance, and often cause the major impairment in people with tic disorders.2 These co-morbid conditions are usually treated first before assessing the level of disability caused by the tics.3
Education and behavioural treatments
Most people with tics do not require pharmacological treatment; education for the individual with tics, their family and the people they interact with, especially schools, is crucial. Treatment aimed primarily at reducing tics is warranted if they cause distress to the patient or are functionally disabling. There has been a resurgence of interest in behavioural programs, and a recent randomised controlled trial of a comprehensive behavioural intervention achieved an effect-size of 0.68 which is comparable with the effect sizes achieved with medication for tics.4 Habit reversal and exposure and response prevention are the behavioural treatments of choice.5
Pharmacological treatments
Studies of pharmacological interventions in TS are difficult to interpret for several reasons.
- There is a large inter-individual variation in tic frequency and severity. Small, randomised studies may include patients that are very different at baseline.
- The severity of tics in a given individual varies markedly over time, making it difficult to separate drug effect from natural variation.
- The bulk of the literature consists of case reports, case series, open studies and underpowered, randomised studies. Publication bias is also likely to be an issue.
- A high proportion of patients have co-morbid psychiatric illness. It can be difficult to disentangle any direct effect on tics from an effect on the co-morbid illness. This makes it difficult to interpret studies that report improvements in global functioning rather than specific reductions in tics.
- Large numbers of individuals attending clinics with TS appear to use complementary or alternative therapies and around 50% report benefit from these.6
- The placebo effect in clinical trials of tic disorders is not as large as previously thought.7
Most of the published literature concerns children and adolescents.
Adrenergic α2-agonists
Clonidine has been shown in open studies to reduce the severity and frequency of tics, but in one study this effect did not seem to be convincingly larger than the placebo.8 Other studies have shown more substantial reductions in tics.9–12 Guanfacine has been shown to lead to a 30% reduction in tic-rating-scale scores.13 In the UK, only clonidine is readily available. Therapeutic doses of clonidine are in the order of 3–5 μg/kg, and the dose should be built up gradually. Main side-effects are sedation, postural hypotension and depression. Patients and their families should be informed not to stop clonidine suddenly because of the risk of rebound hypertension.
Antipsychotics
Adverse effects of antipsychotics may outweigh beneficial effects in the treatment of tics and so it is recommended that clonidine is tried first. Antipsychotics may however be more effective than clonidine in alleviating tics in some individuals.
A number of first-generation antipsychotics have been used in TS.14 In a recent Cochrane review, pimozide demonstrated robust efficacy in a meta analysis of six trials.15 In these trials, pimozide was compared with haloperidol (one trial), placebo (one trial), haloperidol and placebo (two trials) and risperidone (two trials) and was found to be more effective than placebo, as effective as risperidone and slightly less effective than haloperidol in reducing tics. It was associated with fewer adverse reactions compared with haloperidol but did not differ from risperidone in that respect. ECG monitoring is essential for pimozide and haloperidol. Haloperidol is often poorly tolerated. Given their side-effect profile, most authors recommend the use of second-generation rather than first-generation antipsychotics in the treatment of TS.14
Recent studies are suggestive that aripiprazole is an effective and well tolerated treatment of children with TS (and also tics16). A 10-week multicentre double-blind randomised placebo-controlled trial (n = 61) demonstrated the efficacy of aripiprazole in tic reduction in TS. Aripiprazole treatment was associated with significantly decreased serum prolactin concentration, increased mean body weight (by 1.6 kg), body mass index, and waist circumference.17 Several case series are also in support of the use of aripiprazole.18–21 A study evaluating the metabolic side-effects of aripiprazole (n = 25) and pimozide (n = 25) in TS over a 24-month period demonstrated that treatment was not associated with significant increase in body mass index. However, pimozide treatment was associated with increases in blood sugar which did not plateau from 12 to 24 months, aripiprazole treatment was associated with increased cholesterolaemia and both medications were associated with increased triglyceridaemia.22
Risperidone has, in addition to the studies mentioned above, also been shown to be more effective than placebo in a small (n = 34), randomised study.23 Fatigue and increased appetite were problematic in the risperidone arm and a mean weight gain of 2.8 kg over 8 weeks was reported. A small double-blind crossover study suggested that olanzapine24 may be more effective than pimozide. One small randomised, controlled trial found risperidone and clonidine to be equally effective.25 Sulpiride has been shown to be effective and relatively well tolerated,26 as has ziprasidone.27 Open studies support the efficacy of quetiapine28 and olanzapine.29,30 One very small crossover study (n = 7) found no effect for clozapine.31
Overall, metabolic side-effects and weight gain are common with second-generation antipsychotics so benefit/risk ratios need careful discussion.14
Other drugs
A small, double-blind, placebo-controlled, crossover trial of baclofen was suggestive of beneficial effects in overall impairment rather than a specific effect on tics.32 The numerical benefits shown in this study did not reach statistical significance. Similarly, a double-blind, placebo-controlled trial of nicotine augmentation of haloperidol found beneficial effects in overall impairment rather than a specific effect on tics.33 These benefits persisted for several weeks after nicotine (in the form of patches) was withdrawn. Nicotine patches were associated with a high prevalence of nausea and vomiting (71% and 40% respectively). The authors suggest that pro re nata (prn) use may be appropriate. Pergolide (a D1-D2-D3 agonist) given in low dose significantly reduced tics in a double-blind, placebo-controlled, crossover study in children and adolescents.34 Side-effects included sedation, dizziness, nausea and irritability. Pergolide was also evaluated in a randomised trial in children and adolescents with chronic tics and TS, and showed significant tic reduction compared with placebo.35 Flutamide, an antiandrogen, has been the subject of a small RCT in adults with TS. Modest, short-lived effects were seen in motor but not phonic tics.36 A small randomised controlled trial has shown significant advantages for metoclopramide over placebo37 and for topiramate over placebo.38 A recent meta-analysis identified 14 randomised controlled trials (all from China) comparing topiramate with haloperidol or tiapride. It concluded that due to the overall low quality of the study designs, there is not enough evidence to support the routine use of topiramate in clinical practice.39
Case reports or case series describing positive effects for ondansetron,40 clomifene,41 tramadol,42 ketanserin,43 cyproterone,44 levetiracetam45 and cannabis46 have been published. A recent Cochrane Review of cannabinoids concluded that there was little if any current evidence for efficacy.47 Tetrabenazine may be useful as an add-on treatment.48 Many other drugs have been reported to be effective in single case reports. Patients in these reports all had co-morbid psychiatric illness, making it difficult to determine the effect of these drugs on TS alone.
Botulinum toxin has been used to treat bothersome or painful focal motor tics, particularly those affecting neck muscles.14
There may be a sub-group of children who develop tics/and or OCD in association with streptococcal infection. This group has been given the acronym PANDAS (Paediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcus).49 This is thought to be an autoimmune-mediated effect, and there have been trials of immunomodulatory therapy in these children. However, current clinical consensus is that tics or OCD should be treated in the usual way unless a child is part of a research trial. A normal course of antibiotic treatment should be given for any identified active infection (e.g. Strep sore throat) in a child who presents acutely with new onset tics and/or OCD.
References
- Murphy TK et al. Practice parameter for the assessment and treatment of children and adolescents with tic disorders. J Am Acad Child Adolesc Psychiatry 2013; 52:1341–1359.
- Cath DC et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part I: assessment. Eur Child Adolesc Psychiatry 2011; 20:155–171.
- Singer HS. Treatment of tics and tourette syndrome. Curr Treat Options Neurol 2010; 12:539–561.
- Piacentini J et al. Behavior therapy for children with Tourette disorder: a randomized controlled trial. JAMA 2010; 303:1929–1937.
- Verdellen C et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part III: behavioural and psychosocial interventions. Eur Child Adolesc Psychiatry 2011; 20:197–207.
- Kompoliti K et al. Complementary and alternative medicine use in Gilles de la Tourette syndrome. Mov Disord 2009; 24:2015–2019.
- Cubo E et al. Impact of placebo assignment in clinical trials of tic disorders. Mov Disord 2013; 28:1288–1292.
- Goetz CG et al. Clonidine and Gilles de la Tourette's syndrome: double-blind study using objective rating methods. Ann Neurol 1987; 21:307–310.
- Leckman JF et al. Clonidine treatment of Gilles de la Tourette's syndrome. Arch Gen Psychiatry 1991; 48:324–328.
- Tourette's Syndrome Study Group. Treatment of ADHD in children with tics: a randomized controlled trial. Neurology 2002; 58:527–536.
- Du YS et al. Randomized double-blind multicentre placebo-controlled clinical trial of the clonidine adhesive patch for the treatment of tic disorders. Aust N Z J Psychiatry 2008; 42:807–813.
- Hedderick EF et al. Double-blind, crossover study of clonidine and levetiracetam in Tourette syndrome. Pediatr Neurol 2009; 40:420–425.
- Scahill L et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry 2001; 158:1067–1074.
- Roessner V et al. Pharmacological treatment of tic disorders and Tourette Syndrome. Neuropharmacology 2013; 68:143–149.
- Pringsheim T et al. Pimozide for tics in Tourette's syndrome. Cochrane Database Syst Rev 2009; CD006996.
- Yoo HK et al. An open-label study of the efficacy and tolerability of aripiprazole for children and adolescents with tic disorders. J Clin Psychiatry 2007; 68:1088–1093.
- Yoo HK et al. A multicenter, randomized, double-blind, placebo-controlled study of aripiprazole in children and adolescents with Tourette's disorder. J Clin Psychiatry 2013; 74:e772–e780.
- Davies L et al. A case series of patients with Tourette's syndrome in the United Kingdom treated with aripiprazole. Hum Psychopharmacol 2006; 21:447–453.
- Seo WS et al. Aripiprazole treatment of children and adolescents with Tourette disorder or chronic tic disorder. J Child Adolesc Psychopharmacol 2008; 18:197–205.
- Murphy TK et al. Open label aripiprazole in the treatment of youth with tic disorders. J Child Adolesc Psychopharmacol 2009; 19:441–447.
- Wenzel C et al. Aripiprazole for the treatment of Tourette syndrome: a case series of 100 patients. J Clin Psychopharmacol 2012; 32:548–550.
- Rizzo R et al. Metabolic effects of aripiprazole and pimozide in children with Tourette syndrome. Pediatr Neurol 2012; 47:419–422.
- Scahill L et al. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology 2003; 60:1130–1135.
- Onofrj M et al. Olanzapine in severe Gilles de la Tourette syndrome: a 52-week double-blind cross-over study vs. low-dose pimozide. J Neurol 2000; 247:443–446.
- Gaffney GR et al. Risperidone versus clonidine in the treatment of children and adolescents with Tourette's syndrome. J Am Acad Child Adolesc Psychiatry 2002; 41:330–336.
- Robertson MM et al. Management of Gilles de la Tourette syndrome using sulpiride. Clin Neuropharmacol 1990; 13:229–235.
- Sallee FR et al. Ziprasidone treatment of children and adolescents with Tourette's syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry 2000; 39:292–299.
- Mukaddes NM et al. Quetiapine treatment of children and adolescents with Tourette's disorder. J Child Adolesc Psychopharmacol 2003; 13:295–299.
- Budman CL et al. An open-label study of the treatment efficacy of olanzapine for Tourette's disorder. J Clin Psychiatry 2001; 62:290–294.
- McCracken JT et al. Effectiveness and tolerability of open label olanzapine in children and adolescents with Tourette syndrome. J Child Adolesc Psychopharmacol 2008; 18:501–508.
- Caine ED et al. The trial use of clozapine for abnormal involuntary movement disorders. Am J Psychiatry 1979; 136:317–320.
- Singer HS et al. Baclofen treatment in Tourette syndrome: a double-blind, placebo-controlled, crossover trial. Neurology 2001; 56:599–604.
- Silver AA et al. Transdermal nicotine and haloperidol in Tourette's disorder: a double-blind placebo-controlled study. J Clin Psychiatry 2001; 62:707–714.
- Gilbert DL et al. Tourette's syndrome improvement with pergolide in a randomized, double-blind, crossover trial. Neurology 2000; 54:1310–1315.
- Gilbert DL et al. Tic reduction with pergolide in a randomized controlled trial in children. Neurology 2003; 60:606–611.
- Peterson BS et al. A double-blind, placebo-controlled, crossover trial of an antiandrogen in the treatment of Tourette's syndrome. J Clin Psychopharmacol 1998; 18:324–331.
- Nicolson R et al. A randomized, double-blind, placebo-controlled trial of metoclopramide for the treatment of Tourette's disorder. J Am Acad Child Adolesc Psychiatry 2005; 44:640–646.
- Jankovic J et al. A randomised, double-blind, placebo-controlled study of topiramate in the treatment of Tourette syndrome. J Neurol Neurosurg Psychiatry 2010; 81:70–73.
- Yang CS et al. Topiramate for Tourette's syndrome in children: a meta-analysis. Pediatr Neurol 2013; 49:344–350.
- Toren P et al. Ondansetron treatment in patients with Tourette's syndrome. Int Clin Psychopharmacol 1999; 14:373–376.
- Sandyk R. Clomiphene citrate in Tourette's syndrome. Int J Neurosci 1988; 43:103–106.
- Shapira NA et al. Novel use of tramadol hydrochloride in the treatment of Tourette's syndrome. J Clin Psychiatry 1997; 58:174–175.
- Bonnier C et al. Ketanserin treatment of Tourette's syndrome in children. Am J Psychiatry 1999; 156:1122–1123.
- Izmir M et al. Cyproterone acetate treatment of Tourette's syndrome. Can J Psychiatry 1999; 44:710–711.
- Awaad Y et al. Use of levetiracetam to treat tics in children and adolescents with Tourette syndrome. Mov Disord 2005; 20:714–718.
- Sandyk R et al. Marijuana and Tourette's syndrome. J Clin Psychopharmacol 1988; 8:444–445.
- Curtis A et al. Cannabinoids for Tourette's Syndrome. Cochrane Database Syst Rev 2009; CD006565.
- Porta M et al. Tourette's syndrome and role of tetrabenazine: review and personal experience. Clin Drug Invest 2008; 28:443–459.
- Martino D et al. The PANDAS subgroup of tic disorders and childhood-onset obsessive-compulsive disorder. J Psychosom Res 2009; 67:547–557.
Melatonin in the treatment of insomnia in children and adolescents
Insomnia is a common symptom in childhood. Underlying causes may be behavioural (inappropriate sleep associations or bedtime resistance) physiological (delayed sleep phase syndrome) or related to underlying mood disorders (anxiety, depression and bipolar disorder). All forms of insomnia are more common in children with learning difficulties, autism, ADHD and sensory impairments (particularly visual). Although behavioural interventions should be the primary intervention and have a robust evidence base, exogenous melatonin is now the 'first-line' medication prescribed for childhood insomnia.1
Melatonin is a hormone that is produced by the pineal gland in a circadian manner. The evening rise in melatonin, enabled by darkness, precedes the onset of natural sleep by about 2 hours.2 Melatonin is involved in the induction of sleep and in synchronisation of the circadian system.
There are a wide variety of unlicensed fast-release, slow-release and liquid preparations of melatonin. Many products rely on food-grade rather than pharmaceutical grade melatonin and some are very expensive. BioMelatonin is an immediate-release melatonin preparation of pharmaceutical grade which is soluble in water and so obviates the need for expensive liquid preparations. A prolonged release formulation of melatonin (Circadin) was licensed in the UK in April 2008 as a short-term treatment of insomnia in patients over 55 years of age. It has not been evaluated in children. Many children are unable to swallow tablets and use in this population will be 'off-label'. Despite these limitations the MHRA recommends prescription of this licensed formulation where possible.3 Lack of any 'head to head' studies means that there are still no data on whether, or when, immediate or slow release melatonin preparations should be used. Sense would dictate that fast-release melatonin improves sleep latency whilst slow-release improves sleep time (and so a combined approach might be optimal). Nonetheless, there is very little evidence to support this and experience suggests that (1) Circadin can also effectively decrease sleep latency, and (2) sleep duration long term is only minimally altered by any form of melatonin. There are additionally a number of melatonin analogues already produced, or in development4 although they are virtually never used in the paediatric population, with no evidence from equivalence studies of superiority over melatonin itself.
Efficacy
Two meta-analyses on the use of melatonin in sleep disorders have been published.5,6 Both pooled data from studies in children and adults. The first considered melatonin in primary sleep disorders (not accompanied by any medical or psychiatric disorder likely to account for the sleep problem) and showed improvements in the time taken to fall asleep of 11.7 minutes across the group, but nearly 40 minutes if delayed sleep phase syndrome was the underlying cause. The study considering secondary sleep disorders in this heterogeneous group found no significant effect on sleep latency.
Since these meta-analyses, many smaller RCTs comparing melatonin with placebo in children have been published.7–13 Studies have considered diverse groups including children with sleep phase delay, ADHD, autistic spectrum disorders, intellectual disability and epilepsy. Results are surprisingly consistent considering the different underlying disorders. Children in these studies fall asleep about 30 minutes quicker (26.9–34) and their total time asleep increases by a similar (19.8–48) amount of time. The effect size for sleep latency is much greater than for total sleep time confirming that melatonin is of most use for sleep initiation, rather than sleep maintenance. Importantly, over time a number of children who fall asleep earlier on melatonin will also start to wake up earlier on melatonin. The two largest randomised controlled studies to date considered the use of melatonin for children with ASD and neurodevelopmental delay.14,15 Both employed a behavioural intervention, although with different designs. Together they demonstrated the value of a sleep behavioural intervention before melatonin treatment, and the value of continuing the behavioural intervention during melatonin administration. Both studies showed similar effectiveness of melatonin for sleep latency, but total sleep time was increased more in the study that used a combined slow/fast release preparation of melatonin.
Side-effects
Many of the children who have received melatonin in RCTs and published case series had developmental problems and/or sensory deficits. The scope for detecting subtle adverse effects in this population is limited. Screening for side-effects was not routine in all studies. Early reports included a very small case series cases where melatonin was been reported to worsen seizures16 and exacerbate asthma17,18 in the short term. Other reported side-effects include headache, depression, restlessness, confusion, nausea, tachycardia and pruritis.19,20 In the more recent largest placebo-controlled studies to date involving children with learning difficulty, autism and epilepsy,11,13,14 there were no excess adverse effects in the treatment group, and in particular seizures were not worsened.
Dose
The cut-off point between physiological and pharmacological doses in children is less than 500 μg. Physiological doses of melatonin may result in very high receptor occupancy. The doses used in RCTs and published case series vary hugely, between 500 μg and 5 mg being the most common, although much lower and higher doses have been used. The optimal dose is unknown and there is no evidence to support a direct relationship between dose and response.21 In one large RCT 18% of children seemed to respond to a 500 μg dose but others seemed to require much higher doses (12 mg).14 Increasing doses above 5 mg is likely to be utilising the sedative effects of melatonin, rather than its sleep-phase shifting properties. This might be necessary and still helpful for some children with severe and bilateral brain injury. The use of salivary melatonin measurements is likely to become important in identifying those children with the most delayed sleep phase (likely to have the best response to exogenous melatonin) and those children who are slow metabolisers of melatonin in whom serum levels accumulate during the daytime (particularly on higher doses) and eventually reduce efficiency.
See Figure 5.2 for a summary of recommendations for the use of melatonin.
Figure 5.2 Melatonin—summary of recommendations.
References
- Gringras P. When to use drugs to help sleep. Arch Dis Child 2008; 93:976–981.
- Macchi MM et al. Human pineal physiology and functional significance of melatonin. Front Neuroendocrinol 2004; 25:177–195.
- Medicines and Healthcare Products Regulatory Agency. Restrictions on the import of unlicensed Melatonin products following the grant of a marketing authorisation for Circadin® 2 mg tablets. London: MHRA; 2008. http://www.mhra.gov.uk
- Arendt J et al. Melatonin and its agonists: an update. Br J Psychiatry 2008; 193:267–269.
- Buscemi N et al. The efficacy and safety of exogenous melatonin for primary sleep disorders. A meta-analysis. J Gen Intern Med 2005; 20:1151–1158.
- Buscemi N et al. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis. BMJ 2006; 332:385–393.
- Van der Heijden KB et al. Effect of melatonin on sleep, behavior, and cognition in ADHD and chronic sleep-onset insomnia. J Am Acad Child Adolesc Psychiatry 2007; 46:233–241.
- Wasdell MB et al. A randomized, placebo-controlled trial of controlled release melatonin treatment of delayed sleep phase syndrome and impaired sleep maintenance in children with neurodevelopmental disabilities. J Pineal Res 2008; 44:57–64.
- Braam W et al. Melatonin treatment in individuals with intellectual disability and chronic insomnia: a randomized placebo-controlled study. J Intellect Disabil Res 2008; 52:256–264.
- Gupta M et al. Add-on melatonin improves sleep behavior in children with epilepsy: randomized, double-blind, placebo-controlled trial. J Child Neurol 2005; 20:112–115.
- Coppola G et al. Melatonin in wake-sleep disorders in children, adolescents and young adults with mental retardation with or without epilepsy: a double-blind, cross-over, placebo-controlled trial. Brain Dev 2004; 26:373–376.
- Weiss MD et al. Sleep hygiene and melatonin treatment for children and adolescents with ADHD and initial insomnia. J Am Acad Child Adolesc Psychiatry 2006; 45:512–519.
- Garstang J et al. Randomized controlled trial of melatonin for children with autistic spectrum disorders and sleep problems. Child Care Health Dev 2006; 32:585–589.
- Gringras P et al. Melatonin for sleep problems in children with neurodevelopmental disorders: randomised double masked placebo controlled trial. BMJ 2012; 345:e6664.
- Cortesi F et al. Controlled-release melatonin, singly and combined with cognitive behavioural therapy, for persistent insomnia in children with autism spectrum disorders: a randomized placebo-controlled trial. J Sleep Res 2012; 21:700–709.
- Sheldon SH. Pro-convulsant effects of oral melatonin in neurologically disabled children. Lancet 1998; 351:1254.
- Maestroni GJ. The immunoneuroendocrine role of melatonin. J Pineal Res 1993; 14:1–10.
- Sutherland ER et al. Elevated serum melatonin is associated with the nocturnal worsening of asthma. J Allergy Clin Immunol 2003; 112:513–517.
- Chase JE et al. Melatonin: therapeutic use in sleep disorders. Ann Pharmacother 1997; 31:1218–1226.
- Jan JE et al. Melatonin treatment of sleep-wake cycle disorders in children and adolescents. Dev Med Child Neurol 1999; 41:491–500.
- Sack RL et al. Sleep-promoting effects of melatonin: at what dose, in whom, under what conditions, and by what mechanisms? Sleep 1997; 20:908–915.
Rapid tranquillisation in children and adolescents
As in adults, a comprehensive mental state assessment and appropriately implemented treatment plan along with staff skilled in the use of de-escalation techniques and appropriate placement of the patient are key to minimising the need for enforced parenteral medication.
Healthcare professionals undertaking rapid tranquillisation (RT) and/or restraint in children and adolescents should be trained and competent in undertaking these procedures in this population, and should be clear about the legal context for any restrictive practices they employ. Be particularly cautious when considering highpotency antipsychotic medication (such as haloperidol) especially in those who have not taken antipsychotic medication before, because of the increased risk of acute dystonic reactions in this age group.1
A wide dose range is given here for medication used in RT. Caution is required, especially for younger children, but in older adolescents consider the use of adult doses, especially in those who are not drug naïve and where doses in the lower end of the quoted dose range have proved ineffective.
Oral medication should always be offered (and repeated if necessary if the young person is willing to take it), before resorting to parenteral treatment (see Table 5.7). Monitoring after RT is the same as in adults (see section on 'Acutely disturbed or violent behaviour' in Chapter 7).
Table 5.7 Recommended drugs for rapid tranquillsation if the oral route is refused or has proven ineffective
|
Medication
|
Dose
|
Onset of action
|
Comment
|
|
Olanzapine IM2,3
|
2.5-10 mg
|
15-30 minutes
|
Possibly increased risk of respiratory depression when administered with benzodiazepines, particularly if alcohol has been consumed. Separate administration by at least one hour
|
|
Haloperidol IM4
|
< 12 years: 0.025-0.075 mg/kg/dose (max 2.5 mg) IM
Adolescents > 12 years can receive the adult dose (2.5-5 mg)
|
20-30 minutes
|
Must have parenteral anticholinergics present in case of laryngeal spasm (young people more vulnerable to severe dystonia)
Adult data suggest co-administration of promethazine may reduce EPS risk5
ECG essential
|
|
Lorazepam* IM6,7
|
< 12 years: 0.5-1 mg
> 12 years: 0.5-2 mg
|
20-40 minutes
|
Slower onset of action than midazolam
Flumazenil is the reversing agent for all benzodiazepines
|
|
Midazolam* IM, IV or buccal7,8
|
0.1-0.15 mg/kg (IM)
Buccal midazolam 300 μg/kg or
6-10 years = 7.5 mg
> 10 years = 10 mg
|
10-20 minutes IM
(1-3 minutes IV)
|
Quicker onset and shorter duration of action than lorazepam or diazepam
IV administration should only be used (usually as a last resort) with extreme caution and where resuscitation facilities are available
Shorter onset and duration of action than haloperidol
When given as buccal liquid, onset of action is 15-30 minutes.9 Some published data in mental health but only in adults.10 Buccal liquid is unlicensed for this use
|
|
Diazepam* IV
(not for IM administration)11
|
0.1 mg/kg/dose by slow IV injection. Max 40 mg total daily dose for < 12 years and 60 mg for > 12 years
|
1-3 minutes
|
Long half-life that does not correlate with length of sedation. Possibility of accumulation
Never give as IM injection
|
|
Ziprasidone IM12-15
(not UK)
|
10-20 mg
|
15-30 minutes IM
|
Apparently effective. QT prolongation is of concern in this patient group
ECG essential
|
|
Aripiprazole IM16,17
|
9.75 mg
|
15-30 minutes
|
Evidence of effectiveness in adults but no data for children and adolescents
|
|
Promethazine IM
|
< 12 years: 5-25 mg (max 50 mg/day)
> 12 years: 25-50 mg (max 100 mg/day)
|
Up to 60 minutes
|
An effective sedative, although has a slow onset of action. Useful if the cause of behavioural disturbance is unknown and there is concern about the use of antipsychotic medication in a child or young person
|
|
* Note that young people are particularly vulnerable to disinhibitory reactions with benzodiazepines.
ECG, electrocardiogram; EPS, extrapyramidal side-effects; IM, intramuscular; IV, intravenous.
|
References
- National Institute for Health and Care Excellence. Psychosis and schizophrenia in children and young people: Recognition and management. Clinical Guideline 15, 2013. http://www.nice.org/
- Breier A et al. A double-blind, placebo-controlled dose-response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophrenia. Arch Gen Psychiatry 2002; 59:441–448.
- Lindborg SR et al. Effects of intramuscular olanzapine vs. haloperidol and placebo on QTc intervals in acutely agitated patients. Psychiatry Res 2003; 119:113–123.
- Powney MJ et al. Haloperidol for psychosis-induced aggression or agitation (rapid tranquillisation). Cochrane Database Syst Rev 2012; 11: CD009377.
- TREC Collaborative Group. Rapid tranquillisation for agitated patients in emergency psychiatric rooms: a randomised trial of midazolam versus haloperidol plus promethazine. BMJ 2003; 327:708–713.
- Sorrentino A. Chemical restraints for the agitated, violent, or psychotic pediatric patient in the emergency department: controversies and recommendations. Curr Opin Pediatr 2004; 16:201–205.
- Nobay F et al. A prospective, double-blind, randomized trial of midazolam versus haloperidol versus lorazepam in the chemical restraint of violent and severely agitated patients. Acad Emerg Med 2004; 11:744–749.
- Kennedy RM et al. The "ouchless emergency department". Getting closer: advances in decreasing distress during painful procedures in the emergency department. Pediatr Clin North Am 1999; 46:1215–1247.
- Schwagmeier R et al. Midazolam pharmacokinetics following intravenous and buccal administration. Br J Clin Pharmacol 1998; 46:203–206.
- Taylor D et al. Buccal midazolam for agitation on psychiatric intensive care wards. Int J Psychiatry Clin Pract 2008; 12:309–311.
- Nunn K, Dey C. Medication Table. In: Nunn K, Dey C, eds. The Clinician's Guide to Psychotropic Prescribing in Children and Adolescents. Sydney: Glade Publishing; 2003, pp. 383–452.
- Khan SS et al. A naturalistic evaluation of intramuscular ziprasidone versus intramuscular olanzapine for the management of acute agitation and aggression in children and adolescents. J Child Adolesc Psychopharmacol 2006; 16:671–677.
- Staller JA. Intramuscular ziprasidone in youth: a retrospective chart review. J Child Adolesc Psychopharmacol 2004; 14:590–592.
- Hazaray E et al. Intramuscular ziprasidone for acute agitation in adolescents. J Child Adolesc Psychopharmacol 2004; 14:464–470.
- Barzman DH et al. A retrospective chart review of intramuscular ziprasidone for agitation in children and adolescents on psychiatric units: prospective studies are needed. J Child Adolesc Psychopharmacol 2007; 17:503–509.
- Sanford M et al. Intramuscular aripiprazole: a review of its use in the management of agitation in schizophrenia and bipolar I disorder. CNS Drugs 2008; 22:335–352.
- National Institute for Health and Clinical Excellence. Aripiprazole for schizophrenia in people aged 15 to 17 years. Technology Appraisal 13, 2011. http://guidance.nice.org.uk/TA213
Doses of commonly used psychotropic drugs in children and adolescents
See Table 5.8 for doses of commonly used psychotropic drugs in children and adolescents.
Table 5.8 Starting doses of commonly used psychotropic drugs in children and adolescents
|
Drug
|
Dose
|
Comment
|
|
Antipsychotics
|
|
Aripiprazole
|
2 mg daily
|
Increase to 5-15 mg daily according to response
|
|
Clozapine
|
6.25-12.5 mg
|
Use plasma levels to determine maintenance dose
|
|
Haloperidol
|
0.5-1.0 mg daily
|
Little evidence for benefit of doses > 4 mg a day in any condition
|
|
Olanzapine
|
2.5-5 mg
|
Use plasma levels to determine maintenance dose
|
|
Quetiapine
|
25 mg
|
Effective dose usually in the range 150-200 mg daily
|
|
Risperidone
|
0.25-2 mg
|
Adjust dose according to response and adverse effects
|
|
Antidepressants
|
|
Amitriptyline
|
5-10 mg at night
|
Effective dose in neuropathic pain and nocturnal enuresis 10-50 mg at night
|
|
Escitalopram
|
5 mg
|
Effective dose 10-20 mg
|
|
Fluoxetine
|
5-10 mg/day
|
Adjust dose according to response and adverse effects
|
|
Sertraline
|
25-50 mg
|
Effective dose 50-100 mg, sometimes higher
|
|
Mood stabilisers
|
|
Carbamazepine
|
5 mg/kg/day in divided doses
|
Use plasma levels to determine maintenance dose
|
|
Lithium
|
100-200 mg/day lithium carbonate
|
Use plasma levels to determine maintenance dose
|
|
Valproate
|
10-20 mg/kg/day in divided doses
|
Use plasma levels to determine maintenance dose
|
|
Suggested approximate oral starting doses (see primary literature for doses in individual indications). Lower dose in suggested range is for children weighing less than 25 kg.
|


