This chapter covers the treatment of schizophrenia with antipsychotic drugs, the adverse effect profile of these drugs and how adverse effects can be managed. It also discusses the use of clozapine and other drugs in the treatment of refractory schizophrenia, the adverse effects of clozapine and the treatment of these effects.
ANTIPSYCHOTIC DRUGS
General introduction
Classification
Before the 1990s antipsychotics (or major tranquillisers as they were then known) were classified according to their chemistry. The first antipsychotic, chlorpromazine, was a phenothiazine compound—a tricyclic structure incorporating a nitrogen and a sulphur atom. Further phenothiazines were generated and marketed, as were chemically similar thioxanthenes such as flupentixol. Later, entirely different chemical structures were developed according to pharmacological paradigms. These included butyrophenones (haloperidol), diphenylbutylpiperidines (pimozide) and substituted benzamides (sulpiride).
Chemical classification remains useful but is made somewhat redundant by the large range of chemical entities now available and by the absence of clear structure–activity relationships for newer drugs. The chemistry of older drugs does relate to their propensity to cause movement disorder. Piperazine phenothiazines (e.g. fluphenazine), butyrophenones and thioxanthines are most likely to cause extrapyramidal side-effects (EPS), and piperidine phenothiazines (e.g. pipotiazine) and benzamides least likely. Aliphatic phenothiazines (e.g. chlorpromazine) and diphenybutylpiperidines are perhaps somewhere in between.
Relative propensity for EPS was originally the primary factor behind typical/atypical classification. Clozapine has long been known as an atypical antipsychotic on the basis of its inability to cause EPS and its failure in animal-based antipsychotic screening tests. Its re-marketing in 1990 signalled the beginning of a mass of introductions of other drugs claimed, with varying degrees of accuracy, also to be atypical. Of these, perhaps only clozapine and quetiapine are 'fully' atypical, seemingly having no propensity whatever for EPS. Others show dose-related effects, although therapeutic activity can usually be gained without EPS. This is perhaps the real distinction between typical and atypical drugs: the ease with which a dose can be chosen (within the licensed dosage range) which is effective but which does not cause EPS (compare haloperidol with olanzapine).
The typical/atypical dichotomy does not lend itself well to classification of antipsychotics in the middle ground of EPS propensity. Thioridazine was widely described as atypical in the 1980s but is a 'conventional' phenothiazine. Sulpiride was marketed as an atypical but is often classified as typical. Risperidone, at its maximum dose of 16 mg/day (10 mg in the US) is just about as 'typical' as a drug can be. Alongside these difficulties is the fact that there is nothing either pharmacologically or chemically which clearly binds these so-called atypicals together as a group, save a general, but not universal finding, of preference for D2 receptors outside the striatum. Nor are atypicals characterised by improved efficacy over older drugs (clozapine and one or two others excepted) or the absence of hyperprolactinaemia (which is probably worse with risperidone and amisulpride than with typical drugs).
In an attempt to get round some of these problems, typicals and atypicals were re-classified as firstor second-generation antipsychotics (FGA/SGA). All drugs introduced since 1990 are classified as SGAs (i.e. all atypicals) but the new nomenclature dispenses with any connotations regarding atypically, whatever that may mean. However, the FGA/SGA classification remains problematic because neither group is defined by anything other than time of introduction—hardly the most sophisticated pharmacological classification system. Perhaps more importantly, date of introduction is often wildly distant from date of first synthesis. Clozapine is one of the oldest antipsychotics (synthesised in 1959) while olanzapine is hardly in its first flush of youth having first been patented in 1971. These two drugs are of course SGAs; apparently the most modern of antipsychotics.
In this edition of The Guidelines we conserve the FGA/SGA distinction more because of convention than some scientific basis. Also we feel that most people know which drugs belong to each group—it thus serves as a useful shorthand. However, it is clearly more sensible to consider the properties of individual antipsychotics when choosing drugs to prescribe, or in discussions with patients and carers.
Choosing an antipsychotic
The NICE guideline for medicines adherence1 recommends that patients should be as involved as possible in decisions about the choice of medicines that are prescribed for them, and that clinicians should be aware that illness beliefs, and beliefs about medicines, influence adherence. Consistent with this general advice that covers all of healthcare, the NICE guideline for schizophrenia emphasises the importance of patient choice rather than specifically recommending a class or individual antipsychotic as first line treatment.2
Antipsychotics are effective in both the acute and maintenance treatment of schizophrenia and other psychotic disorders. They differ in their pharmacology, kinetics, overall efficacy/effectiveness and tolerability, but perhaps more importantly, response and tolerability differs between patients. This variability of individual response means that there is no clear first line antipsychotic suitable for all.
Relative efficacy
Further to the publication of CATIE3 and CUtLASS,4 the World Psychiatric Association reviewed the evidence relating to the relative efficacy of 51 first-generation antipsychotics and 11 second-generation antipsychotics and concluded that, if differences in EPS could be minimised (by careful dosing) and anticholinergic use avoided, there is no convincing evidence to support any advantage for SGAs over FGAs.5 As a class, SGAs may have a lower propensity for EPS and tardive dyskinesia6 but this is somewhat offset by a higher propensity for metabolic side-effects. A recent metaanalysis of antipsychotics for first episode psychosis7 found few differences between FGAs and SGAs as groups of drugs but minor advantages for olanzapine and amisulpride individually.
When individual non-clozapine SGAs are compared with each other, it would appear that olanzapine is more effective than aripiprazole, risperidone, quetiapine and ziprasidone, and that risperidone has the edge over quetiapine and ziprasidone.8 FGA-controlled trials also suggest an advantage for olanzapine, risperidone and amisulpride over older drugs.9,10 A recent network meta-analysis11 broadly confirmed these findings, ranking amisulpride second behind clozapine and olanzapine third. These three drugs were the only ones to show clear efficacy advantages over haloperidol. The magnitude of these differences is small (but potentially substantial enough to be clinically important)11 and must be weighed against the very different side-effect profiles associated with individual antipsychotics.
Both FGAs and SGAs are associated with a number of adverse effects. These include weight gain, dyslipidaemia, increases in plasma glucose/diabetes,12,13 hyperprolactinaemia, hip fracture,14 sexual dysfunction, EPS including neuroleptic malignant syndrome (NMS),15 anticholinergic effects, venous thromboembolism (VTE),16 sedation and postural hypotension. The exact profile is drug-specific (see individual sections on adverse effects), although comparative data are not robust17 (see Leucht meta-analysis11 for rankings of some adverse effect risks). Adverse effects are a common reason for treatment discontinuation18 particularly when efficacy is poor.11 Patients do not always spontaneously report side-effects however,19 and psychiatrists' views of the prevalence and importance of adverse effects differs markedly from patient experience.20 Systematic enquiry along with a physical examination and appropriate biochemical tests is the only way accurately to assess their presence and severity or perceived severity. Patient-completed checklists such as the Glasgow Antipsychotic Side-effect Scale (GASS)21 can be a useful first step in this process. The clinician-completed Antipsychotic Non-Neurological Side-Effects Rating Scale (ANNSERS) facilitates more detailed assessment.22
Non-adherence to antipsychotic treatment is common and here the guaranteed medication delivery associated with depot preparations is potentially advantageous. In comparison with oral antipsychotics, there is a strong suggestion that depots are associated with a reduced risk of relapse and rehospitalisation.23–25
In patients whose symptoms have not responded adequately to sequential trials of two or more antipsychotic drugs, clozapine is the most effective treatment26–28 and its use in these circumstances is recommended by NICE.2 The biological basis for the superior efficacy of clozapine is uncertain.29 Olanzapine should probably be one of the two drugs used before clozapine.8,30
References
- National Institute for Health and Clinical Excellence. Medicines adherence: involving patients in decisions about prescribed medicines and supporting adherence. Clinical Guideline CG76, 2009. http://www.nice.org.uk/
- National Institute for Health and Clinical Excellence. Schizophrenia: core interventions in the treatment and management of schizophrenia in adults in primary and secondary care (update). Clinical Guideline 82, 2009. http://www.nice.org.uk/
- Lieberman JA et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005; 353:1209–1223.
- Jones PB et al. Randomized controlled trial of the effect on Quality of Life of secondvs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry 2006; 63:1079–1087.
- Tandon R et al. World Psychiatric Association Pharmacopsychiatry Section statement on comparative effectiveness of antipsychotics in the treatment of schizophrenia. Schizophr Res 2008; 100:20–38.
- Tarsy D et al. Epidemiology of tardive dyskinesia before and during the era of modern antipsychotic drugs. Handb Clin Neurol 2011; 100:601–616.
- Zhang JP et al. Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol 2013; 16:1205–1218.
- Leucht S et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry 2009; 166:152–163.
- Davis JM et al. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry 2003; 60:553–564.
- Leucht S et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 2009; 373:31–41.
- Leucht S et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013; 382:951–962.
- Manu P et al. Prediabetes in patients treated with antipsychotic drugs. J Clin Psychiatry 2012; 73:460–466.
- Rummel-Kluge C et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res 2010; 123:225–233.
- Sorensen HJ et al. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol 2013; 23:872–878.
- Trollor JN et al. Comparison of neuroleptic malignant syndrome induced by firstand second-generation antipsychotics. Br J Psychiatry 2012; 201:52–56.
- Masopust J et al. Risk of venous thromboembolism during treatment with antipsychotic agents. Psychiatry Clin Neurosci 2012; 66:541–552.
- Pope A et al. Assessment of adverse effects in clinical studies of antipsychotic medication: survey of methods used. Br J Psychiatry 2010; 197:67–72.
- Falkai P. Limitations of current therapies: why do patients switch therapies? Eur Neuropsychopharmacol 2008; 18 Suppl 3:S135–S139.
- Yusufi B et al. Prevalence and nature of side effects during clozapine maintenance treatment and the relationship with clozapine dose and plasma concentration. Int Clin Psychopharmacol 2007; 22:238–243.
- Day JC et al. A comparison of patients' and prescribers' beliefs about neuroleptic side-effects: prevalence, distress and causation. Acta Psychiatr Scand 1998; 97:93–97.
- Waddell L et al. A new self-rating scale for detecting atypical or second-generation antipsychotic side effects. J Psychopharmacol 2008; 22:238–243.
- Ohlsen RI et al. Interrater reliability of the Antipsychotic Non-Neurological Side-Effects Rating Scale measured in patients treated with clozapine. J Psychopharmacol 2008; 22:323–329.
- Tiihonen J et al. Effectiveness of antipsychotic treatments in a nationwide cohort of patients in community care after first hospitalisation due to schizophrenia and schizoaffective disorder: observational follow-up study. BMJ 2006; 333:224.
- Leucht C et al. Oral versus depot antipsychotic drugs for schizophrenia—a critical systematic review and meta-analysis of randomised long-term trials. Schizophr Res 2011; 127:83–92.
- Leucht S et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet 2012; 379:2063–2071.
- Kane J et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45:789–796.
- McEvoy JP et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry 2006; 163:600–610.
- Lewis SW et al. Randomized controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophr Bull 2006; 32:715–723.
- Stone JM et al. Review: The biological basis of antipsychotic response in schizophrenia. J Psychopharmacol 2010; 24:953–964.
- Agid O et al. An algorithm-based approach to first-episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry 2011; 72:1439–1444.
General principles of prescribing
- There is evidence to suggest that some antipsychotics are more effective than others: clozapine is the treatment of choice for refractory illness, and olanzapine, amisulpride, and perhaps risperidone, are more effective than other SGAs and FGAs.1,2 Antipsychotics differ markedly with respect to their side-effect profiles2 and patients differ in the side-effects they are and are not willing to tolerate. It is therefore important that the patient is involved in the choice of antipsychotic drug.
- The lowest possible dose should be used. For each patient, the dose should be titrated to the lowest known to be effective (see section on 'Minimum effective doses' in this chapter); dose increases should then take place only after one or two weeks of assessment during which the patient shows poor or no response. With depot medication, where no loading dose is given, plasma levels rise substantially for 6–12 weeks after initiation, even without a change in dose. Dose increases during this time are therefore inappropriate and difficult to evaluate.
- There is no evidence that high doses of antipsychotics have any advantages over standard doses but high doses are clearly associated with a greater side-effect burden3 (see section on 'High-dose antipsychotics: prescribing and monitoring' in this chapter). The vast majority of patients should receive a standard dose.
- For the large majority of patients, the use of a single antipsychotic (with or without additional mood stabiliser or sedatives) is recommended (see section on 'Combined antipsychotics' in this chapter). Apart from exceptional circumstances (e.g. clozapine augmentation) antipsychotic polypharmacy should be avoided because of the risk of an increased frequency and severity of adverse effects, particularly that associated with QT prolongation and sudden cardiac death.4
- Combinations of antipsychotics should only be used where response to a single antipsychotic (including clozapine) has been clearly demonstrated to be inadequate. In such cases, the effect of the combination against target symptoms and the side-effects should be carefully evaluated and documented. Where there is no clear benefit, treatment should revert to single antipsychotic therapy.
- In general, antipsychotics should not be used as 'prn' sedatives. Short courses of benzodiazepines or general sedatives (e.g. promethazine) are recommended.
- Responses to antipsychotic drug treatment should be assessed by recognised rating scales and be documented in patients' records.
- Those receiving antipsychotics should undergo close monitoring of physical health (including blood pressure, pulse, ECG, plasma glucose and plasma lipids) (see later sections in this chapter) and regular assessment of adverse effects. The latter may be facilitated by the use of rating scales: for example, GASS5 can be completed by the patient and broadly captures the most common side-effects associated with antipsychotic drugs, while ANNSERS6 is completed by the clinician and allows detailed assessment of non-neurological side-effects. Systematic inquiry reveals considerably more adverse effects than patients spontaneously report.7
References
- Leucht S et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 2009; 373:31–41.
- Leucht S et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013; 382:951–962.
- Royal College of Psychiatrists. Consensus statement on high-dose antipsychotic medication. Council Report CRXX, 2014.
- Ray WA et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009; 360:225–235.
- Waddell L et al. A new self-rating scale for detecting atypical or second-generation antipsychotic side effects. J Psychopharmacol 2008; 22:238–243.
- Ohlsen RI et al. Interrater reliability of the Antipsychotic Non-Neurological Side-Effects Rating Scale measured in patients treated with clozapine. J Psychopharmacol 2008; 22:323–329.
- Yusufi B et al. Prevalence and nature of side effects during clozapine maintenance treatment and the relationship with clozapine dose and plasma concentration. Int Clin Psychopharmacol 2007; 22:238–243.
Minimum effective doses
Table 2.1 suggests the minimum dose of antipsychotic likely to be effective in schizophrenia (first or multi-episode). At least some patients will respond to the dose suggested, although others may require higher doses. Given the variation in individual response, all doses should be considered approximate. Primary references are provided where available, but consensus opinion has also been used. Only oral treatment with commonly used drugs is covered.
Table 2.1 Antipsychotics: minimum effective dose/day
| Drug |
First episode |
Multi-episode |
| FGAs |
|
|
| Chlorpromazine |
200 mg* |
300 mg |
| Haloperidol1–6 |
2 mg |
4 mg |
| Sulpiride7 |
400 mg* |
800 mg |
| Trifluoperazine8 |
10 mg* |
15 mg |
| SGAs |
|
|
| Amisulpride9–12 |
400 mg* |
Unclear ?400 mg |
| Aripiprazole13–16 |
10 mg |
10 mg |
| Asenapine17 |
10 mg* |
10 mg |
| Iloperidone6,18 |
4 mg* |
8 mg |
| Lurasidone19 |
37 mg base/40 mg HCl |
37mg base/40mg HCl |
| Olanzapine5,6,20–22 |
5 mg |
7.5 mg |
| Quetiapine23–28 |
150 mg* |
300 mg |
| Risperidone4,29–31 |
2 mg |
3 mg |
| Sertindole32 |
Not appropriate |
12 mg |
| Ziprasidone6,33–35 |
40 mg* |
80 mg |
|
* Estimate—too few data available.
FGA, first-generation antipsychotic; HCl, hydrochloride; SGA, second-generation antipsychotic.
|
References
- Oosthuizen P et al. Determining the optimal dose of haloperidol in first-episode psychosis. J Psychopharmacol 2001; 15:251–255.
- McGorry PD. Recommended haloperidol and risperidone doses in first-episode psychosis. J Clin Psychiatry 1999; 60:794–795.
- Waraich PS et al. Haloperidol dose for the acute phase of schizophrenia. Cochrane Database Syst Rev 2002; CD001951.
- Schooler N et al. Risperidone and haloperidol in first-episode psychosis: a long-term randomized trial. Am J Psychiatry 2005; 162:947–953.
- Keefe RS et al. Long-term neurocognitive effects of olanzapine or low-dose haloperidol in first-episode psychosis. Biol Psychiatry 2006; 59:97–105.
- Liu CC et al. Aripiprazole for drug-naive or antipsychotic-short-exposure subjects with ultra-high risk state and first-episode psychosis: an open-label study. J Clin Psychopharmacol 2013; 33:18–23.
- Soares BG et al. Sulpiride for schizophrenia. Cochrane Database Syst Rev 2000; CD001162.
- Armenteros JL et al. Antipsychotics in early onset Schizophrenia: Systematic review and meta-analysis. Eur Child Adolesc Psychiatry 2006; 15:141–148.
- Mota NE et al. Amisulpride for schizophrenia. Cochrane Database Syst Rev 2002; CD001357.
- Puech A et al. Amisulpride, and atypical antipsychotic, in the treatment of acute episodes of schizophrenia: a dose-ranging study vs. haloperidol. The Amisulpride Study Group. Acta Psychiatr Scand 1998; 98:65–72.
- Moller HJ et al. Improvement of acute exacerbations of schizophrenia with amisulpride: a comparison with haloperidol. PROD-ASLP Study Group. Psychopharmacology 1997; 132:396–401.
- Sparshatt A et al. Amisulpride - dose, plasma concentration, occupancy and response: implications for therapeutic drug monitoring. Acta Psychiatr Scand 2009; 120:416–428.
- Taylor D. Aripiprazole: a review of its pharmacology and clinical utility. Int J Clin Pract 2003; 57:49–54.
- Cutler AJ et al. The efficacy and safety of lower doses of aripiprazole for the treatment of patients with acute exacerbation of schizophrenia. CNS Spectr 2006; 11:691–702.
- Mace S et al. Aripiprazole: dose-response relationship in schizophrenia and schizoaffective disorder. CNS Drugs 2008; 23:773–780.
- Sparshatt A et al. A systematic review of aripiprazole—dose, plasma concentration, receptor occupancy and response: implications for therapeutic drug monitoring. J Clin Psychiatry 2010; 71:1447–1456.
- Citrome L. Role of sublingual asenapine in treatment of schizophrenia. Neuropsychiatr Dis Treat 2011; 7:325–339.
- Crabtree BL et al. Iloperidone for the management of adults with schizophrenia. Clin Ther 2011; 33:330–345.
- Leucht S et al. Dose equivalents for second-generation antipsychotics: the minimum effective dose method. Schizophr Bull 2014; 40:314–326.
- Sanger TM et al. Olanzapine versus haloperidol treatment in first-episode psychosis. Am J Psychiatry 1999; 156:79–87.
- Kasper S. Risperidone and olanzapine: optimal dosing for efficacy and tolerability in patients with schizophrenia. Int Clin Psychopharmacol 1998; 13:253–262.
- Bishara D et al. Olanzapine: a systematic review and meta-regression of the relationships between dose, plasma concentration, receptor occupancy, and response. J Clin Psychopharmacol 2013; 33:329–335.
- Small JG et al. Quetiapine in patients with schizophrenia. A highand low-dose double-blind comparison with placebo. Seroquel Study Group. Arch Gen Psychiatry 1997; 54:549–557.
- Peuskens J et al. A comparison of quetiapine and chlorpromazine in the treatment of schizophrenia. Acta Psychiatr Scand 1997; 96:265–273.
- Arvanitis LA et al. Multiple fixed doses of "Seroquel" (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. Biol Psychiatry 1997; 42:233–246.
- Kopala LC et al. Treatment of a first episode of psychotic illness with quetiapine: an analysis of 2 year outcomes. Schizophr Res 2006; 81:29–39.
- Sparshatt A et al. Quetiapine: dose-response relationship in schizophrenia. CNS Drugs 2008; 22:49–68.
- Sparshatt A et al. Relationship between daily dose, plasma concentrations, dopamine receptor occupancy, and clinical response to quetiapine: a review. J Clin Psychiatry 2011; 72:1108–1123.
- Lane HY et al. Risperidone in acutely exacerbated schizophrenia: dosing strategies and plasma levels. J Clin Psychiatry 2000; 61:209–214.
- Williams R. Optimal dosing with risperidone: updated recommendations. J Clin Psychiatry 2001; 62:282–289.
- Ezewuzie N et al. Establishing a dose-response relationship for oral risperidone in relapsed schizophrenia. J Psychopharmacol 2006; 20:86–90.
- Lindstrom E et al. Sertindole: efficacy and safety in schizophrenia. Expert Opin Pharmacother 2006; 7:1825–1834.
- Bagnall A et al. Ziprasidone for schizophrenia and severe mental illness. Cochrane Database Syst Rev 2000; CD001945.
- Taylor D. Ziprasidone—an atypical antipsychotic. Pharm J 2001; 266:396401.
- Joyce AT et al. Effect of initial ziprasidone dose on length of therapy in schizophrenia. Schizophr Res 2006; 83:285–292.
Further reading
Davis JM et al. Dose response and dose equivalence of antipsychotics. J Clin Psychopharmacol 2004; 24:192–208.
Quick reference for licensed maximum doses
Table 2.2 lists the EU-licensed maximum doses of antipsychotics, according to the EMA labelling (as of December 2014).
Table 2.2 EU-licensed maximum doses of antipsychotics, according to the EMA labelling (December 2014)
| Drug |
Maximum dose |
| FGAs - oral |
|
| Chlorpromazine |
1000 mg/day |
| Flupentixol |
18 mg/day |
| Haloperidol |
20 mg/day |
| Levomepromazine |
1000 mg/day |
| Pericyazine |
300 mg/day |
| Perphenazine |
24 mg/day |
| Pimozide |
20 mg/day |
| Sulpiride |
2400 mg/day |
| Trifluoperazine |
None (suggest 30 mg/day) |
| Zuclopenthixol |
150 mg/day |
| SGAs - oral |
|
| Amisulpride |
1200 mg/day |
| Aripiprazole |
30 mg/day |
| Asenapine |
20 mg (sublingual) |
| Clozapine |
900 mg/day |
| Iloperidone* |
24 mg/day |
| Lurasidone |
148 mg base/160 mg HCl |
| Olanzapine |
20 mg/day |
| Paliperidone |
12 mg/day |
| Quetiapine |
750 mg/day schizophrenia |
| |
800 mg/day bipolar affective disorder |
| Risperidone |
16 mg/day |
| Sertindole |
2 4 mg/day |
| Ziprasidone* |
160 mg/day |
| Depots |
|
| Aripiprazole depot |
400 mg/month |
| Flupentixol depot |
400 mg/week |
| Fluphenazine depot |
50 mg/week |
| Haloperidol depot |
300 mg every 4 weeks |
| Paliperidone depot |
150 mg/month |
| Pipotiazine depot |
200 mg every 4 weeks |
| Risperidone |
50 mg every 2 weeks |
| Zuclopenthixol depot |
600 mg/week |
|
* US labelling.
FGA, first-generation antipsychotic; HCl, hydrochloride; SGA, second-generation antipsychotic.
|
Equivalent doses
Antipsychotic drugs vary greatly in potency (which is not the same as efficacy) and this is usually expressed as differences in 'neuroleptic' or 'chlorpromazine' 'equivalents'. Knowledge of equivalent doses is useful when switching between FGAs with different potencies and similar pharmacological actions, but in the absence of individual dose–response relationships being known. Some of the estimates relating to neuroleptic equivalents are based on early dopamine binding studies and some on clinical experience or expert panel opinion. Licensed maximum doses for antipsychotic drugs bear little relationship to their 'neuroleptic equivalents'—these maxima represent wildly different neuroleptic equivalents.
Table 2.3 gives some approximate equivalent doses for FGAs.1–3 The values should be seen as a rough guide when transferring from one conventional drug to another. An early review of progress is essential.
It is inappropriate to convert SGA doses into 'equivalents' since, unlike with FGAs, the dose–response relationship is usually well-defined for these drugs and because, with different pharmacological actions, switching between drugs may not be sensible. Dosage guidelines are discussed under each individual drug. A rough guide is given in Table 2.4 below.3–6 Clozapine is not included because its action is clearly not equivalent to any other antipsychotic.
Table 2.3 First-generation antipsychotics—equivalent doses
| Drug |
Equivalent dose (consensus) |
Range of values in literature |
| Chlorpromazine |
100 mg/day |
– |
| Flupentixol |
3 mg/day |
2–3 mg/day |
| Flupentixol depot |
10 mg/week |
10–20 mg/week |
| Fluphenazine |
2 mg/day |
1–5 mg/day |
| Fluphenazine depot |
5 mg/week |
1–12.5 mg/week |
| Haloperidol |
2 mg/day |
1.5–5 mg/day |
| Haloperidol depot |
15 mg/week |
5–25 mg/week |
| Perphenazine |
10 mg/day |
10 mg/day |
| Pimozide |
2 mg/day |
2 mg/day |
| Pipotiazine depot |
10 mg/week |
10–12.5 mg/week |
| Sulpiride |
200 mg/day |
200–300 mg/day |
| Trifluoperazine |
5 mg/day |
2.5–5 mg/day |
| Zuclopenthixol |
25 mg/day |
25–60 mg/day |
| Zuclopenthixol depot |
100 mg/week |
40–100 mg/week |
Table 2.4 Second-generation antipsychotics—equivalent doses
| Drug |
Approximate equivalent dose (per day, unless stated) |
| Aripiprazole |
10 mg |
| Asenapine |
10 mg |
| Iloperidone |
8 mg |
| Lurasidone |
37 mg base/40 mg HCl |
| Olanzapine |
7.5-10 mg |
| Paliperidone palmitate |
75 mg/month |
| Quetiapine |
300 mg |
| Risperidone oral |
3 mg |
| Risperidone LAI |
37.5 mg/2 weeks |
| Sertindole |
12 mg |
| Ziprasidone |
40 mg |
Comparing potencies of FGAs with SGAs introduces yet more uncertainty in respect to dose equivalence. Very approximately, 100 mg chlorpromazine is equivalent to 1.5 mg risperidone.3
References
- Foster P. Neuroleptic equivalence. Pharm J 1989; 243:431–432.
- Atkins M et al. Chlorpromazine equivalents: a consensus of opinion for both clinical and research implications. Psychiatr Bull 1997; 21:224–226.
- Patel MX et al. How to compare doses of different antipsychotics: a systematic review of methods. Schizophr Res 2013; 149:141–148.
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 2003; 64:663–667.
- Gardner DM et al. International consensus study of antipsychotic dosing. Am J Psychiatry 2010; 167:686–693.
- Leucht S et al. Dose equivalents for second-generation antipsychotics: the minimum effective dose method. Schizophr Bull 2014; 40:314–326.
High-dose antipsychotics: prescribing and monitoring
'High dose' can result from the prescription of either:
- a single antipsychotic in a dose that is above the recommended maximum, or
- two or more antipsychotics that, when expressed as a percentage of their respective maximum recommended doses and added together, result in a cumulative dose of > 100%.
Efficacy
There is no firm evidence that high doses of antipsychotics are any more effective than standard doses. This holds true for the use of antipsychotics in rapid tranquillisation, the management of acute psychotic episodes, chronic aggression and relapse prevention. In the UK, approximately one-quarter to one-third of hospitalised patients are prescribed high-dose antipsychotics, the vast majority through the cumulative effect of combinations.1,2 The common practice of prescribing antipsychotic drugs on a prn basis makes a major contribution.1 The national audit of schizophrenia conducted in the UK in 2013, reported on prescribing practice for over 5000 predominantly community-based patients; overall 10% were prescribed a high dose of antipsychotics.3
Reviews of the dose–response effects of a variety of antipsychotics have revealed very little evidence for increasing doses above accepted licensed ranges.2,4,5 Effect appears to be optimal at low doses: 4 mg/day risperidone;6300 mg/day quetiapine,7 olanzapine 10 mg,8,9 etc. Similarly, 100 mg two-weekly risperidone depot offers no benefits over 50 mg two-weekly,10 and 320 mg/day ziprasidone11 is no better than 160 mg/day. All currently available antipsychotics (with the possible exception of clozapine) exert their antipsychotic effect primarily through antagonism (or partial agonism) at post-synaptic dopamine receptors. There is increasing evidence that in some patients with schizophrenia, symptoms do not seen to be driven through dysfunction of dopamine pathways;12,13 and so increasing dopamine blockade in such patients is clearly futile.
There are a small number of randomised controlled trials (RCTs) that examine the efficacy of high versus standard doses in patients with treatment-resistant schizophrenia.14,15 Some demonstrated benefit16 but the majority of these studies are old, the number of patients randomised is small, and study design is poor by current standards. Some studies used daily doses equivalent to more than 10 g chlorpromazine. In a study of patients with first-episode schizophrenia, increasing the dose of olanzapine to up to 30 mg/day and the dose of risperidone to up to 10 mg/day in non-responders to standard doses, yielded only a 4% absolute increase in overall response rate; switching to an alternative antipsychotic, including clozapine was considerably more successful.17 One small (n = 12) open study of high dose quetiapine (up to 1400 mg/day) found modest benefits in a third of subjects18 (other, larger studies of quetiapine have shown no benefit for higher doses7,19,20). A further small (n = 40) RCT of high dose olanzapine (up to 45 mg/day) versus clozapine, high dose olanzapine suggested similar efficacy to clozapine.21 In all studies, the side-effect burden associated with high dose treatment was considerable.
Adverse effects
The majority of side-effects associated with antipsychotic treatment are dose-related. These include EPS, sedation, postural hypotension, anticholinergic effects, QTc prolongation and sudden cardiac death.22,23 High-dose antipsychotic treatment clearly worsens adverse effect incidence and severity.11,20,24,25 There is some evidence that dose reduction from very high (mean 2253 mg chlorpromazine equivalents per day) to high (mean 1315 mg chlorpromazine equivalents per day) dose leads to improvements in cognition and negative symptoms.26
Recommendations:
- The use of high dose antipsychotics should be an exceptional clinical practice and only ever employed when an adequate trial of standard treatments, including clozapine, have failed.
- Documentation of target symptoms, response and side-effects, ideally using validated rating scales, should be standard practice so that there is ongoing consideration of the risk benefit ratio for the patient. Close physical monitoring (including ECG) is essential.
Prescribing high-dose antipsychotics
Before using high doses, ensure that:
- sufficient time has been allowed for response
- at least two different antipsychotics have been tried sequentially (one FGA, and if possible, olanzapine)
- clozapine has failed or not been tolerated due to agranulocytosis or other serious adverse effect. Most other side-effects can be managed. A very small proportion of patients may also refuse clozapine outright
- compliance is not in doubt (use of blood tests, liquids/dispersible tablets, depot preparations, etc)
- adjunctive medications such as antidepressants or mood stabilisers are not indicated
- psychological approaches have failed or are not appropriate.
The decision to use high doses should:
- be made by a senior psychiatrist
- involve the multidisciplinary team
- be done, if possible, with patient's informed consent.
Process
- Rule out contraindications (ECG abnormalities, hepatic impairment).
- Consider and minimise any risks posed by concomitant medication (e.g. potential to cause QTc prolongation, electrolyte disturbance or pharmacokinetic interactions via CYP inhibition).
- Document the decision to prescribe high doses in the clinical notes along with a description of target symptoms. The use of an appropriate rating scale is advised.
- Adequate time for response should be allowed after each dosage increment before a further increase is made.
Monitoring
- Physical monitoring should be carried out as outlined in the section on 'Monitoring' in this chapter.
- All patients on high doses should have regular ECGs (base-line, when steady-state serum levels have been reached after each dosage increment, and then every 6 to 12 months). Additional biochemical/ECG monitoring is advised if drugs that are known to cause electrolyte disturbances or QTc prolongation are subsequently co-prescribed.
- Target symptoms should be assessed after 6 weeks and 3 months. If insufficient improvement in these symptoms has occurred, the dose should be decreased to the normal range.
References
- Paton C et al. High-dose and combination antipsychotic prescribing in acute adult wards in the UK: the challenges posed by p.r.n. prescribing. Br J Psychiatry 2008; 192:435–439.
- Royal College of Psychiatrists. Consensus statement on high-dose antipsychotic medication. College Report CR190 2014: in press.
- Patel MX et al. Quality of prescribing for schizophrenia: evidence from a national audit in England and Wales. Eur Neuropsychopharmacol 2014; 24:499–509.
- Davis JM et al. Dose response and dose equivalence of antipsychotics. J Clin Psychopharmacol 2004; 24:192–208.
- Gardner DM et al. International consensus study of antipsychotic dosing. Am J Psychiatry 2010; 167:686–693.
- Ezewuzie N et al. Establishing a dose-response relationship for oral risperidone in relapsed schizophrenia. J Psychopharmacol 2006; 20:86–90.
- Sparshatt, A et al. Quetiapine: dose-response relationship in schizophrenia. CNS Drugs 2008; 22:49–68.
- Kinon BJ et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder: a randomized, doubleblind, fixed-dose study. J Clin Psychopharmacol 2008; 28:392–400.
- Bishara D et al. Olanzapine: a systematic review and meta-regression of the relationships between dose, plasma concentration, receptor occupancy, and response. J Clin Psychopharmacol 2013; 33:329–335.
- Meltzer HY et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res 2014; 154:14–22.
- Goff DC et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms: the ZEBRAS study. J Clin Psychopharmacol 2013; 33:485–490.
- Kapur S et al. Relationship between dopamine D2 occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry 2000; 157:514–520.
- Demjaha A et al. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am J Psychiatry 2012; 169:1203–1210
- Hirsch SR et al. Clinical use of high-dose neuroleptics. Br J Psychiatry 1994; 164:94–96
- Thompson C. The use of high-dose antipsychotic medication. Br J Psychiatry 1994; 164:448–458.
- Aubree JC et al. High and very high dosage antipsychotics: a critical review. J Clin Psychiatry 1980; 41:341–350.
- Agid O et al. An algorithm-based approach to first-episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry 2011; 72:1439–1444.
- Boggs DL et al. Quetiapine at high doses for the treatment of refractory schizophrenia. Schizophr Res 2008; 101:347–348.
- Lindenmayer JP et al. A randomized, double-blind, parallel-group, fixed-dose, clinical trial of quetiapine at 600 versus 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol 2011; 31:160–168.
- Honer WG et al. A randomized, double-blind, placebo-controlled study of the safety and tolerability of high-dose quetiapine in patients with persistent symptoms of schizophrenia or schizoaffective disorder. J Clin Psychiatry 2012; 73:13–20.
- Meltzer HY et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry 2008; 69:274–285.
- Ray WA et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009; 360:225–235.
- Weinmann S et al. Influence of antipsychotics on mortality in schizophrenia: systematic review. Schizophr Res 2009; 113:1–11.
- Bollini P et al. Antipsychotic drugs: is more worse? A meta-analysis of the published randomized control trials. Psychol Med 1994; 24:307–316.
- Baldessarini RJ et al. Significance of neuroleptic dose and plasma level in the pharmacological treatment of psychoses. Arch Gen Psychiatry 1988; 45:79–90.
- Kawai N et al. High-dose of multiple antipsychotics and cognitive function in schizophrenia: the effect of dose-reduction. Prog Neuropsychopharmacol Biol Psychiatry 2006; 30:1009–1014.
Antipsychotic prophylaxis
First episode of psychosis
Antipsychotics provide effective protection against relapse, at least in the short to medium term. A meta-analysis of placebo-controlled trials found that 26% of first episode patients randomised to receive maintenance antipsychotic relapsed after 6–12 months compared with 61% randomised to receive placebo.1 After 1–2 years of being well on antipsychotic medication, the risk of relapse remains high (figures of 10–15% per month have been quoted), but this area is less well researched.2 Although the current consensus is that antipsychotics should be prescribed for 1–2 years after a first episode of schizophrenia,3,4 Gitlan et al.5 found that withdrawing antipsychotic treatment in line with this consensus led to a relapse rate of almost 80% after one year medication-free and 98% after 2 years. Other studies in first episode patients have found that discontinuing antipsychotics increases the risk of relapse 5-fold6 and confirmed that only a small minority of patients who discontinue remain well 1–2 years later.7–10 However, a 5-year follow-up of a 2-year RCT, during which patients received either maintenance antipsychotic treatment or had their antipsychotic dose reduced or discontinued completely, found that while there was a clear advantage for maintenance treatment with respect to reducing short-term relapse this advantage was lost in the medium-term. Further, the dose-reduction/discontinuation group were receiving lower doses of antipsychotic drugs at follow-up and had better functional outcomes.11 There are numerous interpretations of these outcomes but the most that can be concluded at this stage is dose reduction is a possible option in first episode psychosis.
It should be noted that definitions of relapse usually focus on the severity of positive symptoms, and largely ignore cognitive and negative symptoms: positive symptoms are more likely to lead to hospitalisation while cognitive and negative symptoms (which respond less well, and in some circumstances may even be exacerbated by antipsychotic treatment) have a greater overall impact on quality of life.
With respect to antipsychotic choice: in the context of a RCT, clozapine did not offer any advantage over chlorpromazine in the medium term in first episode patients with non-refractory illness.12 But in a large naturalistic study of patients with a first admission for schizophrenia, clozapine and olanzapine fared better with respect to preventing re-admission than other oral antipsychotics.13 In this same study, the use of a long-acting antipsychotic injection (depot) seemed to offer advantages over oral antipsychotics despite confounding by indication (depots will have been prescribed to poor compliers, oral to good compliers).13 Note that in this study first admission may not be the same as first episode.
In practice, a firm diagnosis of schizophrenia is rarely made after a first episode and the majority of prescribers and/or patients will have at least attempted to stop antipsychotic treatment within one year.14 It is vital that patients, carers and key-workers are aware of the early signs of relapse and how to access help. Antipsychotics should not be considered the only intervention. Psychosocial and psychological interventions are clearly also important.15
Multi-episode schizophrenia
The majority of those who have one episode of schizophrenia will go on to have further episodes. Patients with residual symptoms, a greater side-effect burden and a less positive attitude to treatment are at greater risk of relapse.16 With each subsequent episode, the baseline level of functioning deteriorates17 and the majority of this decline is seen in the first decade of illness. Suicide risk (10%) is also concentrated in the first decade of illness. Antipsychotic drugs, when taken regularly, protect against relapse in the short, medium and long term.1,18 Those who receive targeted antipsychotics (i.e. only when symptoms re-emerge) seem to have a worse outcome than those who receive prophylactic antipsychotics19,20 and the risk of tardive dyskinesia may also be higher. Similarly, low dose antipsychotics are less effective than standard doses.21
Table 2.5 summarises the known benefits and harms associated with maintenance antipsychotic treatment as reported in a meta-analysis by Leucht et al. (2012).1
Depot preparations may have an advantage over oral preparations in maintenance treatment, most likely because of guaranteed medication delivery. Meta-analyses of clinical trials have shown that the relative and absolute risks of relapse with depot maintenance treatment were 30% and 10% lower respectively, than with oral treatment.1,23 Long-acting preparations of antipsychotics may thus be preferred by both prescribers and patients.
A recent meta-analysis concluded that the risk of relapse with newer antipsychotics is similar to that associated with older drugs.1 (Note that lack of relapse is not the same as good functioning.24) The proportion of multi-episode patients who achieve remission is small and may differ between antipsychotic drugs. The CATIE study reported that only 12% of patients treated with olanzapine achieved remission for at least 6 months, compared with 8% treated with quetiapine and 6% with risperidone.25 The advantage seen here for olanzapine is consistent with that seen in an acute efficacy multiple treatments meta-analysis.26
Table 2.5 Benefits and harms associated with maintenance antipsychotic treatment
| Benefits |
Harms |
| Outcome |
Antipsychotic |
Placebo |
NNT |
Adverse effect |
Antipsychotic |
Placebo |
NNH* |
| Relapse at 7–12 months |
27% |
64% |
3 |
Movement disorder |
16% |
9% |
17 |
| Re-admission |
10% |
26% |
5 |
Anticholinergic effects |
24% |
16% |
11 |
| Improvement in mental state |
30% |
12% |
4 |
Sedation |
13% |
9% |
20 |
| Violent/aggressive behaviour |
2% |
12% |
11 |
Weight gain |
10% |
6% |
20 |
|
NNH, number treated for one patient to be harmed; NNT, number needed to treat for one patient to benefit.
* Likely to be a considerable underestimate as adverse effects are rarely systematically assessed in clinical trials.22
|
Patients with schizophrenia often receive a number of sequential antipsychotic drugs during the maintenance phase.27 Such switching is a result of a combination of suboptimal efficacy and poor tolerability. In both CATIE28 and SOHO,29,30 the attrition rate from olanzapine was lower than the attrition rate from other antipsychotic drugs, suggesting that olanzapine may be more effective than other antipsychotic drugs (except clozapine). Note though that olanzapine is associated with a high propensity for metabolic side-effects. In the SOHO study, the relapse rate over a 3 year period was relatively constant, supporting the benefit for maintenance treatment.31,32
In summary:
- relapse rates in patients not receiving antipsychotics are extremely high
- antipsychotics significantly reduce relapse, re-admission and violence/aggression
- long-acting depot formulations provide the best protection against relapse.
Adherence to antipsychotic treatment
Amongst people with schizophrenia, non-adherence with antipsychotic treatment is high. Only 10 days after discharge from hospital up to 25% are partially or non-adherent, rising to 50% at 1 year and 75% at 2 years.33 Not only does non-adherence increase the risk of relapse, it may also increase the severity of relapse and the duration of hospitalisation.33 The risk of suicide attempts also increases four-fold.33
Dose for prophylaxis
Many patients probably receive higher doses than necessary (particularly of the older drugs) when acutely psychotic.34,35 In the longer term a balance needs to be made between effectiveness and adverse-effects. Lower doses of the older drugs (8 mg haloperidol/day or equivalent) are, when compared with higher doses, associated with less severe sideeffects,36 better subjective state and better community adjustment.37 Very low doses increase the risk of psychotic relapse.34,38 There are no data to support the use of lower than standard doses of the newer drugs as prophylaxis. Doses that are acutely effective should generally be continued as prophylaxis39,40 although an exception to this is prophylaxis after a first episode where careful dose reduction is supportable.
How and when to stop41
The decision to stop antipsychotic drugs requires a thorough risk–benefit analysis for each patient. Withdrawal of antipsychotic drugs after long-term treatment should be gradual and closely monitored. The relapse rate in the first 6 months after abrupt withdrawal is double that seen after gradual withdrawal (defined as slow taper down over at least 3 weeks for oral antipsychotics or abrupt stopping of depot preparations).42 Abrupt withdrawal may also lead to discontinuation symptoms (e.g. headache, nausea, insomnia) in some patients.43
The following factors should be considered.41
- Is the patient symptom-free, and if so, for how long? Long-standing, non-distressing symptoms which have not previously been responsive to medication may be excluded.
- What is the severity of adverse-effects (EPS, tardive dyskinesia, sedation, obesity, etc.)?
- What was the previous pattern of illness? Consider the speed of onset, duration and severity of episodes and any danger posed to self and others.
- Has dosage reduction been attempted before, and, if so, what was the outcome?
- What are the patient's current social circumstances? Is it a period of relative stability, or are stressful life events anticipated?
- What is the social cost of relapse (e.g. is the patient the sole breadwinner for a family)?
- Is the patient/carer able to monitor symptoms, and, if so, will they seek help?
As with first-episode patients, patients, carers and key-workers should be aware of the early signs of relapse and how to access help. Be aware that targeted relapse treatment is much less effective than continuous prophylaxis.9 Those with a history of aggressive behaviour or serious suicide attempts and those with residual psychotic symptoms should be considered for life-long treatment.
Key points that patients should know
- Antipsychotics do not 'cure' schizophrenia. They treat symptoms in the same way that insulin treats diabetes.
- Some antipsychotic drugs may be more effective than others.
- Many antipsychotic drugs are available. Different drugs suit different patients. Perceived adverse-effects should always be discussed, so that the best tolerated drug can be found.
- Long-term treatment is generally required to prevent relapses.
- Antipsychotics should never be stopped suddenly.
- Psychological and psychosocial interventions increase the chance of staying well.15
Alternative views
While it is clear that antipsychotics effectively reduce symptom severity and rates of relapse, a minority view is that antipsychotics might also sensitise patients to psychosis. Thus, relapse on withdrawal can be seen as a type of discontinuation reaction resulting from super-sensitivity of (probably) dopamine receptors. This phenomenon might explain better outcomes seen in first episode patients who receive lower doses of antipsychotics.
The concept of 'super-sensitivity psychosis' was much discussed decades ago44,45 although one rarely sees mention of it now. It is also striking that dopamine antagonists used for non-psychiatric conditions can induce withdrawal psychosis.46–48 Whilst these theories and observations do not alter recommendations made in this section, they do emphasise the need for using the lowest possible dose of antipsychotic in all patients.
References
- Leucht S et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet 2012; 379:2063–2071.
- Nuechterlein KH et al. The early course of schizophrenia and long-term maintenance neuroleptic therapy. Arch Gen Psychiatry 1995; 52:203–205.
- American Psychiatric Association. Guideline Watch (September 2009): Practice Guideline for the Treatment of Patients With Schizophrenia. 2009. http://www.psychiatryonline.com/content.aspx?aid=501001
- Sheitman BB et al. The evaluation and treatment of first-episode psychosis. Schizophr Bull 1997; 23:653–661.
- Gitlin M et al. Clinical outcome following neuroleptic discontinuation in patients with remitted recent-onset schizophrenia. Am J Psychiatry 2001; 158:1835–1842.
- Robinson D et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1999; 56:241–247.
- Wunderink L et al. Guided discontinuation versus maintenance treatment in remitted first-episode psychosis: relapse rates and functional outcome. J Clin Psychiatry 2007; 68:654–661.
- Chen EY et al. Maintenance treatment with quetiapine versus discontinuation after one year of treatment in patients with remitted first episode psychosis: randomised controlled trial. BMJ 2010; 341:c4024.
- Gaebel W et al. Relapse prevention in first-episode schizophrenia—maintenance vs intermittent drug treatment with prodrome-based early intervention: results of a randomized controlled trial within the German Research Network on Schizophrenia. J Clin Psychiatry 2011; 72:205–218.
- Caseiro O et al. Predicting relapse after a first episode of non-affective psychosis: a three-year follow-up study. J Psychiatr Res 2012; 46:1099–1105.
- Wunderink L et al. Recovery in remitted first-episode psychosis at 7 years of follow-up of an early dose reduction/discontinuation or maintenance treatment strategy: long-term follow-up of a 2-year randomized clinical trial. JAMA Psychiatry 2013; 70:913–920.
- Girgis RR et al. Clozapine v. chlorpromazine in treatment-naive, first-episode schizophrenia: 9-year outcomes of a randomised clinical trial. Br J Psychiatry 2011; 199:281–288.
- Tiihonen J et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry 2011; 168:603–609.
- Johnson DAW et al. Professional attitudes in the UK towards neuroleptic maintenance therapy in schizophrenia. Psychiatr Bull 1997; 21:394–397.
- National Institute for Health and Clinical Excellence. Schizophrenia: core interventions in the treatment and management of schizophrenia in adults in primary and secondary care (update). Clinical Guideline 82, 2009. http://www.nice.org.uk/
- Schennach R et al. Predictors of relapse in the year after hospital discharge among patients with schizophrenia. Psychiatr Serv 2012; 63:87–90.
- Wyatt RJ. Neuroleptics and the natural course of schizophrenia. Schizophr Bull 1991; 17:325–351.
- Almerie MQ et al. Cessation of medication for people with schizophrenia already stable on chlorpromazine. Schizophr Bull 2008; 34:13–14.
- Jolley AG et al. Trial of brief intermittent neuroleptic prophylaxis for selected schizophrenic outpatients: clinical and social outcome at two years. Br Med J 1990; 301:837–842.
- Herz MI et al. Intermittent vs maintenance medication in schizophrenia. Two-year results. Arch Gen Psychiatry 1991; 48:333–339.
- Schooler NR et al. Relapse and rehospitalization during maintenance treatment of schizophrenia. The effects of dose reduction and family treatment. Arch Gen Psychiatry 1997; 54:453–463.
- Pope A et al. Assessment of adverse effects in clinical studies of antipsychotic medication: survey of methods used. Br J Psychiatry 2010; 197:67–72.
- Leucht C et al. Oral versus depot antipsychotic drugs for schizophrenia—a critical systematic review and meta-analysis of randomised long-term trials. Schizophr Res 2011; 127:83–92.
- Schooler NR. Relapse prevention and recovery in the treatment of schizophrenia. J Clin Psychiatry 2006; 67 Suppl 5:19–23.
- Levine SZ et al. Extent of attaining and maintaining symptom remission by antipsychotic medication in the treatment of chronic schizophrenia: evidence from the CATIE study. Schizophr Res 2011; 133:42–46.
- Leucht S et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013; 382:951–962.
- Burns T et al. Maintenance antipsychotic medication patterns in outpatient schizophrenia patients: a naturalistic cohort study. Acta Psychiatr Scand 2006; 113:126–134.
- Lieberman JA et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005; 353:1209–1223.
- Haro JM et al. Three-year antipsychotic effectiveness in the outpatient care of schizophrenia: observational versus randomized studies results. Eur Neuropsychopharmacol 2007; 17:235–244.
- Haro JM et al. Antipsychotic type and correlates of antipsychotic treatment discontinuation in the outpatient treatment of schizophrenia. Eur Psychiatry 2006; 21:41–47.
- Ciudad A et al. The Schizophrenia Outpatient Health Outcomes (SOHO) study: 3-year results of antipsychotic treatment discontinuation and related clinical factors in Spain. Eur Psychiatry 2008; 23:1–7.
- Suarez D et al. Overview of the findings from the European SOHO study. Expert Rev Neurother 2008; 8:873–880.
- Leucht S et al. Epidemiology, clinical consequences, and psychosocial treatment of nonadherence in schizophrenia. J Clin Psychiatry 2006; 67 Suppl 5:3–8.
- Baldessarini RJ et al. Significance of neuroleptic dose and plasma level in the pharmacological treatment of psychoses. Arch Gen Psychiatry 1988; 45:79–90.
- Harrington M et al. The results of a multi-centre audit of the prescribing of antipsychotic drugs for in-patients in the UK. Psychiatr Bull 2002; 26:414–418.
- Geddes J et al. Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. Br Med J 2000; 321:1371–1376.
- Hogarty GE et al. Dose of fluphenazine, familial expressed emotion, and outcome in schizophrenia. Results of a two-year controlled study. Arch Gen Psychiatry 1988; 45:797–805.
- Marder SR et al. Lowand conventional-dose maintenance therapy with fluphenazine decanoate. Two-year outcome. Arch Gen Psychiatry 1987; 44:518–521.
- Rouillon F et al. Strategies of treatment with olanzapine in schizophrenic patients during stable phase: results of a pilot study. Eur Neuropsychopharmacol 2008; 18:646–652.
- Wang CY et al. Risperidone maintenance treatment in schizophrenia: a randomized, controlled trial. Am J Psychiatry 2010; 167:676–685.
- Wyatt RJ. Risks of withdrawing antipsychotic medications. Arch Gen Psychiatry 1995; 52:205–208.
- Viguera AC et al. Clinical risk following abrupt and gradual withdrawal of maintenance neuroleptic treatment. Arch Gen Psychiatry 1997; 54:49–55.
- Chouinard G et al. Withdrawal symptoms after long-term treatment with low-potency neuroleptics. J Clin Psychiatry 1984; 45:500–502.
- Chouinard G et al. Neuroleptic-induced supersensitivity psychosis: clinical and pharmacologic characteristics. Am J Psychiatry 1980; 137:16–21.
- Kirkpatrick B et al. The concept of supersensitivity psychosis. J Nerv Ment Dis 1992; 180:265–270.
- CHAFFIN DS. Phenothiazine-induced acute psychotic reaction: the "psychotoxicity" of a drug. Am J Psychiatry 1964; 121:26–32.
- Lu ML et al. Metoclopramide-induced supersensitivity psychosis. Ann Pharmacother 2002; 36:1387–1390.
- Roy-Desruisseaux J et al. Domperidone-induced tardive dyskinesia and withdrawal psychosis in an elderly woman with dementia. Ann Pharmacother 2011; 45:e51.
Combined antipsychotics
A recent systematic review of the efficacy of monotherapy with antipsychotic drugs concluded that the magnitude of the clinical improvement that is seen is generally modest.1 It is therefore unsurprising that the main clinical rationale for prescribing combined antipsychotics is to improve residual psychotic symptoms2,3 but there is no good objective evidence that combined antipsychotics (that do not include clozapine) offer any efficacy advantage over the use of a single antipsychotic. There are three negative RCTs of non-clozapine combinations4 while the 'evidence base' supporting such combinations consists for the most part of small open studies and case series.4,5 Placebo response and reporting bias (nobody reports the failure of polypharmacy) are clearly important factors in this flimsy evidence base. Two-thirds of patients established on combined antipsychotics can be successfully switched to monotherapy6 while one-third fare poorly.
Some antipsychotic polypharmacy makes scientific sense. It has been shown that co-prescribed aripiprazole reduces weight in those given clozapine7 and normalises prolactin in those on haloperidol8 and risperidone long-acting injection (LAI)9 (although not amisulpride10). Polypharmacy with aripiprazole in such circumstances may thus represent worthwhile, evidence-based practice, albeit in the absence of regulatory trials demonstrating safety. In many cases, however, using aripiprazole alone might be a more logical choice.
Evidence for harm is perhaps more compelling. There are a number of published reports of clinically significant side-effects such as an increased prevalence of EPS,11 severe EPS,12 increased metabolic side-effects,13 sexual dysfunction,14 increased risk of hip fracture,15 paralytic ileus,16 grand mal seizures,17 prolonged QTc18 and arrhythmias3 associated with combined antipsychotics. Switching to monotherapy has been shown to lead to worthwhile improvements in cognitive functioning.19 With respect to systematic studies, one that followed a cohort of patients with schizophrenia prospectively over a 10-year period found that receiving more than one antipsychotic concurrently was associated with substantially increased mortality20 while there was no association between mortality and any measure of illness severity. These findings imply that it is the co-prescription of antipsychotics that increase mortality, rather than the more severe or refractory illness for which they are prescribed. Another study that followed-up 99 patients with schizophrenia over a 25 year period found that those who were prescribed three antipsychotics simultaneously were twice as likely to die as those who were prescribed only one.21 A negative case-control and a database study22,23 also exist. Combined antipsychotics have also been associated with longer hospital stay and more frequent adverse effects.24 It follows that it should be standard practice to document the rationale for combined antipsychotics in individual cases in clinical records along with a clear account of any benefits and side-effects. Medico-legally, that would seem to be wise although in practice it is rarely done.25
Despite the adverse risk–benefit balance, prescriptions for combined antipsychotics are commonly seen,26 are often long-term,27 and the prevalence of such prescribing is not decreasing.28 A UK quality improvement programme conducted through the Prescribing Observatory for Mental Health (POMH-UK) found that combined antipsychotics were prescribed for 43% of patients in acute adult wards in the UK at baseline and 39% at re-audit one year later.29 In the majority of cases, the second antipsychotic was prescribed prn and the most common reason given for prescribing in this way was to manage behavioural disturbance.29 National surveys have repeatedly shown that up to 50% of patients prescribed atypical antipsychotics receive a typical drug as well.29–32 Anticholinergic medication is then often required.31 Combined antipsychotics are associated with younger age, male gender, increased illness severity, acuity, complexity and chronicity, poorer functioning, inpatient status and a diagnosis of schizophrenia.2,28,33,34 These associations largely reinforce the idea that polypharmacy is used where monotherapy proves inadequate.
The situation in the community is different. A recent, systematic, audit of 5000 community patients from nearly 60 different NHS Trusts in the UK shows that 60% of the patients received a single antipsychotic (FGA or SGA; oral or injectable) and a further 18% received clozapine, and 5% received no antipsychotics at a given time—suggesting that less than one in five received antipsychotic combinations. This highlights a clear difference between inpatient and outpatient practices—probably a reflection of patient selection, disease severity and prescribing cultures.35
Combining antipsychotic drugs is clearly an established custom and practice. A questionnaire survey of US psychiatrists found that, in patients who did not respond to a single antipsychotic, two-thirds of psychiatrists switched to another single antipsychotic, while a third added a second antipsychotic. Those who switched were more positive about outcomes than those who augmented.36 A further questionnaire study conducted in Denmark revealed that almost two-thirds of psychiatrists would rather combine antipsychotics than prescribe clozapine.37 One observational study found that patients who derived no benefit from antipsychotic monotherapy were more likely to be switched to an alternative antipsychotic, while those who partially responded were more likely to have a second antipsychotic added.38 This may partly explain why some patients are prescribed combined antipsychotics early in a treatment episode3,39 and the use of combined antipsychotics in a third of patients prior to initiating clozapine.40 Combined antipsychotics are likely to involve depots/LAIs,41 quetiapine33 and FGAs,29 perhaps due to the frequent use of the last of these as prn. Initiatives to reduce the prevalence of combined antipsychotic prescribing appear to have only modest effects.6,29
Overall, on the basis of lack of evidence for efficacy, and the potential for serious adverse effects such as QT prolongation (common to almost all antipsychotics), routine use of combined antipsychotics should be avoided. Note, however, that clozapine augmentation strategies often involve combining antipsychotics and this is perhaps the sole therapeutic area where such practice is supportable.42–46 See section on 'Clozapine augmentation' in this chapter. While there is little evidence for starting polypharmacy, stopping it is not easy. As mentioned above, switching to monotherapy, even when done in a graded fashion, does entail a slightly higher risk of an exacerbation—although it is usually rewarded with lesser side-effects and the exacerbations can be successfully managed.47
Summary
- There is very little evidence supporting the efficacy of combining non-clozapine antipsychotics.
- Substantial evidence supports the potential for harm, and so the use of combined antipsychotics should generally be avoided.
- Combined antipsychotics are often prescribed and this practice is resistant to change.
- As a minimum requirement, all patients who are prescribed combined antipsychotics should have their side-effects systematically assessed (including ECG monitoring) and any beneficial effect on symptoms carefully documented.
- Some antipsychotic polypharmacy (e.g. combinations with aripiprazole) show clear benefits for tolerability but not efficacy.
References
- Lepping P et al. Clinical relevance of findings in trials of antipsychotics: systematic review. Br J Psychiatry 2011; 198:341–345.
- Correll CU et al. Antipsychotic polypharmacy: a comprehensive evaluation of relevant correlates of a long-standing clinical practice. Psychiatr Clin North Am 2012; 35:661–681.
- Grech P et al. Long-term antipsychotic polypharmacy: how does it start, why does it continue? Ther Adv Psychopharmacol 2012; 2:5–11.
- Tracy DK et al. Antipsychotic polypharmacy: still dirty, but hardly a secret. A systematic review and clinical guide. Curr Psychopharmacol 2013; 2:143–171.
- Barnes TR et al. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs 2011; 25:383–399.
- Tani H et al. Interventions to reduce antipsychotic polypharmacy: a systematic review. Schizophr Res 2013; 143:215–220.
- Fleischhacker WW et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol 2010; 13:1115–1125.
- Shim JC et al. Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebocontrolled trial. Am J Psychiatry 2007; 164:1404–1410.
- Trives MZ et al. Effect of the addition of aripiprazole on hyperprolactinemia associated with risperidone long-acting injection. J Clin Psychopharmacol 2013; 33:538–541.
- Chen CK et al. Differential add-on effects of aripiprazole in resolving hyperprolactinemia induced by risperidone in comparison to benzamide antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34:1495–1499.
- Carnahan RM et al. Increased risk of extrapyramidal side-effect treatment associated with atypical antipsychotic polytherapy. Acta Psychiatr Scand 2006; 113:135–141.
- Gomberg RF. Interaction between olanzapine and haloperidol. J Clin Psychopharmacol 1999; 19:272–273.
- Suzuki T et al. Effectiveness of antipsychotic polypharmacy for patients with treatment refractory schizophrenia: an open-label trial of olanzapine plus risperidone for those who failed to respond to a sequential treatment with olanzapine, quetiapine and risperidone. Hum Psychopharmacol 2008; 23:455–463.
- Hashimoto Y et al. Effects of antipsychotic polypharmacy on side-effects and concurrent use of medications in schizophrenic outpatients. Psychiatry Clin Neurosci 2012; 66:405–410.
- Sorensen HJ et al. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol 2013; 23:872–878.
- Dome P et al. Paralytic ileus associated with combined atypical antipsychotic therapy. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31:557–560.
- Hedges DW et al. New-onset seizure associated with quetiapine and olanzapine. Ann Pharmacother 2002; 36:437–439.
- Beelen AP et al. Asymptomatic QTc prolongation associated with quetiapine fumarate overdose in a patient being treated with risperidone. Hum Exp Toxicol 2001; 20:215–219.
- Hori H et al. Switching to antipsychotic monotherapy can improve attention and processing speed, and social activity in chronic schizophrenia patients. J Psychiatr Res 2013; 47:1843–1848.
- Waddington JL et al. Mortality in schizophrenia. Antipsychotic polypharmacy and absence of adjunctive anticholinergics over the course of a 10-year prospective study. Br J Psychiatry 1998; 173:325–329.
- Joukamaa M et al. Schizophrenia, neuroleptic medication and mortality. Br J Psychiatry 2006; 188:122–127.
- Baandrup L et al. Antipsychotic polypharmacy and risk of death from natural causes in patients with schizophrenia: a population-based nested case-control study. J Clin Psychiatry 2010; 71:103–108.
- Tiihonen J et al. Polypharmacy with antipsychotics, antidepressants, or benzodiazepines and mortality in schizophrenia. Arch Gen Psychiatry 2012; 69:476–483.
- Centorrino F et al. Multiple versus single antipsychotic agents for hospitalized psychiatric patients: case-control study of risks versus benefits. Am J Psychiatry 2004; 161:700–706.
- Taylor D et al. Co-prescribing of atypical and typical antipsychotics—prescribing sequence and documented outcome. Psychiatr Bull 2002; 26:170–172.
- Harrington M et al. The results of a multi-centre audit of the prescribing of antipsychotic drugs for in-patients in the UK. Psychiatr Bull 2002; 26:414–418.
- Procyshyn RM et al. Persistent antipsychotic polypharmacy and excessive dosing in the community psychiatric treatment setting: a review of medication profiles in 435 Canadian outpatients. J Clin Psychiatry 2010; 71:566–573.
- Gallego JA et al. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res 2012; 138:18–28.
- Paton C et al. High-dose and combination antipsychotic prescribing in acute adult wards in the UK: the challenges posed by p.r.n. prescribing. Br J Psychiatry 2008; 192:435–439.
- Taylor,D et al. A prescription survey of the use of atypical antipsychotics for hospital inpatients in the United Kingdom. Int J Psychiatry Clin Pract 2000; 4:41–46.
- Paton C et al. Patterns of antipsychotic and anticholinergic prescribing for hospital inpatients. J Psychopharmacol 2003; 17:223–229.
- De Hert M et al. Pharmacological treatment of hospitalised schizophrenic patients in Belgium. Int J Psychiatry Clin Pract 2006; 10:285–290.
- Novick D et al. Antipsychotic monotherapy and polypharmacy in the treatment of outpatients with schizophrenia in the European Schizophrenia Outpatient Health Outcomes Study. J Nerv Ment Dis 2012; 200:637–643.
- Baandrup L et al. Association of antipsychotic polypharmacy with health service cost: a register-based cost analysis. Eur J Health Econ 2012; 13:355–363.
- Patel MX et al. Quality of prescribing for schizophrenia: evidence from a national audit in England and Wales. Eur Neuropsychopharmacol 2014; 24:499–509.
- Kreyenbuhl J et al. Adding or switching antipsychotic medications in treatment-refractory schizophrenia. Psychiatr Serv 2007; 58:983–990.
- Nielsen J et al. Psychiatrists' attitude towards and knowledge of clozapine treatment. J Psychopharmacol 2010; 24:965–971.
- Ascher-Svanum H et al. Comparison of patients undergoing switching versus augmentation of antipsychotic medications during treatment for schizophrenia. Neuropsychiatr DisTreat 2012; 8:113–118.
- Goren JL et al. Antipsychotic prescribing pathways, polypharmacy, and clozapine use in treatment of schizophrenia. Psychiatr Serv 2013; 64:527–533.
- Howes OD et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry 2012; 201:481–485.
- Aggarwal NK et al. Prevalence of concomitant oral antipsychotic drug use among patients treated with long-acting, intramuscular, antipsychotic medications. J Clin Psychopharmacol 2012; 32:323–328.
- Shiloh R et al. Sulpiride augmentation in people with schizophrenia partially responsive to clozapine. A double-blind, placebo-controlled study. Br J Psychiatry 1997; 171:569–573.
- Josiassen RC et al. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry 2005; 162:130–136.
- Paton C et al. Augmentation with a second antipsychotic in patients with schizophrenia who partially respond to clozapine: a meta-analysis. J Clin Psychopharmacol 2007; 27:198–204.
- Barbui C et al. Does the addition of a second antipsychotic drug improve clozapine treatment? Schizophr Bull 2009; 35:458–468.
- Taylor DM et al. Augmentation of clozapine with a second antipsychotic - a meta-analysis of randomized, placebo-controlled studies. Acta Psychiatr Scand 2009; 119:419–425.
- Essock SM et al. Effectiveness of switching from antipsychotic polypharmacy to monotherapy. Am J Psychiatry 2011; 168:702–708.
Negative symptoms
The aetiology of negative symptoms is complex and it is important to determine the most likely cause in any individual case before embarking on a treatment regime. Negative symptoms can be either primary (transient or enduring) or secondary to positive symptoms (e.g. asociality secondary to paranoia), EPS (e.g. bradykinesia, lack of facial expression), depression (e.g. social withdrawal) or institutionalisation.1 Secondary negative symptoms are obviously best dealt with by treating the relevant cause (EPS, depression, etc.).
The EUFEST (European First Episode Schizophrenia Trial) study found that 7% of first episode patients had persistent negative symptoms and that these were associated with a longer DUP (duration of untreated psychosis) and a deleterious effect on global functioning.2 Negative symptoms are seen to a varying degree in up to three-quarters of people with established schizophrenia,3 with up to 20% having persistent primary negative symptoms.4 The findings of EUFEST related to global functioning have also been reported in those with established illness.5
The literature pertaining to the pharmacological treatment of negative symptoms largely consists of sub-analyses of acute efficacy studies, correlation analysis and path analyses.6 Few studies specifically recruit patients with persistent negative symptoms.
In general:
- The earlier a psychotic illness is effectively treated, the less likely is the development of negative symptoms over time.7,8 In first-episode patients, response of negative symptoms to antipsychotic treatment may be determined by 5HT1A genotype.9
- Older antipsychotics have a modest effect against primary negative symptoms10,11 but some can cause secondary negative symptoms (via EPS).
- Some SGAs have been shown to be statistically superior to FGAs in the treatment of negative symptoms,12 in the context of overall treatment response in non-selected populations.11,13 However the effect size is small12 and unlikely to be clinically meaningful. Interestingly, in a recent meta-analysis, quetiapine (which has one of the best EPS profiles14) was the only SGA to fare worse than haloperidol in head-to-head studies that addressed efficacy against negative symptoms.11
- Data support the effectiveness of amisulpride in primary negative symptoms,15,16 but not clear superiority over low dose haloperidol.17 There are many, mostly small RCTs in the literature reporting equivalent efficacy for different SGAs, e.g. quetiapine and olanzapine;18 ziprasidone and amisulpride,19 asenapine and olanzapine (note that the discontinuation rate in this study was considerably higher with asenapine than with olanzapine).20 A well-conducted study appeared to show superiority for olanzapine (only at 5 mg/day) over amisulpride.21 A further small study shows superiority of olanzapine over haloperidol but the magnitude of the effect was modest.22
- Low serum folate23 and glycine24 concentrations have been found in patients with predominantly negative symptoms. Supplementation with folate and oral B12 has been demonstrated to lead to modest improvements in those with specific functional variants of folate-related genes.25
With respect to non-antipsychotic pharmacological interventions, several drugs that modulate glutamate pathways have been directly tested; there are negative RCTs of glycine,26 d-serine,27 modafanil,28 and armodafanil29 augmentation of antipsychotics, minocycline30,31 and of LY2140023 monotherapy32 (an agonist at mGlu 2/3 receptors) and a small preliminary positive RCT of pregnenolone.33 With respect to decreasing glutamate transmission, there is a positive meta-analysis of lamotrigine augmentation of clozapine34 and a positive35 and negative36 RCT of memantine (the negative study being much the larger of the two).
With respect to antidepressant augmentation of an antipsychotic for negative symptoms, a Cochrane review concluded that this may be an effective strategy for reducing affective flattening, alogia and avolition,37 while a meta-analysis of selective serotonin reuptake inhibitor (SSRI) augmentation of an antipsychotic was less positive.38 A more recent meta-analysis that included available data for all antidepressants was cautiously optimistic39 but the authors noted that their findings were not definitive. A meta-analysis supports the efficacy of mirtazapine and mianserin (postulated to be related to their α2-adrenergic antagonist effects).25
Meta-analyses support the efficacy of augmentation of an antipsychotic with Ginkgo biloba40 and a COX-2 inhibitor (albeit with a small effect size)41 while small RCTs have demonstrated some benefit for selegiline,42,43 pramiprexole,44 testosterone (applied topically),45 ondansetron46 and granisetron.47 Data for repetitive transcranial magnetic stimulation are mixed.48–50 A large (n=250) RCT in adults51 and a smaller RCT in elderly patients52 each found no benefit for donepezil and there is a further negative RCT of galantamine.53
Patients who misuse psychoactive substances experience fewer negative symptoms than patients who do not.54 It is not clear if this cause or effect.
Recommendations
The following recommendations are derived from the British Association for Psychopharmacology (BAP) schizophrenia guideline.55
- Psychotic illness should be identified and treated as early as possible as this may offer some protection against the development of negative symptoms.
- For any given patient, the antipsychotic that gives the best balance between overall efficacy and adverse effects should be used.
Where negative symptoms persist beyond an acute episode of psychosis:
- Ensure EPS (specifically bradykinesia) and depression are detected and treated if present, and consider the contribution of the environment to negative symptoms (e.g. insitutionalisation, lack of stimulation).
- Consider augmentation of antipsychotic treatment with an antidepressant such as an SSRI, or mirtazapine ensuring that choice is based on minimising the potential for compounding side-effects through pharmacokinetic or pharmacodynamic drug interactions.
- If clozapine is prescribed, consider augmenting with lamotrigine or a suitable second antipsychotic.
- There are insufficient data to make recommendations about other pharmacological strategies, but pregnenolone, minocycline, selegiline, pramiprexole, Ginkgo biloba, testosterone and ondansetron may have potential.
References
- Carpenter WT. The treatment of negative symptoms: pharmacological and methodological issues. Br J Psychiatry 1996; 168:17–22.
- Galderisi S et al. Persistent negative symptoms in first episode patients with schizophrenia: results from the European First Episode Schizophrenia Trial. Eur Neuropsychopharmacol 2013; 23:196–204.
- Selten JP et al. Distress attributed to negative symptoms in schizophrenia. Schizophr Bull 2000; 26:737–744.
- Bobes J et al. Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: findings from the CLAMORS study. J Clin Psychiatry 2010; 71:280–286.
- Rabinowitz J et al. Negative symptoms have greater impact on functioning than positive symptoms in schizophrenia: analysis of CATIE data. Schizophr Res 2012; 137:147–150.
- Buckley PF et al. Pharmacological treatment of negative symptoms of schizophrenia: therapeutic opportunity or cul-de-sac? Acta Psychiatr Scand 2007; 115:93–100.
- Waddington JL et al. Sequential cross-sectional and 10-year prospective study of severe negative symptoms in relation to duration of initially untreated psychosis in chronic schizophrenia. Psychol Med 1995; 25:849–857.
- Melle I et al. Prevention of negative symptom psychopathologies in first-episode schizophrenia: two-year effects of reducing the duration of untreated psychosis. Arch Gen Psychiatry 2008; 65:634–640.
- Reynolds GP et al. Effect of 5-HT1A receptor gene polymorphism on negative and depressive symptom response to antipsychotic treatment of drug-naive psychotic patients. Am J Psychiatry 2006; 163:1826–1829.
- Levine SZ et al. Delayedand early-onset hypotheses of antipsychotic drug action in the negative symptoms of schizophrenia. Eur Neuropsychopharmacol 2012; 22:812–817.
- Darba J et al. Efficacy of second-generation-antipsychotics in the treatment of negative symptoms of schizophrenia: a meta-analysis of randomized clinical trials. Rev Psiquiatr Salud Ment 2011; 4:126–143.
- Leucht S et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 2009; 373:31–41.
- Erhart SM et al. Treatment of schizophrenia negative symptoms: future prospects. Schizophr Bull 2006; 32:234–237.
- Leucht S et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013; 382:951–962.
- Boyer P et al. Treatment of negative symptoms in schizophrenia with amisulpride. Br J Psychiatry 1995; 166:68–72.
- Danion JM et al. Improvement of schizophrenic patients with primary negative symptoms treated with amisulpride. Amisulpride Study Group. Am J Psychiatry 1999; 156:610–616.
- Speller JC et al. One-year, low-dose neuroleptic study of in-patients with chronic schizophrenia characterised by persistent negative symptoms. Amisulpride v. haloperidol. Br J Psychiatry 1997; 171:564–568.
- Stauffer VL et al. Responses to antipsychotic therapy among patients with schizophrenia or schizoaffective disorder and either predominant or prominent negative symptoms. Schizophr Res 2012; 134:195–201.
- Olie JP et al. Ziprasidone and amisulpride effectively treat negative symptoms of schizophrenia: results of a 12-week, double-blind study. Int Clin Psychopharmacol 2006; 21:143–151.
- Buchanan RW et al. Asenapine versus olanzapine in people with persistent negative symptoms of schizophrenia. J Clin Psychopharmacol 2012; 32:36–45.
- Lecrubier Y et al. The treatment of negative symptoms and deficit states of chronic schizophrenia: olanzapine compared to amisulpride and placebo in a 6-month double-blind controlled clinical trial. Acta Psychiatr Scand 2006; 114:319–327.
- Lindenmayer JP et al. A randomized controlled trial of olanzapine versus haloperidol in the treatment of primary negative symptoms and neurocognitive deficits in schizophrenia. J Clin Psychiatry 2007; 68:368–379.
- Goff DC et al. Folate, homocysteine, and negative symptoms in schizophrenia. Am J Psychiatry 2004; 161:1705–1708.
- Sumiyoshi T et al. Prediction of the ability of clozapine to treat negative symptoms from plasma glycine and serine levels in schizophrenia. Int J Neuropsychopharmacol 2005; 8:451–455.
- Hecht EM et al. Alpha-2 receptor antagonist add-on therapy in the treatment of schizophrenia; a meta-analysis. Schizophr Res 2012; 134:202–206.
- Buchanan RW et al. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry 2007; 164:1593–1602.
- Weiser M et al. A multicenter, add-on randomized controlled trial of low-dose d-serine for negative and cognitive symptoms of schizophrenia. J Clin Psychiatry 2012; 73:e728–e734.
- Pierre JM et al. A randomized, double-blind, placebo-controlled trial of modafinil for negative symptoms in schizophrenia. J Clin Psychiatry 2007; 68:705–710.
- Kane JM et al. Adjunctive armodafinil for negative symptoms in adults with schizophrenia: a double-blind, placebo-controlled study. Schizophr Res 2012; 135:116–122.
- Levkovitz Y et al. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry 2010; 71:138–149.
- Chaudhry IB et al. Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J Psychopharmacol 2012; 26:1185–1193.
- Patil ST et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med 2007; 13:1102–1107.
- Marx CE et al. Proof-of-concept trial with the neurosteroid pregnenolone targeting cognitive and negative symptoms in schizophrenia. Neuropsychopharmacology 2009; 34:1885–1903.
- Tiihonen J et al. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res 2009; 109:10–14.
- Rezaei F et al. Memantine add-on to risperidone for treatment of negative symptoms in patients with stable schizophrenia: randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol 2013; 33:336–342.
- Lieberman JA et al. A randomized, placebo-controlled study of memantine as adjunctive treatment in patients with schizophrenia. Neuropsychopharmacology 2009; 34:1322–1329.
- Rummel C et al. Antidepressants for the negative symptoms of schizophrenia. Cochrane Database Syst Rev 2006; 3:CD005581.
- Sepehry AA et al. Selective serotonin reuptake inhibitor (SSRI) add-on therapy for the negative symptoms of schizophrenia: a meta-analysis. J Clin Psychiatry 2007; 68:604–610.
- Singh SP et al. Efficacy of antidepressants in treating the negative symptoms of chronic schizophrenia: meta-analysis. Br J Psychiatry 2010; 197:174–179.
- Singh V et al. Review and meta-analysis of usage of ginkgo as an adjunct therapy in chronic schizophrenia. Int J Neuropsychopharmacol 2010; 13:257–271.
- Sommer IE et al. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry 2012; 73:414–419.
- Singh V et al. Review and meta-analysis of usage of ginkgo as an adjunct therapy in chronic schizophrenia. Int J Neuropsychopharmacol 2010; 13:257–271.
- Amiri A et al. Efficacy of selegiline add on therapy to risperidone in the treatment of the negative symptoms of schizophrenia: a double-blind randomized placebo-controlled study. Hum Psychopharmacol 2008; 23:79–86.
- Kelleher JP et al. Pilot randomized, controlled trial of pramipexole to augment antipsychotic treatment. Eur Neuropsychopharmacol 2012; 22:415–418.
- Ko YH et al. Short-term testosterone augmentation in male schizophrenics: a randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol 2008; 28:375–383.
- Zhang ZJ et al. Beneficial effects of ondansetron as an adjunct to haloperidol for chronic, treatment-resistant schizophrenia: a double-blind, randomized, placebo-controlled study. Schizophr Res 2006; 88:102–110.
- Khodaie-Ardakani MR et al. Granisetron as an add-on to risperidone for treatment of negative symptoms in patients with stable schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res 2013; 47:472–478.
- Novak T et al. The double-blind sham-controlled study of high-frequency rTMS (20Hz) for negative symptoms in schizophrenia: Negative results. Neuro Endocrinol Lett 2006; 27:209–213.
- Mogg A et al. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: a randomized controlled pilot study. Schizophr Res 2007; 93:221–228.
- Prikryl R et al. Treatment of negative symptoms of schizophrenia using repetitive transcranial magnetic stimulation in a double-blind, randomized controlled study. Schizophr Res 2007; 95:151–157.
- Keefe RSE et al. Efficacy and safety of donepezil in patients with schizophrenia or schizoaffective disorder: significant placebo/practice effects in a 12-week, randomized, double-blind, placebo-controlled trial. Neuropsychopharmacology 2007; 33:1217–1228.
- Mazeh D et al. Donepezil for negative signs in elderly patients with schizophrenia: an add-on, double-blind, crossover, placebo-controlled study. Int Psychogeriatr 2006; 18:429–436.
- Conley RR et al. The effects of galantamine on psychopathology in chronic stable schizophrenia. Clin Neuropharmacol 2009; 32:69–74.
- Potvin S et al. A meta-analysis of negative symptoms in dual diagnosis schizophrenia. Psychol Med 2006; 36:431–440.
- Barnes TR. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2011; 25:567–620.
Monitoring
Table 2.6 summarises suggested monitoring for those receiving antipsychotics. More detail and background is provided in specific sections in this chapter.
Table 2.6 Monitoring of metabolic parameters for patients receiving antipsychotic drugs
Parameter/
test |
Suggested frequency |
Action to be taken if results outside reference range |
Drugs with special precautions |
Drugs for which monitoring is not required |
Urea and electrolytes
(including creatinine or estimated GFR) |
Baseline and yearly as part of a routine physical health check |
Investigate all abnormalities detected |
Amisulpride and sulpiride renally excreted - consider reducing dose if GFR reduced |
None |
Full blood count
(FBC)1-6 |
Baseline and yearly as part of a routine physical health check and to detect chronic bone marrow suppression (small risk associated with some antipsychotics) |
Stop suspect drug if neutrophils fall below 1.5 x 109/L
Refer to specialist medical care if neutrophils below 0.5 x 109/L
Note high frequency of benign ethnic neutropenia in certain ethnic groups |
Clozapine - FBC weekly for 18 weeks, then fortnightly up to one year, then monthly (schedule varies from country to country) |
None |
Blood lipids7,8
(cholesterol; triglycerides)
Fasting sample, if possible |
Baseline, at 3 months then yearly to detect antipsychotic-induced changes, and generally monitor physical health |
Offer lifestyle advice
Consider changing antipsychotic and/or initiating statin therapy |
Clozapine, olanzapine, quetiapine, phenothiazines - 3 monthly for first year, then yearly |
Some antipsychotics (e.g. aripiprazole) not clearly associated with dyslipidaemia but prevalence is high in this patient group9-11 so all patients should be monitored |
Weight7,8,11
(include waist size and BMI, if possible) |
Baseline, frequently for 3 months then yearly to detect antipsychotic-induced changes, and generally monitor physical health |
Offer lifestyle advice
Consider changing antipsychotic and/or dietary/pharmacological intervention |
Clozapine, olanzapine - 3 monthly for first year, then yearly |
Aripiprazole, ziprasidone and lurasidone not clearly associated with weight gain but monitoring recommended nonetheless - obesity prevalence high in this patient group |
Plasma glucose
(fasting sample, if possible) |
Baseline, at 4-6 months, then yearly to detect antipsychotic-induced changes, and generally monitor physical health |
Offer lifestyle advice
Obtain fasting sample or non-fasting and HbA1C
Refer to GP or specialist |
Clozapine, olanzapine, chlorpromazine -test at baseline, one month, then 4-6 monthly |
Some antipsychotics not clearly associated with IFG but prevalence is high in this patient group12,13 so all patients should be monitored |
| ECG |
Baseline and after dose increases
(ECG changes rare in practice14) on admission to hospital and before discharge if drug regimen changed
If an antipsychotic associated with moderate-high risk of QTc prolongation is prescribed |
Discuss with/refer to cardiologist if abnormality detected |
Haloperidol, pimozide, sertindole - ECG mandatory
Ziprasidone - ECG mandatory in some situations |
Risk of sudden cardiac death increased with most antipsychotics15 Ideally, all patients should be offered an ECG at least yearly |
| Blood pressure |
Baseline; frequently during dose titration to detect antipsychotic-induced changes, and generally monitor physical health |
If severe hypotension or hypertension (clozapine) observed, slow rate of titration
Consider switching to another antipsychotic if symptomatic postural hypotension
Treat hypertension in line with NICE guidelines |
Clozapine, chlorpromazine and quetiapine most likely to be associated with postural hypotension |
Amisulpride, aripiprazole, lurasidone, trifluoperazine, sulpiride |
| Prolactin |
Baseline, then at 6 months, then yearly to detect antipsychotic-induced changes |
Switch drugs if hyperprolactinaemia confirmed and symptomatic
Consider tests of bone mineral density (e.g. DEXA scanning) for those with chronically raised prolactin. |
Amisulpride, risperidone and paliperidone particularly associated with hyperprolactinaemia |
Asenapine, aripiprazole, clozapine, lurasidone, quetiapine, olanzapine (< 20 mg), ziprasidone usually do not elevate prolactin, but worth measuring if symptoms arise |
| Liver function tests (LFTs)16-18 |
Baseline, then yearly as part of a routine physical health check and to detect chronic antipsychotic-induced changes (rare) |
Stop suspect drug if LFTs indicate hepatitis (transaminases x 3 normal) or functional damage (PT/albumin change) |
Clozapine and chlorpromazine associated with hepatic failure |
Amisulpride, sulpiride |
Creatine phosphokinase
(CPK) |
Baseline, then if neuroleptic malignant syndrome (NMS) suspected |
See section on 'Neuroleptic malignant syndrome' |
NMS more likely with first-generation antipsychotics |
None |
Other tests:
Patients on clozapine may benefit from an EEG19,20 as this may help determine the need for valproate (although interpretation is obviously complex). Those on quetiapine should have thyroid function tests yearly although the risk of abnormality is very small.21,22 |
|
BMI, body mass index; DEXA, dual-energy X-ray absorptiometry; ECG, electrocardiogram; EEG, electrocephalogram; GFR, glomerular filtration rate; IFG, impaired fasting glucose; PT, prothrombin time.
|
References
- Burckart GJ et al. Neutropenia following acute chlorpromazine ingestion. Clin Toxicol 1981; 18:797–801.
- Grohmann R et al. Agranulocytosis and significant leucopenia with neuroleptic drugs: results from the AMUP program. Psychopharmacology 1989; 99 Suppl:S109–S112.
- Esposito D et al. Risperidone-induced morning pseudoneutropenia. Am J Psychiatry 2005; 162:397.
- Montgomery J. Ziprasidone-related agranulocytosis following olanzapine-induced neutropenia. Gen Hosp Psychiatry 2006; 28:83–85.
- Cowan C et al. Leukopenia and neutropenia induced by quetiapine. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31:292–294.
- Buchman N et al. Olanzapine-induced leukopenia with human leukocyte antigen profiling. Int Clin Psychopharmacol 2001; 16:55–57.
- Marder SR et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry 2004; 161:1334–1349.
- Fenton WS et al. Medication-induced weight gain and dyslipidemia in patients with schizophrenia. Am J Psychiatry 2006; 163:1697–1704.
- Weissman EM et al. Lipid monitoring in patients with schizophrenia prescribed second-generation antipsychotics. J Clin Psychiatry 2006; 67:1323–1326.
- Cohn TA et al. Metabolic monitoring for patients treated with antipsychotic medications. Can J Psychiatry 2006; 51:492–501.
- Paton C et al. Obesity, dyslipidaemias and smoking in an inpatient population treated with antipsychotic drugs. Acta Psychiatr Scand 2004; 110:299–305.
- Taylor D et al. Undiagnosed impaired fasting glucose and diabetes mellitus amongst inpatients receiving antipsychotic drugs. J Psychopharmacol 2005; 19:182–186.
- Citrome L et al. Incidence, prevalence, and surveillance for diabetes in New York State psychiatric hospitals, 1997-2004. Psychiatr Serv 2006; 57:1132–1139.
- Novotny T et al. Monitoring of QT interval in patients treated with psychotropic drugs. Int J Cardiol 2007; 117:329–332.
- Ray WA et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009; 360:225–235.
- Hummer M et al. Hepatotoxicity of clozapine. J Clin Psychopharmacol 1997; 17:314–317.
- Erdogan A et al. Management of marked liver enzyme increase during clozapine treatment: a case report and review of the literature. Int J Psychiatry Med 2004; 34: 83–89.
- Regal RE et al. Phenothiazine-induced cholestatic jaundice. Clin Pharm 1987; 6:787–794.
- Centorrino F et al. EEG abnormalities during treatment with typical and atypical antipsychotics. Am J Psychiatry 2002; 159:109–115.
- Gross A et al. Clozapine-induced QEEG changes correlate with clinical response in schizophrenic patients: a prospective, longitudinal study. Pharmacopsychiatry 2004; 37:119–122.
- Twaites BR et al. The safety of quetiapine: results of a post-marketing surveillance study on 1728 patients in England. J Psychopharmacol 2007; 21:392–399.
- Kelly DL et al. Thyroid function in treatment-resistant schizophrenia patients treated with quetiapine, risperidone, or fluphenazine. J Clin Psychiatry 2005; 66:80–84.
Relative adverse effects—a rough guide
A rough guide to the relative adverse effects of antipsychotic drugs is shown in Table 2.7.
The table is made up of approximate estimates of relative incidence and/or severity, based on clinical experience, manufacturers' literature and published research. See individual sections for more precise information.
Other side-effects not mentioned in this table do occur. Please see dedicated sections on other side-effects included in this book for more information.
Table 2.7 Relative adverse effects of antipsychotic drugs
| Drug |
Sedation |
Weight gain |
Akathisia |
Parkin-
sonism |
Anti-
cholinergic |
Hypoten-
sion |
Prolactin elevation |
| Amisulpride |
– |
+ |
+ |
+ |
– |
– |
+++ |
| Aripiprazole |
– |
– |
+ |
– |
– |
– |
– |
| Asenapine |
+ |
+ |
+ |
– |
– |
– |
+ |
| Benperidol |
+ |
+ |
+ |
+++ |
+ |
+ |
+++ |
| Chlorpromazine |
+++ |
++ |
+ |
++ |
++ |
+++ |
+++ |
| Clozapine |
+++ |
+++ |
– |
– |
+++ |
+++ |
– |
| Flupentixol |
+ |
++ |
++ |
++ |
++ |
+ |
+++ |
| Fluphenazine |
+ |
+ |
++ |
+++ |
++ |
+ |
+++ |
| Haloperidol |
+ |
+ |
+++ |
+++ |
+ |
+ |
++ |
| Iloperidone |
– |
++ |
+ |
+ |
– |
+ |
– |
| Loxapine |
++ |
+ |
+ |
+++ |
+ |
++ |
+++ |
| Lurasidone |
+ |
– |
+ |
+ |
– |
– |
+ |
| Olanzapine |
++ |
+++ |
+ |
– |
+ |
+ |
+ |
| Paliperidone |
+ |
++ |
+ |
+ |
+ |
++ |
+++ |
| Perphenazine |
+ |
+ |
++ |
+++ |
+ |
+ |
+++ |
| Pimozide |
+ |
+ |
+ |
+ |
+ |
+ |
+++ |
| Pipothiazine |
++ |
++ |
+ |
++ |
++ |
++ |
+++ |
| Promazine |
+++ |
++ |
+ |
+ |
++ |
++ |
++ |
| Quetiapine |
++ |
++ |
– |
– |
+ |
++ |
– |
| Risperidone |
+ |
++ |
+ |
+ |
+ |
++ |
+++ |
| Sertindole |
– |
+ |
– |
– |
– |
+++ |
– |
| Sulpiride |
– |
+ |
+ |
+ |
– |
– |
+++ |
| Trifluoperazine |
+ |
+ |
+ |
+++ |
+ |
+ |
+++ |
| Ziprasidone |
+ |
– |
+ |
– |
– |
+ |
+ |
| Zuclopenthixol |
++ |
++ |
++ |
++ |
++ |
+ |
+++ |
| +++ high incidence/severity, ++ moderate, + low,—very low. |
Treatment algorithms for schizophrenia

Figure 2.1 Treatment of first-episode schizophrenia.

Figure 2.2 Treatment of relapse or acute exacerbation of schizophrenia (full adherence to medication confirmed).
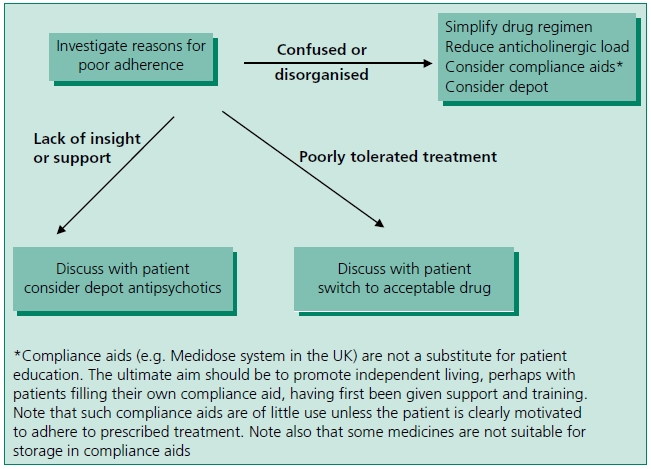
Figure 2.3 Treatment of relapse or acute exacerbation of schizophrenia (adherence doubtful or known to be poor).
Notes
- First-generation drugs may be slightly less efficacious than some SGAs.3,4 FGAs should probably be reserved for second-line use because of the possibility of poorer outcome compared with FGAs and the higher risk of movement disorder, particularly tardive dyskinesia.5,6
- Choice is, however, based largely on comparative adverse effect profile and relative toxicity. Patients seem able to make informed choices based on these factors7,8 although in practice they may only very rarely be involved in drug choice.9
- Where there is prior treatment failure (but not confirmed treatment refractoriness) olanzapine or risperidone may be better options than quetiapine.10 Olanzapine, because of the wealth of evidence suggesting slight superiority over other antipsychotics, should always be tried before clozapine unless contraindicated.11–14
- Where there is confirmed treatment resistance (failure to respond to at least two antipsychotics) evidence supporting the use of clozapine (and only clozapine) is overwhelming.15,16
References
- Agid O et al. The "delayed onset" of antipsychotic action—an idea whose time has come and gone. J Psychiatry Neurosci 2006; 31:93–100.
- Agid O et al. An algorithm-based approach to first-episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry 2011; 72:1439–1444.
- Davis JM et al. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry 2003; 60:553–564.
- Leucht S et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 2009; 373:31–41.
- Schooler N et al. Risperidone and haloperidol in first-episode psychosis: a long-term randomized trial. Am J Psychiatry 2005; 162:947–953.
- Oosthuizen PP et al. Incidence of tardive dyskinesia in first-episode psychosis patients treated with low-dose haloperidol. J Clin Psychiatry 2003; 64:1075–1080.
- Whiskey E et al. Evaluation of an antipsychotic information sheet for patients. Int J Psychiatry Clin Pract 2005; 9:264–270.
- Stroup TS et al. Results of phase 3 of the CATIE schizophrenia trial. Schizophr Res 2009; 107:1–12.
- Olofinjana B et al. Antipsychotic drugs - information and choice: a patient survey. Psychiatr Bull 2005; 29:369–371.
- Stroup TS et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006; 163:611–622.
- Haro JM et al. Remission and relapse in the outpatient care of schizophrenia: three-year results from the Schizophrenia Outpatient Health Outcomes study. J Clin Psychopharmacol 2006; 26:571–578.
- Novick D et al. Recovery in the outpatient setting: 36-month results from the Schizophrenia Outpatients Health Outcomes (SOHO) study. Schizophr Res 2009; 108:223–230.
- Tiihonen J et al. Effectiveness of antipsychotic treatments in a nationwide cohort of patients in community care after first hospitalisation due to schizophrenia and schizoaffective disorder: observational follow-up study. BMJ 2006; 333:224.
- Leucht S et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry 2009; 166:152–163.
- McEvoy JP et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry 2006; 163:600–610.
- Lewis SW et al. Randomized controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophr Bull 2006; 32:715–723.
First-generation antipsychotics—place in therapy
'Typical' and 'atypical' antipsychotics are not categorically differentiated. Typical (firstgeneration) drugs are those which can be expected to give rise to acute EPS, hyperprolactinaemia and, in the longer term, tardive dyskinesia. Atypicals (second-generation antipsychotics), by any sensible definition, might be expected not to be associated with these adverse effects. However, some atypicals show dose-related EPS, some induce hyperprolactinaemia (often to a greater extent than with FGAs) and all may eventually give rise to tardive dyskinesia. To complicate matters further, it has been suggested that the therapeutic and adverse effects of typical drugs can be separated by careful dosing1–thus making FGAs potentially 'atypical' (although there is much evidence to the contrary2–4).
Given these observations, it seems unwise and unhelpful to consider so-called typical and atypical drugs as distinct groups of drugs. The essential difference between the two groups is the size of the therapeutic index in relation to acute EPS; for instance haloperidol has an extremely narrow index (probably less than 0.5 mg/day); olanzapine a wide index (20–40 mg/day).
FGAs still play an important role in schizophrenia and offer a valid alternative to atypicals where atypicals are poorly tolerated or where typicals are preferred by patients themselves. Typicals may be less effective than some non-clozapine SGAs (amisulpride, olanzapine and risperidone may be more efficacious5,6). CATIE7 and CUtLASS,8 however, found few important differences between SGAs and FGAs (mainly sulpiride and perphenazine). The main drawbacks of FGAs are, of course, acute EPS, hyperprolactinaemia and tardive dyskinesia. Hyperprolactinaemia is probably unavoidable in practice and, even when not symptomatic, may grossly affect hypothalamic function.9 It is also associated with sexual dysfunction,10 but be aware that the autonomic effects of some atypicals may also cause sexual dysfunction.11 In addition, some SGAs (risperidone, paliperidone, amisulpride) increase prolactin more than FGAs.12
Tardive dyskinesia probably occurs much more frequently with FGAs than SGAs13–16 (notwithstanding difficulties in defining what is atypical), although there remains some uncertainty.16–18 Careful observation of patients and the prescribing of the lowest effective dose are essential to help reduce the risk of this serious adverse event.19,20 Even with these precautions, the risk of tardive dyskinesia with FGAs may be unacceptably high.21
A good example of the relative merits of SGAs and a carefully dosed FGA is the recent trial comparing paliperidone palmitate with haloperidol decanoate.22 Paliperidone produced more weight gain and prolactin change, but haloperidol was associated with significantly more akathisia and parkinsonism and numerically more tardive dyskinesia. Actual efficacy was identical.
References
- Oosthuizen P et al. Determining the optimal dose of haloperidol in first-episode psychosis. J Psychopharmacol 2001; 15:251–255.
- Zimbroff DL et al. Controlled, dose-response study of sertindole and haloperidol in the treatment of schizophrenia. Sertindole Study Group. Am J Psychiatry 1997; 154:782–791.
- Jeste DV et al. Incidence of tardive dyskinesia in early stages of low-dose treatment with typical neuroleptics in older patients. Am J Psychiatry 1999; 156:309–311.
- Meltzer HY et al. The effect of neuroleptics on serum prolactin in schizophrenic patients. Arch Gen Psychiatry 1976; 33:279–286.
- Davis JM et al. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry 2003; 60:553–564.
- Leucht S et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 2009; 373:31–41.
- Lieberman JA et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005; 353:1209–1223.
- Jones PB et al. Randomized controlled trial of the effect on Quality of Life of secondvs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry 2006; 63:1079–1087.
- Smith S et al. The effects of antipsychotic-induced hyperprolactinaemia on the hypothalamic-pituitary-gonadal axis. J Clin Psychopharmacol 2002; 22:109–114.
- Smith SM et al. Sexual dysfunction in patients taking conventional antipsychotic medication. Br J Psychiatry 2002; 181:49–55.
- Aizenberg D et al. Comparison of sexual dysfunction in male schizophrenic patients maintained on treatment with classical antipsychotics versus clozapine. J Clin Psychiatry 2001; 62:541–544.
- Leucht S et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013; 382:951–962.
- Tollefson GD et al. Blind, controlled, long-term study of the comparative incidence of treatment-emergent tardive dyskinesia with olanzapine or haloperidol. Am J Psychiatry 1997; 154:1248–1254.
- Beasley C et al. Randomised double-blind comparison of the incidence of tardive dyskinesia in patients with schizophrenia during long-term treatment with olanzapine or haloperidol. Br J Psychiatry 1999; 174:23–30.
- Correll CU et al. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry 2004; 161:414–425.
- Novick D et al. Tolerability of outpatient antipsychotic treatment: 36-month results from the European Schizophrenia Outpatient Health Outcomes (SOHO) study. Eur Neuropsychopharmacol 2009; 19:542–550.
- Halliday J et al. Nithsdale Schizophrenia Surveys 23: movement disorders. 20-year review. Br J Psychiatry 2002; 181:422–427.
- Miller DD et al. Extrapyramidal side-effects of antipsychotics in a randomised trial. Br J Psychiatry 2008; 193:279–288.
- Jeste DV et al. Tardive dyskinesia. Schizophr Bull 1993; 19:303–315.
- Cavallaro R et al. Recognition, avoidance, and management of antipsychotic-induced tardive dyskinesia. CNS Drugs 1995; 4:278–293.
- Oosthuizen P et al. A randomized, controlled comparison of the efficacy and tolerability of low and high doses of haloperidol in the treatment of first-episode psychosis. Int J Neuropsychopharmacol 2004; 7:125–131.
- McEvoy JP et al. Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: a randomized clinical trial. JAMA 2014; 311:1978–1987.
Omega-3 fatty acid (fish oils) in schizophrenia
Fish oils contain the omega-3 fatty acids, eicosapentanoic acid (EPA) and docosahexanoic acid (DHA)—also known as polyunsaturated fatty acids or PUFAs. These compounds are thought to be involved in maintaining neuronal membrane structure, in the modulation of membrane proteins and in the production of prostaglandins and leukotrienes.1 High intake of PUFAs seems to protect against psychosis2 and antipsychotic treatment may normalise PUFA deficits.3 Animal models suggest a protective effect for PUFAs.4 They have been suggested as treatments for a variety of psychiatric illnesses5,6 but most research relates to their use in schizophrenia, where case reports,7–9 case series10 and prospective trials appear to suggest useful efficacy.11–15
A meta-analysis of these RCTs16 concluded that EPA has 'no beneficial effect in established schizophrenia', although the estimate of effect size (0.242) approached statistical significance. Since then, a further RCT of 97 subjects in acute psychosis showed no advantage for EPA 2 g daily17 and a relapse prevention study of EPA 2 g+ DHA 1 g a day failed to demonstrate any value for PUFAs over placebo (relapse rate was 90% with PUFAs, 75% with placebo).18
On balance, evidence suggests that EPA (2–3 g daily) is unlikely to be a worthwhile option in schizophrenia when added to standard treatment. Set against doubts over efficacy are the observations that fish oils are relatively cheap, well tolerated (mild gastrointestinal symptoms may occur) and benefit physical health.1,19–22 In addition, a study of 700 mg EPA + 480 mg DHA in adolescents and young adults at high risk of psychosis showed that such treatment greatly reduced emergence of psychotic symptoms compared with placebo23 (although a recent review described this study as 'very low quality evidence'24).
PUFAs are no longer recommended for the treatment of residual symptoms of schizophrenia. If used, careful assessment of response is important and fish oils should be withdrawn if no effect is observed after 3 months' treatment, unless required for their beneficial metabolic effects. In younger people at risk of psychosis there seems to be no reason not to give PUFAs although supporting evidence remains weak.
Recommendations
- Patients at high risk of first-episode psychosis
- Suggest EPA 700 mg/day (2 × Omacor or 6 × Maxepa capsules)
- Residual symptoms of multi-episode schizophrenia (added to antipsychotic)
- Not recommended. If used, suggest dose of EPA 2 g/day (5 × Omacor or 10 × Maxepa capsules)
References
- Fenton WS et al. Essential fatty acids, lipid membrane abnormalities, and the diagnosis and treatment of schizophrenia. Biol Psychiatry 2000; 47:8–21.
- Hedelin M et al. Dietary intake of fish, omega-3, omega-6 polyunsaturated fatty acids and vitamin D and the prevalence of psychotic-like symptoms in a cohort of 33,000 women from the general population. BMC Psych 2010; 10:38.
- Sethom MM et al. Polyunsaturated fatty acids deficits are associated with psychotic state and negative symptoms in patients with schizophrenia. Prostaglandins Leukot Essent Fatty Acids 2010; 83:131–136.
- Zugno AI et al. Omega-3 prevents behavior response and brain oxidative damage in the ketamine model of schizophrenia. Neuroscience 2014; 259:223–231.
- Freeman MP. Omega-3 fatty acids in psychiatry: a review. Ann Clin Psychiatry 2000; 12:159–165.
- Ross BM et al. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis 2007; 6:21–.
- Richardson AJ et al. Red cell and plasma fatty acid changes accompanying symptom remission in a patient with schizophrenia treated with eicosapentaenoic acid. Eur Neuropsychopharmacol 2000; 10:189–193.
- Puri BK et al. Eicosapentaenoic acid treatment in schizophrenia associated with symptom remission, normalisation of blood fatty acids, reduced neuronal membrane phospholipid turnover and structural brain changes. Int J Clin Pract 2000; 54:57–63.
- Su KP et al. Omega-3 fatty acids as a psychotherapeutic agent for a pregnant schizophrenic patient. Eur Neuropsychopharmacol 2001; 11:295–299.
- Sivrioglu EY et al. The impact of omega-3 fatty acids, vitamins E and C supplementation on treatment outcome and side effects in schizophrenia patients treated with haloperidol: an open-label pilot study. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31:1493–1499.
- Mellor JE et al. Schizophrenic symptoms and dietary intake of n-3 fatty acids. Schizophr Res 1995; 18:85–86.
- Peet M et al. Two double-blind placebo-controlled pilot studies of eicosapentaenoic acid in the treatment of schizophrenia. Schizophr Res 2001; 49:243–251.
- Fenton WS et al. A placebo-controlled trial of omega-3 fatty acid (ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. Am J Psychiatry 2001; 158:2071–2074.
- Emsley R et al. Randomized, placebo-controlled study of ethyl-eicosapentaenoic acid as supplemental treatment in schizophrenia. Am J Psychiatry 2002; 159:1596–1598.
- Berger GE et al. Ethyl-eicosapentaenoic acid in first-episode psychosis: a randomized, placebo-controlled trial. J Clin Psychiatry 2007; 68:1867–1875.
- Fusar-Poli P et al. Eicosapentaenoic acid interventions in schizophrenia: meta-analysis of randomized, placebo-controlled studies. J Clin Psychopharmacol 2012; 32:179–185.
- Bentsen H et al. A randomized placebo-controlled trial of an omega-3 fatty acid and vitamins E+ C in schizophrenia. Transl Psychiatry 2013; 3:e335.
- Emsley R et al. A randomized, controlled trial of omega-3 fatty acids plus an antioxidant for relapse prevention after antipsychotic discontinuation in first-episode schizophrenia. Schizophr Res 2014.
- Scorza FA et al. Omega-3 fatty acids and sudden cardiac death in schizophrenia: if not a friend, at least a great colleague. Schizophr Res 2007; 94:375–376.
- Caniato RN et al. Effect of omega-3 fatty acids on the lipid profile of patients taking clozapine. Aust N Z J Psychiatry 2006; 40:691–697.
- Emsley R et al. Safety of the omega-3 fatty acid, eicosapentaenoic acid (EPA) in psychiatric patients: results from a randomized, placebocontrolled trial. Psychiatry Res 2008; 161:284–291.
- Das UN. Essential fatty acids and their metabolites could function as endogenous HMG-CoA reductase and ACE enzyme inhibitors, anti-arrhythmic, anti-hypertensive, anti-atherosclerotic, anti-inflammatory, cytoprotective, and cardioprotective molecules. Lipids Health Dis 2008; 7:37.
- Amminger GP et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry 2010; 67:146–154.
- Stafford MR et al. Early interventions to prevent psychosis: systematic review and meta-analysis. BMJ 2013; 346:f185.
New and developing drugs to treat schizophrenia
Since the introduction of the 'atypical' antipsychotics nearly two decades ago, there have been no major new developments in the drug treatment of schizophrenia. The industry is following three different approaches:
- further refinement of dopamine-based antipsychotics with the aim of reducing side-effect burden
- development of non-dopamine antipsychotics
- development of add-on treatments that target specific aspects of schizophrenia (e.g. negative symptoms, cognitive symptoms, refractory symptoms, etc).
Further refined dopamine-related antipsychotics
Cariprazine
Cariprazine is a D3-prefering D3/D2 partial agonist with limited activity at other receptor types.1,2 The parent drug has a plasma half-life of several days and is metabolised by CYP3A4 to active compounds with even longer plasma half-lives.3 Published data are very limited, but doses of 3–9 mg/day seem to be effective in schizophrenia and bipolar mania with minimal effects on metabolic parameters, prolactin or the ECG.3 Currently (as of late 2014), cariprazine is being evaluated for regulatory approval and is not as yet available for routine clinical use.
Brexpiprazole
Brexpiprazole4 is a D2 partial agonist and 5HT2A antagonist which also inhibits serotonin re-uptake and is chemically related to aripiprazole. It has been investigated as a treatment for attention deficit hyperactivity disorder (ADHD), adjunct in refractory unipolar depression and schizophrenia. Its main adverse effects are akathisia, weight gain and nasopharyngitis (all with placebo-corrected incidences of < 5%). It appears to have no effect on the cardiac QT interval. Currently (as of late 2014), brexipiprazole is being evaluated for regulatory approval and is not as yet available for routine clinical use.
ITI-007
ITI-007 is a new compound being investigated by the drug company Intracellular Therapies—and while the drug was chosen because of its effects on intracellular signalling—it seems to achieve this effect via actions on dopamine and serotonin receptors. The compound has been evaluated in Phase I and Phase II trials in patients with schizophrenia and has shown promise in the treatment of positive and negative symptoms with limited side-effects.5 The compound will need to be evaluated in Phase III trials to confirm efficacy and differential value. Those studies are currently being planned.
Inhaled loxapine
This represents a new form of delivery of antipsychotics for acute dosing. It uses the conventional antipsychotic loxapine, in a powder form and delivers it via a breath-actuated single-use inhaler at doses of 5 mg and 10 mg of loxapine equivalent. Pharmacokinetically, this delivers a peak concentration within 2–3 minutes and a clinically discernible effect within 10 minutes. In addition to side-effects related to loxapine, the delivery method leads to dysgeusia (distorted taste sense or bad taste) in a small number, and bronchospasm rarely. The method does require active patient participation, thus limiting use in acutely agitated situations. The drug is approved for use in the US and Europe but not widely marketed.
Non-dopamine approaches to antipsychotic effect
Phosphodiesterase 10A inhibitors
All antipsychotics block dopamine receptors. One effect of dopamine blockade is an alteration in intracellular cyclic adenosine monophosphate (AMP). Phosphodiesterase (PDE) enzyme systems regulate intracellular cyclic AMP, and since PDE 10A is expressed mainly in cells bearing dopamine receptors, it is possible to bypass the D2 receptors and achieve the same effect (in cells) via PDE 10A inhibitors.6 A number of companies are exploring this strategy to treat schizophrenia. Pfizer has already evaluated PF-02545920 in Phase II trials, without success. Other companies (Amgen [AMG579], Lundbeck [AF111167], Omeros [OMS643762] Roche, Takeda, Forum, ICT) are developing different compounds for the same target.7 None of these agents is available for clinical use as yet.
Add-on treatments for schizophrenia
Bitopertin and others—for negative symptoms
Bitopertin is a glycine re-uptake inhibitor which modulates glutamate and dopamine in the brain.8 Similar agents Org 25935 (Organon) and AMG 747 (Amgen) were also being developed for the treatment for negative symptoms as an adjunct to regulate atypical antipsychotic treatment. Bitopertin is generally well tolerated and has minimal effects on the QT interval.9 By inhibiting the glycine type 1 (GlyT1) transporter, bitopertin also inhibits haemoglobin synthesis and most patients show a dose-related reduction in plasma Hb, although reductions of > 10% are uncommon.10 While the Phase II trials showed evidence of an antipsychotic effect,11 much larger Phase III trials have failed to replicate this effect.12 Bitopertin is not currently available for clinical trials and it is unclear if any of these agents are being developed further.
Bitopertin and others—for refractory symptoms
Bitopertin is also being investigated as an add-on agent for patients for whom the conventional (dopamine-blocking) antipsychotics do not provide a sufficient clinical response on positive symptoms. Phase III studies have been initiated based on mechanistic reasoning, though the early results have not been encouraging.
Cholinergic approaches for cognitive symptoms
It is widely recognised that despite good control of psychosis, most patients suffer with cognitive symptoms which limit their functional potential. A number of pathways have been implicated but there is considerable interest in enhancing cholinergic transmission, especially via the nicotinic alpha-7 receptor.13 Targacept's TC 5619 and Forum's EVP-6124, both of which are partial agonists at this receptor have been actively investigated at scale. After initially encouraging results14 which were not replicated in subsequent larger trials, TC 5619 is not being further developed. After a positive Phase II study in which EVP-6124 was superior to placebo in both cognitive test improvement and functional symptoms improvement it is being evaluated for further Phase III trials. These agents are not available for clinical use.
References
- Kiss B et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther 2010; 333:328–340.
- Seneca N et al. Occupancy of dopamine D(2) and D(3) and serotonin 5-HT(1)A receptors by the novel antipsychotic drug candidate, cariprazine (RGH-188), in monkey brain measured using positron emission tomography. Psychopharmacology (Berl) 2011; 218:579–587.
- Citrome L. Cariprazine: chemistry, pharmacodynamics, pharmacokinetics, and metabolism, clinical efficacy, safety, and tolerability. Expert Opin Drug Metab Toxicol 2013; 9:193–206.
- Citrome L. A review of the pharmacology, efficacy and tolerability of recently approved and upcoming oral antipsychotics: an evidence-based medicine approach. CNS Drugs 2013; 27:879–911.
- Intra-Cellular Therapies. Intra-Cellular Therapies Announces Positive Topline Phase II Clinical Results of ITI-007 for the Treatment of Schizophrenia. http://www.intracellulartherapies.com/press-room/press-releases/10-press-releases/38-dec-9-2013.html, 2013.
- Siuciak JA. The role of phosphodiesterases in schizophrenia : therapeutic implications. CNS Drugs 2008; 22:983–993.
- Schizophrenia Research Forum. Drugs in Trials. http://www.schizophreniaforum.org/res/drc/drug_tables.asp, 2014.
- Alberati D et al. Glycine reuptake inhibitor RG1678: a pharmacologic characterization of an investigational agent for the treatment of schizophrenia. Neuropharmacology 2012; 62:1152–1161.
- Hofmann, C et al. Evaluation of the effects of bitopertin (RG1678) on cardiac repolarization: a thorough corrected QT study in healthy male volunteers. Clin Ther 2012; 34:2061–2071.
- Stark FS et al. Semi-physiologic Population PKPD Model Characterizing the Effect of Bitopertin (RG1678) Glycine Reuptake Inhibitor on Hemoglobin Turnover in Humans. PAGE 21 (2012) Abstr 2553. www.page-meeting.org/?abstract=2553: http://www.page-meeting. org/?abstract=2553, 2013.
- Umbricht D et al. Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: a randomized, double-blind, proof-of-concept study. JAMA Psychiatry 2014; 71:637–646.
- Goff DC. Bitopertin: the good news and bad news. JAMA Psychiatry 2014; 71:621–622.
- Martin LF et al. Schizophrenia and the alpha7 nicotinic acetylcholine receptor. Int Rev Neurobiol 2007; 78:225–246.
- Lieberman, JA et al. A Randomized Exploratory Trial of an Alpha-7 Nicotinic Receptor Agonist (TC-5619) for Cognitive Enhancement in Schizophrenia. Neuropsychopharmacology 2013; 38:968–975.
NICE guidelines for the treatment of schizophrenia1
The 2009 NICE guidelines2 differed importantly from previous guidelines. There was no longer an imperative to prescribe an 'atypical' as first line treatment and it was recommended only that clozapine be 'offered' (rather than prescribed) after the prior failure of two antipsychotics. These semantic differences pointed respectively towards a disillusionment with SGAs and a recognition of the delay in prescribing clozapine in practice. Much emphasis was placed on involving patients and their carers in prescribing decisions. There is some evidence that this is rarely done3 but that it can be done.4 New NICE guidelines appeared in February 2014. Few changes were made to recommendations regarding drug treatment but psychological treatments are now more strongly promoted (perhaps reflecting the make-up of the NICE review panel).
NICE guidelines—a summary
- For people with newly diagnosed schizophrenia, offer oral antipsychotic medication. Provide information and discuss the benefits and side-effect profile of each drug with the service user. The choice of drug should be made by the service user and healthcare professional together, considering:
- the relative potential of individual antipsychotic drugs to cause extrapyramidal side-effects (including akathisia), cardiovascular side-effects, metabolic side-effects (including weight gain), hormonal side-effects and other side-effects (including unpleasant subjective experiences)
- the views of the carer where the service user agrees.
- Before starting antipsychotic medication, offer the person with schizophrenia an electrocardiogram (ECG) if:
- specified in the summary of product characteristics (SPC)
- a physical examination has identified specific cardiovascular risk (such as diagnosis of high blood pressure)
- there is personal history of cardiovascular disease, or
- the service user is being admitted as an inpatient.
- Treatment with antipsychotic medication should be considered an explicit individual therapeutic trial. Include the following:
- record the indications and expected benefits and risks of oral antipsychotic medication, and the expected time for a change in symptoms and appearance of side-effects
- at the start of treatment give a dose at the lower end of the licensed range and slowly titrate upwards within the dose range given in the British National Formulary (BNF) or SPC
- justify and record reasons for dosages outside the range given in the BNF or SPC.
- Monitor and record the following regularly and systematically throughout treatment, but especially during titration:
- efficacy, including changes in symptoms and behaviour
- side-effects of treatment, taking into account overlap between certain side-effects and clinical features of schizophrenia, for example the overlap between akathisia and agitation or anxiety
- adherence
- physical health
- nutritional status, diet and physical activity
- record the rationale for continuing, changing or stopping medication, and the effects of such changes
- carry out a trial of the medication at optimum dosage for 4–6 weeks (although half of this period is probably sufficient if no effect at all is seen).
- Physical monitoring is to be the responsibility of the secondary care team for one year or until the patient is stable.
- Do not use a loading dose of antipsychotic medication (often referred to as 'rapid neuroleptisation'). (Note that this does not apply to loading doses of depot forms of olanzapine and paliperidone.)
- Do not routinely initiate regular combined antipsychotic medication, except for short periods (for example, when changing medication).
- If prescribing chlorpromazine, warn of its potential to cause skin photosensitivity. Advise using sunscreen if necessary.
- Consider offering depot/long-acting injectable antipsychotic medication to people with schizophrenia:
- who would prefer such treatment after an acute episode
- where avoiding covert non-adherence (either intentional or unintentional) to antipsychotic medication is a clinical priority within the treatment plan.
- Offer clozapine to people with schizophrenia whose illness has not responded adequately to treatment despite the sequential use of adequate doses of at least two different antipsychotic drugs alongside psychological therapies. At least one of the drugs should be a non-clozapine second-generation antipsychotic. (see Figure 2.2—we recommend that one of the drugs should be olanzapine).
- For people with schizophrenia whose illness has not responded adequately to clozapine at an optimised dose, healthcare professionals should establish prior compliance with optimised antipsychotic treatment (including measuring drug levels) and engagement with psychological treatment before adding a second antipsychotic to augment treatment with clozapine. An adequate trial of such an augmentation may need to be up to 8–10 weeks (some data suggest 6 weeks may be enough5). Choose a drug that does not compound the common side-effects of clozapine.
References
- National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: treatment and management. Clinical Guideline 178, 2014. http://www.nice.org.uk/Guidance/CG178
- National Institute for Health and Clinical Excellence. Schizophrenia: core interventions in the treatment and management of schizophrenia in adults in primary and secondary care (update). Clinical Guideline 82, 2009. http://www.nice.org.uk/
- Olofinjana B et al. Antipsychotic drugs - information and choice: a patient survey. Psychiatr Bull 2005; 29:369–371.
- Whiskey E et al. Evaluation of an antipsychotic information sheet for patients. Int J Psychiatry Clin Pract 2005; 9:264–270.
- Taylor D et al. Augmentation of clozapine with a second antipsychotic. A meta analysis. Acta Psychiatr Scand 2012; 125:15–24.
Antipsychotic response—to increase the dose, to switch, to add or just wait—what is the right move?
For any clinician taking active care of patients with schizophrenia the single most common clinical dilemma is what to do when the current antipsychotic is not optimal for the patient. This may be for two broad reasons: firstly when the symptoms are well controlled but side-effects are problematic and, secondly, where there is inadequate response. Fortunately, given the diversity of antipsychotics available, it is usually possible to find an antipsychotic that has a side-effect profile that is acceptable to the patient. The more difficult question is when there is inadequate symptom response. If the patient has already had 'adequate' trials of two antipsychotics for 'sufficient' duration then clozapine should clearly be considered. However, the majority of the patients in the clinic are those who are either as yet not ready for clozapine or unwilling to choose that option. In those instances the clinician has four main choices: to increase the dose of the current medication; to switch to another antipsychotic; to add an adjunct medication or just to wait.
When to increase the dose?
While optimal doses of typical antipsychotics were always a matter of debate, the recommended doses of the newer atypical antipsychotics were generally based on careful and extensive clinical trials but even then the consensus on optimal doses has changed with time. For example, when risperidone was first launched it was suggested that optimal titration was from 2 mg to 4 mg to 6 mg or more for all patients, however, practising psychiatrists have tended towards lower doses.1 On the other hand, when quetiapine was introduced, 300 mg was considered the optimal dose and the overall consensus now is towards higher doses,2 although the evidence does not support this shift.2,3 Nonetheless, most clinicians feel comfortable in navigating within the recommended clinical doses. The more critical question is what one should do if one has hit the upper limit of these dose ranges and the patient is tolerating the medication well with limited efficacy benefit.
Dose–response observations
Davis and Chen performed a systematic meta-analysis of the data available up to 2004 and concluded that the average dose that produces maximal benefit was 4 mg for risperidone, 16 mg of olanzapine, 120 mg of ziprasidone and 10–15 mg of aripiprazole (they could not determine such a dose for quetiapine using their method).4 More recent trials have tried to compare 'high-dose' versus the standard dose. Kinon et al5 studied the dose–response relationship of standard and higher doses of olanzapine in a randomized, double-blind, 8-week, fixed-dose study comparing olanzapine 10 mg, 20 mg and 40 mg and found no benefit of the higher doses (i.e. 40 mg was no better than even 10 mg) and found clear evidence for increasing side-effects (weight gain and prolactin) with dose. Similarly, the initial licensing studies of risperidone had compared the usual doses, 2–6 mg/day, to the higher doses, 8–16 mg/day, and had chosen the lower dose ranges as they found no additional benefit at higher doses, but, a clear signal for greater side-effects (extrapyramidal side-effects and prolactin). These more recent studies are in accord with older studies involving fixed-doses of haloperidol.6 However, it is important to keep in mind that these doses are extracted from group evidence where patients are assigned to different doses, which is a different question from the clinical one where one considers increasing a dose only in those who have failed an initial dose. To our knowledge only one study has systematically addressed this question in its clinically relevant dimension. Kinon et al.7 examined patients who failed to respond to the (then) standard dose of fluphenazine (20 mg) and tested three strategies: increasing dose to 80 mg, switching to haloperidol or watchful waiting (on the original dose). All three strategies were equivalent in terms of efficacy. Thus, it seems that at a group level (as opposed to an individual level) there is little evidence to support treatment beyond the recommended doses. This evidence from structured trials is corroborated by the practice norms—Hermes and Rosenheck examined the CATIE data to identify clinical factors that predicted the physician's decision to increase the dose and found that decisions for dose change (within the therapeutic ranges) were only weakly associated with clinical measures.8
Plasma level variations
However, group level evidence cannot completely determine individual decisions. There is significant inter-individual variation in plasma levels in patients treated with antipsychotics. One can often encounter a patient who, when at the higher end of the dose range (say 6 mg of risperidone or 20 mg of olanzapine) would have plasma levels that are well below the range expected for 2 mg risperidone or 10 mg of olanzapine respectively. In such patients, one can make a rational case for increasing the dose, provided the patient is informed and the side-effects are tolerable, to bring the plasma levels to the median optimal range for the particular medication. More details on plasma levels and their interpretation are provided in Chapter 1. However, one often encounters an unresponsive compliant patient, whose dose has reached the ceiling and plasma levels are also sufficient—what next?
Treatment choices
There are essentially three options here, clozapine, switch to another drug or add another (non-clozapine) drug. If the patient meets the criteria for clozapine it is undoubtedly the preferred option. Yet, in the most recent audit of community (not inpatient) practice in the UK, covering some 5000 patients in 60 different NHS Trusts, it was shown that nearly 40% of the patients who met criteria for treatment resistance did not receive clozapine; and of those who did the vast majority (85%) received their clozapine after a much longer wait after the failure of two antipsychotics that is advised in most guidelines.9
Nonetheless, there is a set of patients who do not like the idea of regular blood testing, the side-effect profile and the regular appointments required to receive clozapine. In these patients the choice is to switch to another medication or to add another antipsychotic. The data on switching are sparse. While almost every clinical trial in patients with chronic schizophrenia has entailed the patient switching from one antipsychotic to another - there are no rigorous studies of preferred switch combinations (e.g. if risperidone fails—what next? Olanzapine, quetiapine, aripiprazole or ziprasidone?). If one looks at only the switching trials which have been sponsored by the drug companies—it leads to a rather confusing picture, with the trials results being very closely linked to the sponsors' interest (see 'Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine: an exploratory analysis of head-to-head comparison studies of second-generation antipsychotics'.10)
CATIE, a major US-based publicly funded comparative trial, examined patients who had failed their first atypical antipsychotic and were then randomly assigned to a different second one.11 Patients who were switched to olanzapine and risperidone did better than those switched to quetiapine and ziprasidone. This greater effectiveness is supported by a recent meta-analysis that compared a number of atypicals to first-generation typical antipsychotics and concluded that, other than clozapine, only amisulpride, risperidone and olanzapine were superior to first-generation agents in efficacy;12 and a metaanalysis comparing atypicals amongst themselves which suggests that olanzapine and risperidone (in that order) may be more effective than others.13 This suggests that if a patient has not tried olanzapine or risperidone as yet, it would be a reasonable decision to switch to these drugs provided the side-effect balance is favourable. Between these two drugs, the data is somewhat limited. However, a number of controlled, but open label studies do show an asymmetrical advantage (i.e. switching to olanzapine being more effective, than risperidone)—providing some direction, albeit incomplete.14,15
What to choose for someone who fails olanzapine and risperidone (other than clozapine) is not yet clear. Should one switch (to, say, aripiprazole or ziprasidone or even an older typical agent) or should one add another antipsychotic. It should be borne in mind that after 'switching', adding another antipsychotic is probably the second most common clinical move as 39–43% of patients in routine care are on more than one antipsychotic.16 Often a second antipsychotic is added to get an additional profile (e.g. sedation with quetiapine, or decrease prolactin with the addition of aripiprazole)—these matters are discussed elsewhere. We concern ourselves solely with the addition of an antipsychotic to another antipsychotic to increase efficacy. From a theoretical point of view since all antipsychotics block D2 receptors (unlike antihypertensives, which use different mechanisms) there is limited rationale for addition. Studies of add-ons have often chosen combinations of convenience or based on clinical lore and perhaps the most systematic evidence is available for the addition of antipsychotics to clozapine17–perhaps supported by the rationale that since clozapine has low D2 occupancy, increasing its D2 occupancy may yield additional benefits.18 A meta-analysis of all systematic antipsychotic add-on studies seem to suggest a modest benefit at best—the benefit being more likely when the patient is on clozapine, when a first-generation antipsychotic is added, and when both antipsychotics are used at effective doses.19
However, a move to polypharmacy should not be seen as a one-way street. While there is some evidence for augmentation with another antipsychotic, in general this ought to be avoided. Nonetheless, under some conditions of acute exacerbations or agitation the physician may find this to be the only practicable solution. Or quite often the physician may inherit the care of a patient on antipsychotic polypharmacy. Can this be safely reversed? Essock et al20 provide evidence from a large trial (127 patients who were stable on antipsychotic polypharmacy). They examined the efficacy of returning these patients to their one major antipsychotic. Over a 12-month period this strategy was successful in about two-thirds of the patients. In the cases where the move to monotherapy resulted in a return of symptoms, the most common recourse was to go back to the original polypharmacy, and this was achieved without any significant worsening in this group. As an advantage the monotherapy group was exposed to less medication, had equivalent symptoms and even lost some weight.
When to just 'stay'? A review of the above evidence suggests that no one strategy—increasing the dose, switching or augmenting—is a clear winner in all situations. Increase the dose if plasma levels are low; switch if the patient has not tried olanzapine or risperidone; and if failing on clozapine—augmentation may help. Given the limited efficacy of these manoeuvres perhaps an equally important call by the treating doctor is when to just 'stay' with the current pharmacotherapy and focus on nonpharmacological means: engagement in case-management, targeted psychological treatments and vocational rehabilitation as means of enhancing patient well-being. While it may seem a passive option—staying may often do less harm that aimless switching.
Summary—when treatment fails
- If dose has been optimised, consider watchful waiting.
- Consider increasing antipsychotic dose according to tolerability and plasma levels.
- If this fails, consider switching to olanzapine or risperidone (if not already used).
- If this fails, use clozapine (supporting evidence very strong).
- If clozapine fails, use time-limited augmentation strategies (supporting evidence variable).
References
- Ezewuzie N et al. Establishing a dose-response relationship for oral risperidone in relapsed schizophrenia. J Psychopharmacol 2006; 20:86–90.
- Sparshatt A et al. Quetiapine: dose-response relationship in schizophrenia. CNS Drugs 2008; 22:49–68.
- Honer WG et al. A randomized, double-blind, placebo-controlled study of the safety and tolerability of high-dose quetiapine in patients with persistent symptoms of schizophrenia or schizoaffective disorder. J Clin Psychiatry 2012; 73:13–20.
- Davis JM et al. Dose response and dose equivalence of antipsychotics. J Clin Psychopharmacol 2004; 24:192–208.
- Kinon BJ et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder: a randomized, doubleblind, fixed-dose study. J Clin Psychopharmacol 2008; 28:392–400.
- Van PT et al. A controlled dose comparison of haloperidol in newly admitted schizophrenic patients. Arch Gen Psychiatry 1990; 47:754–758.
- Kinon BJ et al. Treatment of neuroleptic-resistant schizophrenic relapse. Psychopharmacol Bull 1993; 29:309–314.
- Hermes E et al. Predictors of antipsychotic dose changes in the CATIE schizophrenia trial. Psychiatry Res 2012; 199:1–7.
- Patel MX et al. Quality of prescribing for schizophrenia: evidence from a national audit in England and Wales. Eur Neuropsychopharmacol 2014; 24:499–509.
- Heres S et al. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine: an exploratory analysis of head-to-head comparison studies of second-generation antipsychotics. Am J Psychiatry 2006; 163:185–194.
- Stroup TS et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006; 163:611–622.
- Leucht S et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 2009; 373:31–41.
- Leucht S et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry 2009; 166:152–163.
- Hong J et al. Clinical consequences of switching from olanzapine to risperidone and vice versa in outpatients with schizophrenia: 36-month results from the Worldwide Schizophrenia Outpatients Health Outcomes (W-SOHO) study. BMC Psychiatry 2012; 12:218.
- Agid O et al. Antipsychotic response in first-episode schizophrenia: efficacy of high doses and switching. Eur Neuropsychopharmacol 2013; 23:1017–1022.
- Paton C et al. High-dose and combination antipsychotic prescribing in acute adult wards in the UK: the challenges posed by p.r.n. prescribing. Br J Psychiatry 2008; 192:435–439.
- Taylor DM et al. Augmentation of clozapine with a second antipsychotic—a meta-analysis of randomized, placebo-controlled studies. Acta Psychiatr Scand 2009; 119:419–425.
- Kapur S et al. Increased dopamine D2 receptor occupancy and elevated prolactin level associated with addition of haloperidol to clozapine. Am J Psychiatry 2001; 158:311–314.
- Correll CU et al. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull 2009; 35:443–457.
- Essock SM et al. Effectiveness of switching from antipsychotic polypharmacy to monotherapy. Am J Psychiatry 2011; 168:702–708.
Antipsychotic long-acting injections
Antipsychotic long-acting injections (LAIs) are recommended where a patient has expressed preference for such a formulation because of its convenience or where avoidance of covert non-adherence is a clinical priority.1,2 It is estimated that between one-quarter and one-third of people with schizophrenia are prescribed a LAI,3 although this prevalence varies from country to country. Approximately half are also prescribed an oral antipsychotic drug, which often results in so-called high dose prescribing and seems counterintuitive.
Advice on prescribing LAIs
- For FGAs, give a test dose. Because they are long-acting, any adverse effects that result from injection are likely to be long-lived. For FGAs a test dose consisting of a small dose of active drug in a small volume of oil serves a dual purpose; it is a test of the patient's sensitivity to EPS and of any sensitivity to the base oil. For SGAs test doses are not required (less propensity to cause EPS and aqueous base not known to be allergenic).
- Begin with the lowest therapeutic dose. There are few data showing clear dose–response effects for LAIs. There is some information indicating that low doses (within the licensed range) are at least as effective as higher ones.4–6 Low doses are likely to be better tolerated and are certainly less expensive.
- Administer at the longest possible licensed interval. All LAIs can be safely administered at their licensed dosing intervals, bearing in mind the maximum recommended single dose. There is no evidence whatsoever to suggest that shortening the dose interval improves efficacy although this fantasy persists. Moreover, injections are painful, so less frequent administration is desirable. The 'observation' that some patients deteriorate in the days before the next dose is due is not supported by fact. For some hours (or even days with some preparations) plasma levels of antipsychotics continue to fall, albeit slowly, after the next injection. Thus, patients are most at risk of deterioration immediately after a LAI and not before it. Moreover, in trials, relapse seems only to occur 3–6 months after withdrawing therapy; roughly the time required to clear steadystate LAI drug levels from the blood.
- Adjust doses only after an adequate period of assessment. Attainment of peak plasma levels, therapeutic effect and steady-state plasma levels are all delayed with LAIs. Doses may be reduced if adverse effects occur, but should only be increased after careful assessment over at least one month, and preferably longer. The use of adjunctive oral medication to assess dosage requirements of LAIs may be helpful, but is complicated by the slow emergence of antipsychotic effects. Note that at the start of therapy, plasma levels of antipsychotic released from a LAI increase over several weeks to months without increasing the given dose. (This is due to accumulation: steady state is only achieved after 6–8 weeks.) Dose increases during this time to steady-state plasma levels are thus illogical and impossible to evaluate properly. Monitoring and recording of efficacy, side-effects and impact on physical health during therapy is recommended
- Depot preparations are not recommended for those who are antipsychotic-naïve. Tolerance to some LAIs can be established by using the oral form of the same drug for two weeks before starting the LAI. Good examples here are haloperidol, aripiprazole and paliperidone (using oral risperidone).
Differences between LAIs
There are few differences between individual FGA LAIs. Pipotiazine may be associated with relatively less frequent EPS, and fluphenazine with relatively more EPS, but perhaps less weight gain.7 Cochrane reviews have been completed for pipotiazine,8 flupentixol,9 zuclopenthixol,10 haloperidol11 and fluphenazine.12 With the exception of zuclopenthixol (see below), these preparations are equally effective, both with respect to oral antipsychotics and each other. Standard doses are said to be as effective as high doses for flupentixol.9
Table 2.8 Antipsychotic LAIs: suggested doses and frequencies2
| Drug |
Trade Name |
Licenced injection site |
Test dose (mg) |
Dose range (mg/week) |
Dosing interval (weeks) |
Comments |
| Aripiprazole |
Abilify Maintena |
Buttock |
Not required** |
300-400 mg monthly |
Monthly |
Does not increase prolactin |
| Flupentixol decanoate |
Depixol |
Buttock or thigh |
20 |
50 mg every 4 weeks to 400 mg a week |
2-4 |
Maximum licensed dose is very high relative to other LAIs |
| Fluphenazine decanoate |
Modecate |
Gluteal region |
12.5 |
12.5 mg every 2 weeks to 100 mg every 2 weeks |
2-5 |
High EPS |
| Haloperidol decanoate |
Haldol |
Gluteal region |
25* |
50-300 mg every 4 weeks |
4 |
High EPS |
| Olanzapine pamoate |
ZypAdhera |
Gluteal |
Not required** |
150 mg every 4 weeks to 300 mg every 2 weeks |
2-4 |
Note risk of post injection syndrome |
| Paliperidone palmitate |
Xeplion |
Deltoid or gluteal |
Not required** |
50-150 mg monthly |
Monthly |
Loading dose required at treatment initiation |
| Pipothiazine palmitate |
Piportil |
Gluteal region |
25 |
50-200 mg every 4 weeks |
4 |
? Lower incidence of EPS (unproven) |
| Risperidone microspheres |
Risperidal Consta |
Deltoid or gluteal |
Not required** |
25-50 mg every 2 weeks |
2 |
Drug release delayed for 2-3 weeks |
| Zuclopenthixol decanoate |
Clopixol |
Buttock or thigh |
100 |
200 mg every 3 weeks to 600 mg a week |
2-4 |
? Slightly better efficacy than some FGAs |
- The doses above are for adults. Check formal labelling for appropriate doses in the elderly.
- After a test dose, wait 4–10 days then titrate to maintenance dose according to response (see product information for individual drugs).
- Avoid using shorter dose intervals than those recommended except in exceptional circumstances (e.g. long interval necessitates high volume (> 3–4 mL) injection). Maximum licensed single dose overrides longer intervals and lower volumes. For example, zuclopentixol 500 mg every week is licensed whereas 1000 mg every 2 weeks is not (more than the licensed maximum of 600 mg is administered). Always check official manufacturer's information.
* Test dose not stated by manufacturer.
** Tolerability and response to the oral preparation should be established before administering the LAI. With respect to paliperidone LAI, oral risperidone can be used for this purpose.
|
Two differences that do exist between FGA LAIs are:
- zuclopenthixol may be more effective in preventing relapses than others, although this may be at the expense of an increased burden of side-effects13,14
- flupentixol decanoate can be given in very much higher 'neuroleptic equivalent' doses than the other LAI preparations and still remain 'within licensed dosing limits'. It is doubtful that this confers any real therapeutic advantage.
Aripiprazole, paliperidone, risperidone and olanzapine LAIs have a relatively lower propensity for EPS. Risperidone however increases prolactin, and because of its pharmacokinetic profile, dosage adjustment can be complex. Olanzapine can cause significant weight gain and is associated with inadvertent intravascular injection or post injection syndrome15. Unlike risperidone LAI, it is effective within a few days. Paliperidone is also rapidly released and effective within a few days, as is aripiprazole LAI.
Although the use of LAIs does not guarantee good treatment adherence, for those who continue with LAIs, there may be some adherence advantage over oral antipsychotics, which is indicated by a longer time to medication discontinuation.16,17 There is also some evidence to suggest a better global outcome with LAIs as compared with oral antipsychotics with a reduced risk of rehospitalisation.1,16 It has been argued that compliance with oral antipsychotics decreases over time and that relapse rates in patients prescribed depots decrease in comparison to oral antipsychotics only in the longer term.18 That is, depots reveal advantages over oral treatment only after several years.
Table 2.8 summarises suggested doses and frequencies for administration of antipsychotic LAIs.
Intramuscular anticholinergics and LAIs
Antipsychotic LAIs do not produce acute movement disorders at the time of administration:19 this may take hours to days. The administration of intramuscular procyclidine routinely with each dose is illogical, as the effects of the anticholinergic drug will have worn off before plasma antipsychotic levels peak.
References
- National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: treatment and management. Clinical Guideline 178, 2014. http://www.nice.org.uk/Guidance/CG178
- Barnes TR. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2011; 25:567–620.
- Barnes T. Antipsychotic long acting injections: prescribing practice in the UK. Br J Psychiatry 2009; 195:S37–S42.
- Kane JM et al. A multidose study of haloperidol decanoate in the maintenance treatment of schizophrenia. Am J Psychiatry 2002; 159:554–560.
- Taylor D. Establishing a dose-response relationship for haloperidol decanoate. Psychiatr Bull 2005; 29:104–107.
- McEvoy JP et al. Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: a randomized clinical trial. JAMA 2014; 311:1978–1987.
- Taylor D. Psychopharmacology and adverse effects of antipsychotic long acting injections. Br J Psychiatry 2009; 195:S13–S19.
- Dinesh M et al. Depot pipotiazine palmitate and undecylenate for schizophrenia. Cochrane Database Syst Rev 2004; CD001720.
- Mahapatra J et al. Flupenthixol decanoate (depot) for schizophrenia or other similar psychotic disorders. Cochrane Database Syst Rev 2014; 6: CD001470.
- Coutinho Eet al. Zuclopenthixol decanoate for schizophrenia and other serious mental illnesses. Cochrane Database Syst Rev 2000; CD001164.
- Quraishi Set al. Depot haloperidol decanoate for schizophrenia. Cochrane Database Syst Rev 2000; CD001361.
- David Aet al. Depot fluphenazine decanoate and enanthate for schizophrenia. Cochrane Database Syst Rev 2005; CD000307.
- da Silva Freire Coutinho E et al. Zuclopenthixol decanoate for schizophrenia and other serious mental illnesses. Cochrane Database Syst Rev 2006; CD001164.
- Shajahan P et al. Comparison of the effectiveness of depot antipsychotics in routine clinical practice. Psychiatrist 2010; 34:273–279.
- Citrome L. Olanzapine pamoate: a stick in time? Int J Clin Pract 2009; 63:140–150.
- Tiihonen J et al. A Nationwide Cohort Study of Oral and Depot Antipsychotics After First Hospitalization for Schizophrenia. Am J Psychiatry 2011; 168:603–609.
- Leucht C et al. Oral versus depot antipsychotic drugs for schizophrenia—a critical systematic review and meta-analysis of randomised longterm trials. Schizophr Res 2011; 127:83–92.
- Schooler NR. Relapse and rehospitalization: comparing oral and depot antipsychotics. J Clin Psychiatry 2003; 64 Suppl 16:14–17.
- Kane JM et al. Guidelines for depot antipsychotic treatment in schizophrenia. European Neuropsychopharmacology Consensus Conference in Siena, Italy. Eur Neuropsychopharmacol 1998; 8:55–66.
Further reading
Patel MX et al. Antipsychotic long-acting (depot) injections for the treatment of schizophrenia. Br J Psychiatry 2009; 195 Suppl 52:S1–S67.
Depot antipsychotics—pharmacokinetics
Table 2.9 summarises the pharmacokinetics of depot antipsychotics.
Table 2.9 Pharmacokinetics of depot antipsychotics
| Drug |
Trade name |
Time to peak* (days) |
Plasma half-life (days) |
Time to steady state (weeks)† |
| Aripiprazole1 |
Abilify Maintena |
7 |
30–46 |
~20 |
| Flupentixol decanoate2 |
Depixol |
7 |
8–17 |
~9 |
| Fluphenazine decanoate3–5 |
Modecate |
8–12‡ |
10 |
~8 |
| Haloperidol decanoate6,7 |
Haldol |
7 |
21 |
~14 |
| Olanzapine pamoate8,9 |
ZypAdhera |
2–3 |
30 |
~12 |
| Paliperidone palmitate10 |
Xeplion |
13 |
29–45 |
~20 |
| Pipotiazine palmitate11,12 |
Piportil |
7–14 |
15 |
~9 |
| Risperidone microspheres13,14 |
Risperidal Consta |
35 |
4 |
~8 |
| Zuclopenthixol decanoate2,11,15 |
Clopixol |
4–7 |
19 |
~12 |
|
* Time to peak is not the same as time to reach therapeutic plasma concentration but both are dependent on dose. For large (loading) doses, therapeutic activity is often seen before attaining peak levels. For low (test) doses, the initial peak level may be sub-therapeutic.
† Attainment of steady state (SS) follows logarithmic, not linear characteristics: around 90% of SS levels are achieved in three half-lives. Time to attain steady state is independent of dose and dosing frequency (that is, you can't hurry it up by giving more, more often). Loading doses can be used to produce prompt therapeutic plasma levels but time to SS remains the same.
‡ Earlier estimates suggest peak concentrations after only a few hours.15,16 It is likely that fluphenazine decanoate produces two peaks—one on the day of injection and a second slightly higher peak a week or so later.6
|
References
- Mallikaarjun S et al. Pharmacokinetics, tolerability and safety of aripiprazole once-monthly in adult schizophrenia: an open-label, parallelarm, multiple-dose study. Schizophr Res 2013; 150:281–288.
- Jann MW et al. Clinical pharmacokinetics of the depot antipsychotics. Clin Pharmacokinet 1985; 10:315–333.
- Simpson GM et al. Single-dose pharmacokinetics of fluphenazine after fluphenazine decanoate administration. J Clin Psychopharmacol 1990; 10:417–421.
- Balant-Gorgia AE et al. Antipsychotic drugs. Clinical pharmacokinetics of potential candidates for plasma concentration monitoring. Clin Pharmacokinet 1987; 13:65–90.
- Gitlin MJ et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol 1988; 8:53–56.
- Wiles DH et al. Pharmacokinetics of haloperidol and fluphenazine decanoates in chronic schizophrenia. Psychopharmacology (Berl) 1990; 101:274–281.
- Nayak RK et al. The bioavailability and pharmacokinetics of oral and depot intramuscular haloperidol in schizophrenic patients. J Clin Pharmacol 1987; 27:144–150.
- Heres S et al. Pharmacokinetics of olanzapine long-acting injection: the clinical perspective. Int Clin Psychopharmacol 2014;.29:299–312.
- Mitchell M et al. Singleand multiple-dose pharmacokinetic, safety, and tolerability profiles of olanzapine long-acting injection: an openlabel, multicenter, nonrandomized study in patients with schizophrenia. Clin Ther 2013; 35:1890–1908.
- Hoy SM et al. Intramuscular paliperidone palmitate. CNS Drugs 2010; 24:227–244.
- Barnes TR et al. Long-term depot antipsychotics. A risk-benefit assessment. Drug Saf 1994; 10:464–479.
- Ogden DA et al. Determination of pipothiazine in human plasma by reversed-phase high-performance liquid chromatography. J Pharm Biomed Anal 1989; 7:1273–1280.
- Ereshefsky L et al. Pharmacokinetic profile and clinical efficacy of long-acting risperidone: potential benefits of combining an atypical antipsychotic and a new delivery system. Drugs R D 2005; 6:129–137.
- Eerdekens M et al. Pharmacokinetics and tolerability of long-acting risperidone in schizophrenia. Schizophr Res 2004; 70:91–100.
- Viala A et al. Comparative study of the pharmacokinetics of zuclopenthixol decanoate and fluphenazine decanoate. Psychopharmacology (Berl) 1988; 94:293–297.
- Soni SD et al. Plasma levels of fluphenazine decanoate. Effects of site of injection, massage and muscle activity. Br J Psychiatry 1988; 153:382–384.
Management of patients on long-term depots
All patients receiving long-term treatment with antipsychotic medication should be seen by their responsible psychiatrist at least once a year (ideally more frequently) in order to review their progress and treatment. A systematic assessment of side-effects should constitute part of this review. For most people with multi-episode schizophrenia long-term, even lifelong, treatment is necessary. However with long-term depot treatment dose reduction might be considered in stable patients. There is some evidence to suggest that FGA depots are prescribed in excessive doses: haloperidol decanoate is optimally effective at 75 mg every 4 weeks;1,2 other depots almost saturate dopamine receptors at low doses (e.g. flupentixol 40 mg per week3). There is no simple formula for deciding when or whether to reduce the dose of maintenance antipsychotic treatment; therefore, a risk–benefit analysis must be done for every patient. Many patients, it should be noted, are happy to receive depots.4 When considering dose reduction, the following prompts may be helpful.
- Is the patient symptom-free and if so for how long? Long-standing, non-distressing symptoms which have not previously been responsive to medication may be excluded.
- What is the severity of the side-effects (EPS, tardive dyskinesia, obesity, etc.)?
- What is the previous pattern of illness? Consider the speed of onset, duration and severity of episodes and any danger posed to self or others.
- Has dosage reduction been attempted before? If so, what was the outcome?
- What are the patient's current social circumstances? Is it a period of relative stability, or are stressful life events anticipated?
- What is the social cost of relapse (e.g. is the patient the sole breadwinner for a family)?
- Is the patient able to monitor his/her own symptoms? If so, will he/she seek help?
If after consideration of the above, the decision is taken to reduce medication dose, the patient's family should be involved and a clear explanation given of what should be done if symptoms return/worsen. It would then be reasonable to proceed in the following manner.
- If it has not already been done, oral antipsychotic medication should be discontinued first.
- The interval between injections should be increased to up to 4 weeks before decreasing the dose given each time. Note: not with risperidone.
- The dose should be reduced by no more than one-third at any one time. Note: special considerations apply to risperidone.
- Decrements should, if possible, be made no more frequently than every 3 months, preferably every 6 months.
- Discontinuation should not be seen as the ultimate aim of the above process although it sometimes results. NICE5 (2014) now suggest that intermittent treatment (symptom-triggered) is preferable to no treatment.
If the patient becomes symptomatic, this should be seen not as a failure, but rather as an important step in determining the minimum effective dose that the patient requires.
References
- Taylor D. Establishing a dose-response relationship for haloperidol decanoate. Psychiatr Bull 2005; 29:104–107.
- McEvoy JP et al. Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: a randomized clinical trial. JAMA 2014; 311:1978–1987.
- Uchida H et al. Dose and dosing frequency of long-acting injectable antipsychotics: a systematic review of PET and SPECT data and clinical implications. J Clin Psychopharmacol 2014; Epub ahead of print: DOI: 10.1097/JCP.0000000000000065.
- Heres S et al. The attitude of patients towards antipsychotic depot treatment. Int Clin Psychopharmacol 2007; 22:275–282.
- National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: treatment and management. Clinical Guideline 178, 2014. http://www.nice.org.uk/Guidance/CG178
Aripiprazole LAI
Aripiprazole lacks the prolactin-related and metabolic adverse effects of other SGA LAIs and so is a useful alternative to them. Placebo-controlled studies show a good acute and longer term effect1 but aripiprazole LAI has not been compared with other depots. For most patients, a suitable dosing regimen is oral aripiprazole 10–20 mg/day for 14 days (to establish tolerability and response) then 400 mg aripiprazole LAI once monthly. Oral aripiprazole should be continued for 14 days after the first injection. In such a regimen, peak plasma levels are seen at 1–2 weeks after injection and the lowest trough at 4 weeks.2 At steady state, peak plasma levels are up to 50% higher than the first dose peak and trough plasma levels only slightly below the first dose peak.2 Dose adjustments should take this into account. A lower dose of 300 mg a month can be used in those not tolerating 400 mg. A dose of 200 mg a month may only be used for those patients receiving particular enzyme inhibiting drugs. The incidence of akathisia, insomnia, nausea and restlessness is similar to that seen with oral aripiprazole.3,4
There are no formal recommendations for switching to aripiprazole but we present recommendations based on our interpretation of available pharmacokinetic data in Table 2.10.
Table 2.10 Switching to aripiprazole LAI
|
Switching from
|
Aripiprazole LAI regimen
|
|
Oral antipsychotics
|
Cross taper antipsychotic with oral aripiprazole* over 2 weeks. Start LAI, continue aripiprazole oral for 2 weeks then stop
|
|
Depot antipsychotics (not risperidone LAI)
|
Start oral aripiprazole* on day last depot injection was due. Start aripiprazole LAI after 2 weeks then stop oral aripiprazole 2 weeks later
|
|
Risperidone LAI
|
Start oral aripiprazole* 5-6 weeks** after the last risperidone injection. Start aripiprazole LAI 2 weeks later; discontinue oral aripiprazole 2 weeks after that
|
|
* If prior response and tolerability to aripiprazole known, oral aripiprazole may not be required. Switch straight to aripiprazole LAI on the day oral would have started.
** This gap seems excessive, but the last injection of risperidone will provide therapeutic levels 4–6 weeks later; a post-dose peak at 5 weeks.
|
References
- Shirley M et al. Aripiprazole (ABILIFY MAINTENA(R)): a review of its use as maintenance treatment for adult patients with schizophrenia. Drugs 2014; 74:1097–1110.
- Mallikaarjun S et al. Pharmacokinetics, tolerability and safety of aripiprazole once-monthly in adult schizophrenia: an open-label, parallel-arm, multiple-dose study. Schizophr Res 2013; 150:281–288.
- Kane JM et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week, multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2012; 73:617–624.
- Potkin SG et al. Safety and tolerability of once monthly aripiprazole treatment initiation in adults with schizophrenia stabilized on selected atypical oral antipsychotics other than aripiprazole. Curr Med Res Opin 2013; 29:1241–1251.
Olanzapine LAI
Like all esters, olanzapine pamoate (embonate, in some countries) is very poorly water soluble. An aqueous suspension of olanzapine pamoate, when injected intramuscularly, affords both prompt and sustained release of olanzapine. Peak plasma levels are seen within one week of injection1 and efficacy can be demonstrated after only 3 days.2 Only gluteal injection is licensed; deltoid injection is less effective.3 Olanzapine LAI is effective when given every 4 weeks, with 2-weekly administration only required when the highest dose is prescribed. It has not been compared with other long-acting injections. Loading doses are recommended in some dose regimens (Table 2.11). Formal labelling/ SPC suggests that patients be given oral olanzapine to assess response and tolerability. This rarely happens in practice. Oral supplementation after the first depot injection is not necessary.
Switching
Direct switching to olanzapine LAI, ideally following an oral trial, is usually possible. So, when switching from another LAI (but not risperidone) olanzapine oral or LAI can be started on the day the last LAI was due. Likewise for switching from oral treatment—a direct switch is possible but prior antipsychotics are probably best reduced slowly after starting olanzapine (either oral or LAI). When switching from risperidone RLAI, olanzapine should be started, we suggest, 3–4 weeks after the last injection was due (e.g. 5–6 weeks after the last injection).
Post-injection syndrome
Post-injection syndrome occurs when olanzapine pamoate is inadvertently exposed to high blood volumes (probably via accidental intravasation). Olanzapine plasma levels may reach 600 μg/L and delirium and somnolence result.4 The incidence of post-injection syndrome is less than 0.1% of injections; almost all reactions (86%) occur within 1 hour of injection.5 In most countries, olanzapine LAI may only be given in healthcare facilities under supervision and patients need to be kept under observation for 3 hours after the injection is given.
Table 2.11 Olanzapine—dosing schedules
|
Oral olanzapine equivalent
|
Loading dose
|
Maintenance dose (given 8 weeks after the first dose)
|
|
10 mg/day
|
210 mg every 2 weeks 405 mg every 4 weeks
|
300 mg/4 weeks (or 150 mg every 2 weeks)
|
|
15 mg/day
|
300 mg every 2 weeks
|
405 mg/4 weeks (or 210 mg every 2 weeks)
|
|
20 mg/day
|
None - give 300 mg every 2 weeks
|
300 mg every 2 weeks
|
In the EU, the exact wording of the SPC6 is as follows:
After each injection, patients should be observed in a healthcare facility by appropriately qualified personnel for at least 3 hours for signs and symptoms consistent with olanzapine overdose.
Immediately prior to leaving the healthcare facility, it should be confirmed that the patient is alert, oriented, and absent of any signs and symptoms of overdose. If an overdose is suspected, close medical supervision and monitoring should continue until examination indicates that signs and symptoms have resolved. The 3-hour observation period should be extended as clinically appropriate for patients who exhibit any signs or symptoms consistent with olanzapine overdose.
For the remainder of the day after injection, patients should be advised to be vigilant for signs and symptoms of overdose secondary to post-injection adverse reactions, be able to obtain assistance if needed, and should not drive or operate machinery.
This monitoring requirement has undoubtedly adversely affected the popularity of olanzapine LAI. No patient or medical factor has been identified which might predict post-injection syndrome4 except that those experiencing the syndrome are more likely to have previously has an injection-site related adverse effect.7
References
- Heres S et al. Pharmacokinetics of olanzapine long-acting injection: the clinical perspective. Int Clin Psychopharmacol 2014; 29:299–312.
- Lauriello J et al. An 8-week, double-blind, randomized, placebo-controlled study of olanzapine long-acting injection in acutely ill patients with schizophrenia. J Clin Psychiatry 2008; 69:790–799.
- Mitchell M et al. Singleand multiple-dose pharmacokinetic, safety, and tolerability profiles of olanzapine long-acting injection: an open-label, multicenter, nonrandomized study in patients with schizophrenia. Clin Ther 2013; 35:1890–1908.
- McDonnell DP et al. Post-injection delirium/sedation syndrome in patients with schizophrenia treated with olanzapine long-acting injection, II: investigations of mechanism. BMC Psych 2010; 10:45.
- Bushes CJ et al. Olanzapine long-acting injection: review of first experiences of post-injection delirium/sedation syndrome in routine clinical practice. 2013. Eli Lilly Personal Communication.
- Eli Lilly and Company Limited, Eli Lilly and. Summary of Product Characteristics. ZYPADHERA 210 mg, 300 mg, and 405 mg, powder and solvent for prolonged release suspension for injection. 2014. http://www.medicines.org.uk/
- Atkins S et al. A pooled analysis of injection site-related adverse events in patients with schizophrenia treated with olanzapine long-acting injection. BMC Psychiatry 2014; 14:7.
Paliperidone palmitate LAI
Paliperidone was the third SGA to be developed as a LAI. It is the major active metabolite of risperidone: 9-hydroxyrisperidone. Following an intramuscular injection, active paliperidone plasma levels are seen within a few days, therefore co-administration of oral paliperidone or risperidone during initiation is not required.1 Dosing consists of two initiation doses (deltoid) followed by monthly maintenance doses (deltoid or gluteal)—see Table 2.12. Following administration of a single IM dose to the deltoid muscle, on average 28% higher peak concentration is observed compared with IM injection to the gluteal muscle.1 Thus, the two deltoid muscle injections on days 1 and 8 help to quickly attain therapeutic drug concentrations.
Paliperidone LAI has been compared with haloperidol depot given in a loading dose schedule matching that of paliperidone.2 The two formulations were equally effective in preventing relapse but paliperidone increased prolactin to a greater extent and caused more weight gain. Haloperidol caused more akathisia, more acute movement disorder and there was a trend for a higher incidence of tardive dyskinesia. The average dose of haloperidol was around 75 mg a month; a dose rarely used in practice.
Table 2.12 Paliperidone dose and administration information1
|
|
Dose
|
Route
|
|
Initiation
|
|
|
|
Day 1
|
150 mg IM
(234 mg)*
|
Deltoid only
|
|
Day 8 (+/- 4 days)
|
100 mg IM
(156 mg)*
|
Deltoid only
|
|
Maintenance
|
|
|
|
Every month (+/- 7 days) thereafter
|
50-150 mg IM†
(78-234 mg)*
|
Deltoid or gluteal
|
|
* Paliperidone palmitate dose can be expressed in terms of active moiety (50–150 mg) or weight of compound (78–234 mg).
† The maintenance dose is perhaps best judged by consideration of what might be a suitable dose of oral risperidone and then giving paliperidone palmitate in an equivalent dose (see below).
IM, intramuscular.
|
Table 2.13 Approximate dose equivalent1,3
|
Risperidone oral (mg/day) (bioavailability = 70%)4
|
paliperidone oral (mg/day) (bioavailability = 28%)5
|
Risperidone LAI (consta) (mg/2 weeks)
|
paliperidone palmitate (mg/monthly)
|
|
2
|
4
|
25
|
50
|
|
3
|
6
|
37.5
|
75
|
|
4
|
9
|
50
|
100
|
|
6
|
12
|
-
|
150
|
Table 2.14 Switching to paliperidone palmitate
|
Switching from
|
Recommended method of switching
|
Comments
|
|
No treatment
|
Give the two initiation doses: 150 mg IM deltoid on day 1 and 100 mg IM deltoid on day 8 Maintenance dose starts 1 month later
|
In general the lowest most effective maintenance dose should be used The manufacturer recommends a dose of 75 mg monthly for the general adult population.1 This is approximately equivalent to 3 mg/day oral risperidone (see Table 2.13). In practice the modal dose is 100 mg/month6 Maintenance dose adjustments should be made monthly. However the full effect of the dose adjustment may not be apparent for several months3
|
|
Oral paliperidone/ risperidone
|
Give the two initiation doses followed by the maintenance dose (see Table 2.13 and prescribe equivalent dose)
|
Oral paliperidone/risperidone supplementation during initiation is not necessary
|
|
Oral antipsychotics
|
Reduce the dose of the oral antipsychotic over 1-2 weeks following the first injection of paliperidone. Give the two initiation doses followed by the maintenance dose
|
|
|
Depot antipsychotic
|
For risperidone LAI, begin paliperidone 5-6 weeks after the last injection NB. No initiation doses are required
|
Doses of paliperidone palmitate IM may be difficult to predict. The manufacturer recommends a dose of 75 mg monthly for the general adult population. If switching from RLAI see Table 2.13 and prescribe equivalent dose Maintenance dose adjustments should be made monthly. However the full effect of the dose adjustment may not be apparent for several months3
|
|
Antipsychotic polypharmacy with depot
|
Start paliperidone (at the maintenance dose) when the next injection is due NB. No initiation doses are required Reduce the dose of the oral antipsychotic over 1-2 weeks following the first injection of paliperidone
|
Aim to treat the patient with paliperidone palmitate IM as the sole antipsychotic The maintenance dose should be governed as far as possible by the total dose of oral and injectable antipsychotic (see Table 2.13)
|
|
IM, intramuscular; RLAI, risperidone long-acting injection.
|
The second initiation dose may be given 4 days before or after day 8 (after the first initiation dose on day one).3 Similarly the manufacturer recommends that patients may be given maintenance doses up to 7 days before or after the monthly time point.3 This flexibility should help minimise the number of missed doses. There is a complex schedule of recommendations to be adhered to when doses are missed—please see SPC or formal labelling.
Some points to note:
- Paliperidone palmitate is an esterified form of paliperidone and utilises Elan Technologies' NanoCrystal® Technology; is formulated as an aqueous suspension engineered for sustained release.
- Paliperidone palmitate IM does not require cold storage.
- Paliperidone palmitate IM is available as pre-filled syringes and does not require reconstitution or suspension before administration.
- No oral supplementation is required on initiation for paliperidone palmitate.
- No test dose is required for paliperidone palmitate (but patients should (ideally) be currently stabilised on or have previously responded to oral paliperidone or risperidone).
- The median time to maximum plasma concentrations is 13 days.1
The approximate dose equivalents of different formulations of risperidone and paliperidone are shown in Table 2.13. Switching to paliperidone palmitate is shown in Table 2.14.
References
- Janssen Pharmaceuticals. Highlights of Prescribing Information. INVEGA SUSTENNAR (paliperidone palmitate) extended-release injectable suspension, for intramuscular use. 2014. http://www.janssencns.com/sustenna-prescribing-information
- McEvoy JP et al. Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: a randomized clinical trial. JAMA 2014; 311:1978–1987.
- Janssen-Cilag Ltd. Summary of Product Characteristics. XEPLION 50 mg, 75 mg, 100 mg and 150 mg prolonged release suspension for injection. 2013. http://www.medicines.org.uk/
- Janssen-Cilag Ltd, Janssen. Summary of Product Characteristics. Risperdal Tablets, Liquid and Quicklet. 2014. http://www.medicines.org.uk/
- Janssen-Cilag Ltd. Summary of Product Characteristics. INVEGA 1.5 mg, 3 mg, 6 mg, 9 mg, 12 mg prolonged-release tablets. 2013. http://www. medicines.org.uk/
- Attard A et al. Paliperidone palmitate long-acting injection–prospective year-long follow-up of use in clinical practice. Acta Psychiatr Scand 2014; 130:46–51.
Risperidone LAI
Risperidone was the first 'atypical' drug to be made available as a depot, or long-acting, injectable formulation. Doses of 25–50 mg every 2 weeks appear to be as effective as oral doses of 2–6 mg/day.1 The long-acting injection also seems to be well tolerated—fewer than 10% of patients experienced EPS and fewer than 6% withdrew from a long-term trial because of adverse effects.2 Oral risperidone increases prolactin,3 as does RLAI4 but levels appear to reduce somewhat following a switch from oral to injectable risperidone.5–7 Rates of tardive dyskinesia are said to be low.8 There are no direct comparisons with standard depots but switching from FGA depots in stable patients to risperidone LAI has been shown to be less successful than remaining on the FGA depot.9
Uncertainty remains over the dose–response relationship for RLAI. Studies randomising subjects to different fixed doses of RLAI show no differences in response according to dose.10 One randomised, fixed-dose year-long study suggested better outcome for 50 mg every 2 weeks than with 25 mg, although no observed difference reached statistical significance.11 Naturalistic studies indicate doses higher than 25 mg/2 weeks are frequently used.12,13 One study suggested higher doses were associated with better outcome.14,15
Plasma levels afforded by 25 mg/2 weeks seem to be similar to, or even lower than, levels provided by 2 mg/day oral risperidone.16,17 (One study found 9.5% of plasma samples from people apparently receiving risperidone LAI contained no risperidone or 9-hydroxyrisperidone18). Striatal dopamine D2 occupancies are similarly low in people receiving 25 mg/2 weeks.19,20 So, although fixed dose studies have not revealed clear advantages for doses above 25 mg/2 weeks other indicators cast doubt on the assumption that 25 mg/2 weeks will be adequate for all or even most patients. While this conundrum remains unresolved the need for careful dose titration becomes of great importance. This is perhaps most efficiently achieved by establishing the required dose of oral risperidone and converting this dose into the equivalent injection dose. Trials have clearly established that switching from 2 mg oral to 25 mg injection and 4 mg oral to 50 mg injection is usually successful2,21,22 (switching from 4 mg/day to 25 mg/2 weeks increases the risk of relapse23). There remains a question over the equivalent dose for 6 mg oral: in theory, patients should be switched to 75 mg injection but this showed no advantage over lower doses in trials and is in any case above the licensed maximum dose. Paliperidone palmitate 150 mg a month is equivalent to oral risperidone 6 mg/day. In fact, for many reasons paliperidone palmitate (9-hydroxyrisperidone) may be preferred to risperidone injection: it acts acutely, can be given monthly, does not require cold storage and has a wider, more useful dose range (see section on 'Paliperidone palmitate intramuscular long-acting injection' in this chapter).
Risperidone long-acting injection differs importantly from other depots and the following points should be noted.
- Risperidone depot is not an esterified form of the parent drug. It contains risperidone coated in polymer to form microspheres. These microspheres have to be suspended in an aqueous base immediately before use.
- The injection must be stored in a fridge (consider the practicalities for community staff).
Table 2.15 Switching to risperidone long-acting injection (RLAI)
|
Switching from
|
Recommended method of switching
|
Comments
|
No treatment
(new patient or recently non-compliant)
|
Start risperidone oral at 2 mg/day and titrate to effective dose. If tolerated, prescribe equivalent dose of RLAI Continue with oral risperidone for at least 3 weeks then taper over 1-2 weeks. Be prepared to continue oral risperidone for longer
|
Use oral risperidone before giving injection to assure good tolerability Those stabilised on 2 mg/day start on 25 mg/2 weeks Those on higher doses, start on 37.5 mg/2 weeks and be prepared to use 50 mg/2 weeks
|
|
Oral risperidone
|
Prescribe equivalent dose of RLAI
|
See above
|
Oral antipsychotics
(not risperidone)
|
Either:
Switch to oral risperidone and titrate to effective dose. If tolerated, prescribe equivalent dose of RLAI Continue with oral risperidone for at least 3 weeks then taper over 1-2 weeks. Be prepared to continue oral risperidone for longer
or:
Give RLAI and then slowly discontinue oral antipsychotics after 3-4 weeks. Be prepared to continue oral antipsychotics for longer
|
Dose assessment is difficult in those switching from another antipsychotic. Broadly speaking, those on low oral doses should be switched to 25 mg/2weeks. 'Low' in this context means towards the lower end of the licensed dose range or around the minimum dose known to be effective
Those on higher oral doses should receive 37.5 mg or 50 mg every 2 weeks. The continued need for oral antipsychotics after 3-4 weeks may indicate that higher doses of RLAI are required
|
|
Depot antipsychotic
|
Give RLAI one week before the last depot injection is given
|
Dose of RLAI difficult to predict. For those on low doses (see above) start at 25 mg/2 weeks and then adjust as necessary
Start RLAI at 37.5 mg/2 weeks in those previously maintained on doses in the middle or upper range of licensed doses. Be prepared to increase to 50 mg/2 weeks
|
|
Antipsychotic polypharmacy with depot
|
Give RLAI one week before the last depot injection is given
Slowly taper oral antipsychotics 3-4 weeks later. Be prepared to continue oral antipsychotics for longer
|
Aim to treat patient with RLAI as the sole antipsychotic. As before, RLAI dose should be dictated, as far as is possible, by the total dose of oral and injectable antipsychotic
|
- It is available as doses of 25, 37.5 and 50 mg. The whole vial must be used (because of the nature of the suspension). This means that there is limited flexibility in dosing.
- A test dose is not required or sensible. (Testing tolerability with oral risperidone is desirable but not always practical.)
- It takes 3–4 weeks for the first injection to produce therapeutic plasma levels. Peak plasma levels are reached after 5 weeks. Patients must be maintained on a full dose of their previous antipsychotic for at least 3 weeks after the administration of the first risperidone injection. Oral antipsychotic cover is sometimes required for longer (6–8 weeks). If the patient is not already receiving an oral antipsychotic, oral risperidone should be prescribed. (see Table 2.15 for advice on switching from depots.) Patients who refuse oral treatment and are acutely ill should not be given RLAI because of the long delay in drug release.
- Risperidone depot must be administered every 2 weeks. The Product Licence does not allow longer intervals between doses. There is little flexibility to negotiate with patients about the frequency of administration although monthly injections may be effective.24
- The most effective way of predicting response to RLAI is to establish dose and response with oral risperidone.
- Risperidone injection is not considered suitable for patients with treatment refractory schizophrenia although one study showed good effect with 50 mg and 100 mg two weekly.25
For guidance on switching to risperidone long-acting injection see Table 2.15.
References
- Chue P et al. Comparative efficacy and safety of long-acting risperidone and risperidone oral tablets. Eur Neuropsychopharmacol 2005; 15:111–117.
- Fleischhacker WW et al. Treatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry 2003; 64:1250–1257.
- Kleinberg DL et al. Prolactin levels and adverse events in patients treated with risperidone. J Clin Psychopharmacol 1999; 19:57–61.
- Fu DJ et al. Paliperidone palmitate versus oral risperidone and risperidone long-acting injection in patients with recently diagnosed schizophrenia: a tolerability and efficacy comparison. Int Clin Psychopharmacol 2014; 29:45–55.
- Bai YM et al. A comparative efficacy and safety study of long-acting risperidone injection and risperidone oral tablets among hospitalized patients: 12-week randomized, single-blind study. Pharmacopsychiatry 2006; 39:135–141.
- Bai YM et al. Pharmacokinetics study for hyperprolactinemia among schizophrenics switched from risperidone to risperidone long-acting injection. J Clin Psychopharmacol 2007; 27:306–308.
- Peng PW et al. The disparity of pharmacokinetics and prolactin study for risperidone long-acting injection. J Clin Psychopharmacol 2008; 28:726–727.
- Gharabawi GM et al. An assessment of emergent tardive dyskinesia and existing dyskinesia in patients receiving long-acting, injectable risperidone: results from a long-term study. Schizophr Res 2005; 77:129–139.
- Covell NH et al. Effectiveness of switching from long-acting injectable fluphenazine or haloperidol decanoate to long-acting injectable risperidone microspheres: an open-label, randomized controlled trial. J Clin Psychiatry 2012; 73:669–675.
- Kane JM et al. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry 2003; 160:1125–1132.
- Simpson GM et al. A 1-year double-blind study of 2 doses of long-acting risperidone in stable patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry 2006; 67:1194–1203.
- Turner M et al. Long-acting injectable risperidone: safety and efficacy in stable patients switched from conventional depot antipsychotics. Int Clin Psychopharmacol 2004; 19:241–249.
- Taylor DM et al. Early clinical experience with risperidone long-acting injection: a prospective, 6-month follow-up of 100 patients. J Clin Psychiatry 2004; 65:1076–1083.
- Taylor DM et al. Prospective 6-month follow-up of patients prescribed risperidone long-acting injection: factors predicting favourable outcome. Int J Neuropsychopharmacol 2006; 9:685–694.
- Taylor DM et al. Risperidone long-acting injection: a prospective 3-year analysis of its use in clinical practice. J Clin Psychiatry 2009; 70:196–200.
- Nesvag R et al. Serum concentrations of risperidone and 9-OH risperidone following intramuscular injection of long-acting risperidone compared with oral risperidone medication. Acta Psychiatr Scand 2006; 114:21–26.
- Castberg I et al. Serum concentrations of risperidone and 9-hydroxyrisperidone after administration of the long-acting injectable form of risperidone: evidence from a routine therapeutic drug monitoring service. Ther Drug Monit 2005; 27:103–106.
- Bowskill SV et al. Risperidone and total 9-hydroxyrisperidone in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service, 2002–2010. Ther Drug Monit 2012; 34:349–355.
- Gefvert O et al. Pharmacokinetics and D2 receptor occupancy of long-acting injectable risperidone (Risperdal ConstaTM) in patients with schizophrenia. Int J Neuropsychopharmacol 2005; 8:27–36.
- Remington G et al. A PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidone. Am J Psychiatry 2006; 163:396–401.
- Lasser RA et al. Clinical improvement in 336 stable chronically psychotic patients changed from oral to long-acting risperidone: a 12-month open trial. Int J Neuropsychopharmacol 2005; 8:427–438.
- Lauriello J et al. Long-acting risperidone vs. placebo in the treatment of hospital inpatients with schizophrenia. Schizophr Res 2005; 72:249–258.
- Bai YM et al. Equivalent switching dose from oral risperidone to risperidone long-acting injection: a 48-week randomized, prospective, singleblind pharmacokinetic study. J Clin Psychiatry 2007; 68:1218–1225.
- Uchida H et al. Monthly administration of long-acting injectable risperidone and striatal dopamine D2 receptor occupancy for the management of schizophrenia. J Clin Psychiatry 2008; 69:1281–1286.
- Meltzer HY et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res 2014; 154:14–22.
ANTIPSYCHOTICS—ADVERSE EFFECTS
Extrapyramidal side-effects
Details of the extrapyramidal side-effects of antipsychotic drug treatment are shown in Table 2.16.
Table 2.16 Most common extrapyramidal side-effects
|
|
Dystonia
(uncontrolled muscular spasm)
|
Pseudo-parkinsonism
(tremor, etc.)
|
Akathisia
(restlessness)1
|
Tardive dyskinesia
(abnormal movements)
|
|
Signs and symptoms2
|
Muscle spasm in any part of the body, e.g.
- eyes rolling upwards (oculogyric crisis)
- head and neck twisted to the side (torticollis)
- the patient may be unable to swallow or speak clearly
- in extreme cases, the back may arch or the jaw dislocate
Acute dystonia can be both painful and very frightening
|
Symptoms include:
- tremor and/or rigidity
- bradykinesia (decreased facial expression, flat monotone voice, slow body movements, inability to initiate movement)
- bradyphrenia (slowed thinking)
- salivation
Pseudo-parkinsonism can be mistaken for depression or the negative symptoms of schizophrenia
|
A subjectively unpleasant state of inner restlessness where there is a strong desire or compulsion to move, e.g.
- foot stamping when seated
- constantly crossing/ uncrossing legs
- rocking from foot to foot
- constantly pacing up and down
Akathisia can be mistaken for psychotic agitation and has been linked with suicidal ideation3 and aggression towards others4
|
A wide variety of movements can occur such as:
- lip smacking or chewing
- tongue protrusion (fly catching)
- choreiform hand movements (pill rolling or piano playing)
- pelvic thrusting
Severe orofacial movements can lead to difficulty speaking, eating or breathing. Movements are worse when under stress
|
|
Rating scales
|
No specific scale. Small component of general EPS scales
|
Simpson-Angus EPS Rating Scale5
|
Barnes Akathisia Scale6
|
Abnormal Involuntary Movement Scale7 (AIMS)
|
|
Prevalence (with older drugs)
|
Approximately 10%,8 but more common:9
- in young males
- in the neuroleptic-naive
- with high potency drugs (e.g. haloperidol)
Dystonic reactions are rare in the elderly
|
Approximately 20%,10 but more common in:
- elderly females
- those with preexisting neurological damage (head injury, stroke, etc.)
|
Approximately 25%,11 less with SGAs; in decreasing order: aripiprazole, lurasidone, risperidone, olanzapine, quetiapine and clozapine12
|
5% of patients per year of antipsychotic exposure.13 More common in:
- elderly women
- those with affective illness
- those who have had acute EPS early in treatment
Tardive dyskinesia may be associated with neurocognitive deficits14
|
|
Time taken to develop
|
Acute dystonia can occur within hours of starting antipsychotics (minutes if the IM or IV route is used) Tardive dystonia occurs after months to years of antipsychotic treatment
|
Days to weeks after antipsychotic drugs are started or the dose is increased
|
Acute akathisia occurs within hours to weeks of starting antipsychotics or increasing the dose. Tardive akathisia takes longer to develop and can persist after antipsychotics have been withdrawn
|
Months to years Approximately 50% of cases are reversible13,14
|
|
Treatment
|
Anticholinergic drugs given orally, IM or IV depending on the severity of symptoms:9
- remember the patient may be unable to swallow
- response to IV administration will be seen within 5 minutes
- response to IM administration takes around 20 minutes
- tardive dystonia may respond to ECT15
- where symptoms do not respond to simpler measures including switching to an antipsychotic with a low propensity for EPS, botulinuim toxin may be effective16
- rTMS may be helpful17
|
Several options are available depending on the clinical circumstances:
- reduce the antipsychotic dose
- change to an antipsychotic with lower propensity for pseudoparkinsonism (see section on 'Relative adverse effects of antipsychotics' in this chapter) (as antipsychotic monotherapy)
- prescribe an anticholinergic. The majority of patients do not require long-term anticholinergics. Use should be reviewed at least every 3 months. Do not prescribe at night (symptoms usually absent during sleep)
|
Several options are available depending on the clinical circumstances:
- reduce the antipsychotic dose
- change to an antipsychotic drug with lower propensity for akathisia (see section on 'Akathisia and relative adverse effects of antipsychotics')
- a reduction in symptoms may be seen with:18 propranolol 30-80 mg/ day (evidence poor), clonazepam (low dose) 5HT2 antagonists such as: cyproheptadine,15 mirtazapine,18 trazodone,19,20 mianserin,21 and cyproheptadine may help, as may diphenhydramine22
All are unlicenced for this indication Anticholinergics are generally unhelpful23
|
Several options are available depending on the clinical circumstances:
- stop anticholinergic if prescribed
- reduce dose of antipsychotic
- change to an antipsychotic with lower propensity for tardive dyskinesia;24-27 note data are conflicting28,29
- clozapine is the antipsychotic most likely to be associated with resolution of symptoms.30 Quetiapine may also be useful in this regard31
- tetrabenazine and Ginkgo biloba32 have some efficacy as add on treatments. For other treatment options see the review by the American Academy of Neurology33 and the section on 'Tardive dyskinesia' in this chapter
|
|
ECT, electroconvulsive therapy; EPS, extrapyramidal side-effects; IM, intramuscular; IV, intravenous; rTMS, repetitive transcranial magnetic stimulation.
|
EPS are:
- dose-related
- most likely with high doses of high potency FGAs
- less common with other antipsychotics, particularly clozapine, olanzapine, quetiapine and aripiprazole,34 but once present may be persistent.35 Note that CUtLASS reported no difference in EPS between FGAs and SGAs36 (although sulpiride was widely used in the FGA group). Vulnerability to EPS may be genetically determined.37
Beware that in never-medicated patients with first-episode schizophrenia, 1% have dystonia, 8% Parkinsonian symptoms and 11% akathisia.38 Parkinsonian symptoms in such patients are associated with cognitive impairment.39 In never-treated patients with established illness, 9% exhibit spontaneous dyskinesias and 17% Parkinsonian symptoms.40 Patients who experience one type of EPS may be more vulnerable to developing others.41 Substance misuse increases the risk of dystonia, akathisia and tardive dyskinesia.42 Alcohol abuse is associated with akathisia.43
References
- Barnes TRE. The Barnes akathisia scale—revisited. J Psychopharmacol 2003; 17:365–370.
- Gervin M et al. Assessment of drug-related movement disorders in schizophrenia. Adv Psychiatr Treat 2000; 6:332–341.
- Seemuller F et al. Akathisia and suicidal ideation in first-episode schizophrenia. J Clin Psychopharmacol 2012; 32:694–698.
- Leong GB et al. Neuroleptic-induced akathisia and violence: a review. J Forensic Sci 2003; 48:187–189.
- Simpson GM et al. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand 1970; 212:11–19.
- Barnes TRE. A rating scale for drug-induced akathisia. Br J Psychiatry 1989; 154:672–676.
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Washington, DC: US Department of Health, Education, and Welfare 1976:534–537.
- Association AP. Practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry 1997; 154 Suppl 4:1–63.
- van Harten PN et al. Acute dystonia induced by drug treatment. Br Med J 1999; 319:623–626.
- Bollini P et al. Antipsychotic drugs: is more worse? A meta-analysis of the published randomized control trials. Psychol Med 1994; 24:307–316.
- Halstead SM et al. Akathisia: prevalence and associated dysphoria in an in-patient population with chronic schizophrenia. Br J Psychiatry 1994; 164:177–183.
- Hirose S. The causes of underdiagnosing akathisia. Schizophr Bull 2003; 29:547–558.
- Association AP. Tardive dyskinesia: a task force report of the American Psychiatric Association. Hosp Community Psychiatry 1993; 44:190.
- Caroff SN et al. Treatment outcomes of patients with tardive dyskinesia and chronic schizophrenia. J Clin Psychiatry 2011; 72:295–303.
- Miller CH et al. Managing antipsychotic-induced acute and chronic akathisia. Drug Saf 2000; 22:73–81.
- Hennings JM et al. Successful treatment of tardive lingual dystonia with botulinum toxin: case report and review of the literature. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:1167–1171.
- Jankovic J. Treatment of hyperkinetic movement disorders. Lancet Neurol 2009; 8:844–856.
- Poyurovsky M et al. Efficacy of low-dose mirtazapine in neuroleptic-induced akathisia: a double-blind randomized placebo-controlled pilot study. J Clin Psychopharmacol 2003; 23:305–308.
- Stryjer R et al. Treatment of neuroleptic-induced akathisia with the 5-HT2A antagonist trazodone. Clin Neuropharmacol 2003; 26: 137–141.
- Stryjer R et al. Trazodone for the treatment of neuroleptic-induced acute akathisia: a placebo-controlled, double-blind, crossover study. Clin Neuropharmacol 2010; 33:219–222.
- Stryjer R et al. Mianserin for the rapid improvement of chronic akathisia in a schizophrenia patient. Eur Psychiatry 2004; 19:237–238.
- Vinson DR. Diphenhydramine in the treatment of akathisia induced by prochlorperazine. J Emerg Med 2004; 26:265–270.
- Rathbone J et al. Anticholinergics for neuroleptic-induced acute akathisia. The Cochrane database of systematic reviews 2006: CD003727.
- Glazer WM. Expected incidence of tardive dyskinesia associated with atypical antipsychotics. J Clin Psychiatry 2000; 61 Suppl 4:21–26.
- Kinon BJ et al. Olanzapine treatment for tardive dyskinesia in schizophrenia patients: a prospective clinical trial with patients randomized to blinded dose reduction periods. Prog Neuropsychopharmacol Biol Psychiatry 2004; 28:985–996.
- Bai YM et al. Risperidone for severe tardive dyskinesia: a 12-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2003; 64:1342–1348.
- Tenback DE et al. Effects of antipsychotic treatment on tardive dyskinesia: a 6-month evaluation of patients from the European Schizophrenia Outpatient Health Outcomes (SOHO) Study. J Clin Psychiatry 2005; 66:1130–1133.
- Woods SW et al. Incidence of tardive dyskinesia with atypical versus conventional antipsychotic medications: a prospective cohort study. J Clin Psychiatry 2010; 71:463–474.
- Pena MS et al. Tardive dyskinesia and other movement disorders secondary to aripiprazole. Mov Disord 2011; 26:147–152.
- Simpson GM. The treatment of tardive dyskinesia and tardive dystonia. J Clin Psychiatry 2000; 61 Suppl 4:39–44.
- Peritogiannis V et al. Can atypical antipsychotics improve tardive dyskinesia associated with other atypical antipsychotics? Case report and brief review of the literature. J Psychopharmacol 2010; 24:1121–1125.
- Zhang WF et al. Extract of ginkgo biloba treatment for tardive dyskinesia in schizophrenia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2011; 72:615–21.
- Bhidayasiri R et al. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2013; 81:463–469.
- Leucht S et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013; 382:951–962.
- Tenback DE et al. Incidence and persistence of tardive dyskinesia and extrapyramidal symptoms in schizophrenia. J Psychopharmacol 2010; 24:1031–1035.
- Peluso MJ et al. Extrapyramidal motor side-effects of firstand second-generation antipsychotic drugs. Br J Psychiatry 2012; 200:387–392.
- Zivkovic M et al. The association study of polymorphisms in DAT, DRD2, and COMT genes and acute extrapyramidal adverse effects in male schizophrenic patients treated with haloperidol. J Clin Psychopharmacol 2013; 33:593–599.
- Rybakowski JK et al.Extrapyramidal symptoms during treatment of first schizophrenia episode: Results from EUFEST.Eur Neuropsychopharmacol 2014; 24:1500–1505.
- Cuesta MJ et al. Spontaneous Parkinsonism is associated with cognitive impairment in antipsychotic-naive patients with first-episode psychosis: a 6-month follow-up study. Schizophr Bull 2014; 40:1164–1173.
- Pappa S et al. Spontaneous movement disorders in antipsychotic-naive patients with first-episode psychoses: a systematic review. Psychol Med 2009; 39:1065–1076.
- Kim JH et al. Prevalence and characteristics of subjective akathisia, objective akathisia, and mixed akathisia in chronic schizophrenic subjects. Clin Neuropharmacol 2003; 26:312–316.
- Potvin S et al. Substance abuse is associated with increased extrapyramidal symptoms in schizophrenia: a meta-analysis. Schizophr Res 2009; 113:181–188.
- Hansen LK et al. Movement disorders in patients with schizophrenia and a history of substance abuse. Human psychopharmacol 2013; 28:192–197.
Further reading
Dayalu P et al. Antipsychotic-induced extrapyramidal symptoms and their management. Expert Opin Pharmacother 2008; 9:1451–1462.
El Sayeh HG et al. Non-neuroleptic catecholaminergic drugs for neuroleptic-induced tardive dyskinesia. Cochrane Database Syst Rev 2006; CD000458.
Akathisia
Akathisia is a common motor adverse effect of most antipsychotics although some SGAs are less likely to be associated with it. Akathisia is subjectively unpleasant and is fairly strongly linked to the emergence (often sudden) of suicidal ideation.1,2 Figure 2.4 suggests a programme of treatment options for drug-induced akathisia.

Figure 2.4 Treatment options for antipsychotic-induced akathisia.
Notes
- Akathisia is sometimes difficult to diagnose with certainty. A careful history of symptoms, medication and illicit substance use is essential.
- Evaluate efficacy of each treatment option over at least 1 month. Some effect may be seen after a few days but it may take much longer to become apparent in those with chronic akathisia.
- Withdraw previously ineffective akathisia treatments before starting the next option in the algorithm.
- Combinations of treatment may be used in refractory cases if carefully monitored.
- Consider the possibility of tardive akathisia in patients on long-term therapy.
- Other possible treatments for acute akathisia include vitamin B6,15,16 pregabalin,17 diphenhydramine,18 trazodone11,19 and zolmitriptan.20,21 Always read the primary literature before considering any of the options.
- Parenteral midazolam (1.5 mg) has been successfully used to prevent akathisia associated with IV metoclopromide.22
References
- Seemuller F et al. Akathisia and suicidal ideation in first-episode schizophrenia. J Clin Psychopharmacol 2012; 32:694–698.
- Seemuller F et al. The relationship of Akathisia with treatment emergent suicidality among patients with first-episode schizophrenia treated with haloperidol or risperidone. Pharmacopsychiatry 2012; 45:292–296.
- Fleischhacker WW et al. The pharmacologic treatment of neuroleptic-induced akathisia. J Clin Psychopharmacol 1990; 10:12–21.
- Sachdev P. The identification and management of drug-induced akathisia. CNS Drugs 1995; 4:28–46.
- Kumar R et al. Akathisia and second-generation antipsychotic drugs. Curr Opin Psychiatry 2009; 22:293–299.
- Miller DD et al. Extrapyramidal side-effects of antipsychotics in a randomised trial. Br J Psychiatry 2008; 193:279–288.
- Rummel-Kluge C et al. Second-Generation Antipsychotic Drugs and Extrapyramidal Side Effects: A Systematic Review and Meta-analysis of Head-to-Head Comparisons. Schizophr Bull 2012; 38:167–177.
- Kane JM et al. Akathisia: an updated review focusing on second-generation antipsychotics. J Clin Psychiatry 2009; 70:627–643.
- Adler L et al. A controlled assessment of propranolol in the treatment of neuroleptic-induced akathisia. Br J Psychiatry 1986; 149:42–45.
- Fischel T et al. Cyproheptadine versus propranolol for the treatment of acute neuroleptic-induced akathisia: a comparative double-blind study. J Clin Psychopharmacol 2001; 21:612–615.
- Laoutidis ZG et al. 5-HT2A receptor antagonists for the treatment of neuroleptic-induced akathisia: a systematic review and meta-analysis. Int J Neuropsychopharmacol 2014; 17:823–832.
- Poyurovsky M et al. Mirtazapine—a multifunctional drug: low dose for akathisia. CNS Spectr 2011; 16:63.
- Rathbone J et al. Anticholinergics for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev 2006; CD003727.
- Weiss D et al. Cyproheptadine treatment in neuroleptic-induced akathisia. Br J Psychiatry 1995; 167:483–486.
- Miodownik C et al. Vitamin B6 versus mianserin and placebo in acute neuroleptic-induced akathisia: a randomized, double-blind, controlled study. Clin Neuropharmacol 2006; 29:68–72.
- Lerner V et al. Vitamin B6 treatment in acute neuroleptic-induced akathisia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2004; 65:1550–1554.
- De BD et al. Reversal of aripiprazole-induced tardive akathisia by addition of pregabalin. J Neuropsychiatry Clin Neurosci 2013; 25:E9–10.
- Friedman BW et al. A randomized trial of diphenhydramine as prophylaxis against metoclopramide-induced akathisia in nauseated emergency department patients. Ann Emerg Med 2009; 53:379–385.
- Stryjer R et al. Trazodone for the treatment of neuroleptic-induced acute akathisia: a placebo-controlled, double-blind, crossover study. Clin Neuropharmacol 2010; 33:219–222.
- Gross-Isseroff R et al. The 5-HT1D receptor agonist zolmitriptan for neuroleptic-induced akathisia: an open label preliminary study. Int Clin Psychopharmacol 2005; 20:23–25.
- Avital A et al. Zolmitriptan compared to propranolol in the treatment of acute neuroleptic-induced akathisia: A comparative double-blind study. Eur Neuropsychopharmacol 2009; 19:476–482.
- Erdur B et al. A trial of midazolam vs diphenhydramine in prophylaxis of metoclopramide-induced akathisia. Am J Emerg Med 2012; 30:84–91.
Further reading
Poyurovsky M. Acute antipsychotic-induced akathisia revisited. Br J Psychiatry 2010; 196:89–91.
Weight gain
Antipsychotics have long been recognised as weight-inducing agents. Suggested mechanisms include 5HT2C antagonism, H1 antagonism, hyperprolactinaemia and increased serum leptin (leading to leptin desensitisation).1–4 There is no evidence that drugs exert any direct metabolic effect: weight gain seems to result from increased food intake and, in some cases, reduced energy expenditure.5,6 Risk of weight gain appears to be related to clinical response7 (although the association is too small to be clinically important8) and may also have a genetic basis.9,10
All available antipsychotics have been associated with weight gain, although mean weight gained varies substantially between drugs. With all drugs, some patients lose weight, some gain no weight and some gain a great deal of weight. Knowledge of the mean weight gained is often not useful in predicting how much weight an individual might gain. Assessment of relative risk for different drugs is based largely on short term studies. Table 2.17 suggests approximate relative risk of weight gain and the extent of mean weight gain.
See the following section for advice on treating drug-induced weight gain.
Table 2.17 Antipsychotic-induced weight gain11–15
|
Drug
|
Risk/extent of weight gain
|
Clozapine
Olanzapine
|
High
|
Chlorpromazine
Iloperidone
Quetiapine
Risperidone
Paliperidone
|
Moderate
|
Amisulpride
Asenapine
Aripiprazole
Haloperidol
Lurasidone
Sulpiride
Trifluoperazine
Ziprasidone
|
Low
|
References
- Monteleone P et al. Pronounced early increase in circulating leptin predicts a lower weight gain during clozapine treatment. J Clin Psychopharmacol 2002; 22:424–426.
- Herran A et al. Effects of long-term treatment with antipsychotics on serum leptin levels. Br J Psychiatry 2001; 179:59–62.
- McIntyre RS et al. Mechanisms of antipsychotic-induced weight gain. J Clin Psychiatry 2001; 62 Suppl 23:23–29.
- Kroeze WK et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology 2003; 28:519–526.
- Virkkunen M et al. Decrease of energy expenditure causes weight increase in olanzapine treatment—a case study. Pharmacopsychiatry 2002; 35:124–126.
- Sharpe JK et al. Energy expenditure and physical activity in clozapine use: implications for weight management. Aust N Z J Psychiatry 2006; 40:810–814.
- Czobor P et al. Antipsychotic-induced weight gain and therapeutic response: a differential association. J Clin Psychopharmacol 2002; 22:244–251.
- Hermes E et al. The association between weight change and symptom reduction in the CATIE schizophrenia trial. Schizophr Res 2011; 128:166–170.
- Basile VS et al. Genetic dissection of atypical antipsychotic-induced weight gain: novel preliminary data on the pharmacogenetic puzzle. J Clin Psychiatry 2001; 62 Suppl 23:45–66.
- Reynolds GP et al. Polymorphism of the promoter region of the serotonin 5-HT(2C) receptor gene and clozapine-induced weight gain. Am J Psychiatry 2003; 160:677–679.
- Allison D et al. Antipsychotic-induced weight gain: A comprehensive research synthesis. Am J Psychiatry 1999; 156:1686–1696.
- Rummel-Kluge C et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res 2010; 123:225–233.
- Leucht S et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013; 382:951–962.
- Cutler AJ et al. Long-term safety and tolerability of iloperidone: results from a 25-week, open-label extension trial. CNS Spectr 2013; 18:43–54.
- McEvoy JP et al. Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: a randomized clinical trial. JAMA 2014; 311:1978–1987.
Treatment of drug-induced weight gain
Weight gain is an important adverse effect of nearly all antipsychotics with obvious consequences for self-image, morbidity and mortality. Prevention and treatment are therefore matters of clinical urgency.
Monitoring
Patients starting antipsychotic treatment or changing drugs should, as an absolute minimum, be weighed and their weight clearly recorded. Estimates of body mass index and waist circumference should, ideally, also be made at baseline and later at least every 6 months.1 Weekly monitoring of weight is recommended early in treatment, for the first 3 months at least. There is evidence that only a minority patients have anywhere near adequate monitoring of weight.2 Clearly, monitoring of weight parameters is essential to assess the value of preventative and remedial measures.
Treatment and prevention
Most of the relevant literature in this area relates to attempts at reversing antipsychoticrelated weight gain;3 although there are now useful data suggesting that early interventions can prevent or mitigate weight gain.4–6
When weight gain occurs, initial options involve switching drugs or instituting behavioural programmes (or both). Switching always presents a risk of relapse and treatment discontinuation7 but there is fairly strong support for switching to aripiprazole,7–13 ziprasidone14–16 or lurasidone17,18 as a method for reversing weight gain. It is possible that switching to other drugs with a low propensity for weight gain is also beneficial.19,20 Another option is to add aripiprazole to existing treatment—weight loss has been observed when aripiprazole was added to clozapine21–23 and to olanzapine.24 Stopping antipsychotic treatment altogether will reverse weight gain25 but this course of action would not be sensible for the large majority of people with multi-episode schizophrenia. Note that, while some switching and augmentation strategies may minimise further weight gain or facilitate weight loss, the overall effect is generally modest; many patients continue to be overweight. Additional behavioural interventions are often required if BMI is to remain in/move towards the normal range.
A variety of behavioural methods have been proposed and evaluated with fairly good results.26 Methods include calorie restriction,27 low glycaemic index diet,28 Weight Watchers29 and diet/exercise programmes.3,5,6,30–33 A meta-analysis of RCTs showed a robust effect for both prevention and intervention with these methods.34 Pharmacological methods should be considered only where behavioural methods or switching have failed or where obesity presents clear, immediate physical risk to the patient. Some options are described in the table; metformin is now probably considered to be the drug of choice for the prevention and treatment of antipsychotic-induced weight gain. Table 2.18 lists drug treatment options for antipsychotic-induced weight gain (in alphabetical order).
Table 2.18 Drug treatment of antipsychotic-induced weight gain
|
Drug
|
Comments
|
Amantadine35-38
(100-300 mg/day)
|
May attenuate olanzapine-related weight gain. Seems to be well tolerated. May (theoretically, at least) exacerbate psychosis. Weak evidence for benefit.39 Not recommended.
|
Bupropion40,41
(amfebutamone)
|
Seems to be effective in obesity when combined with calorie-restricted diets. Few data of its effects on drug-induced weight gain. Not recommended
|
Fluoxetine42,43
(and other SSRIs)
|
Probably not effective.39 Not recommended
|
H2 antagonists44-48
(e.g. nizatidine 300 mg bd, ranitidine 300 mg bd or famotidine 40 mg/day)
|
Some positive studies but most negative. Effect, if any, is small. Few data supporting a reversal of weight gain.
|
Metformin49,50
(1.5-2.0 g/day)
|
Now a substantial database (in non-diabetic patients) supporting the use of metformin in both reducing and reversing weight gain caused by antipsychotics (mainly olanzapine). Beneficial effects on other metabolic parameters. Some negative studies, but clear and significant effect in meta-analyses.39 Three more positive RCTs published since then.51-53 Ideal for those with weight gain and diabetes or polycystic ovary syndrome. Note that metformin treatment increases the risk of vitamin B12 deficiency54
|
Melatonin55
(3 mg at night)
|
One RCT showing attenuation of olanzapine-induced weight gain.
|
Methylcellulose
(1500 mg ac)
|
Old-fashioned and rather unpalatable preparation. No data in drug-induced weight gain but once fairly widely used. Also acts as a laxative so may be suitable for clozapine-related weight gain
|
Orlistat56-60
(120 mg tds ac/pc)
|
Reliable effect in obesity, especially when combined with calorie restriction. Few published data in drug-induced weight gain but widely used in practice with some success. When used without calorie restriction in psychiatric patients effects are very limited.61,62 Failure to adhere to a low fat diet will result in fatty diarrhoea and possible malabsorbtion of orally administered medication. Good choice for clozapine-induced weight gain where it reduces both weight and the incidence of constipation63
|
Reboxetine64-65
(4-8 mg daily)
|
Attenuates olanzapine-induced weight gain. Reverses some metabolic changes.66 Effective when combined with betahistine67
|
Topiramate68-78
(Up to 300 mg daily)
|
Reliably reduces weight even when drug-induced, but data are mainly observational. Problems may arise because of topiramate's propensity for causing sedation, confusion and cognitive impairment. May be antipsychotic78,79
|
|
Zonisamide80 (150-600 mg/day)
|
Anticonvulsant drug with weight reducing properties. An RCT of 150 mg a day81 showed significant weight reduction in people receiving SGAs
|
|
ac, ante cibum (before meals); bd, bis in die (twice a day); pc, post cibum (after meals); RCT, randomised controlled trial; SGA, second-generation antipsychotic; SSRI, selective serotonin reuptake inhibitor; tds, ter die sumendum (three times a day).
|
References
- Marder SR et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry 2004; 161:1334–1349.
- Paton C et al. Obesity, dyslipidaemias and smoking in an inpatient population treated with antipsychotic drugs. Acta Psychiatr Scand 2004; 110:299–305.
- Kwon JS et al. Weight management program for treatment-emergent weight gain in olanzapine-treated patients with schizophrenia or schizoaffective disorder: A 12-week randomized controlled clinical trial. J Clin Psychiatry 2006; 67:547–553.
- Littrell KH et al. The effects of an educational intervention on antipsychotic-induced weight gain. J Nurs Scholarsh 2003; 35:237–241.
- Mauri M et al. Effects of an educational intervention on weight gain in patients treated with antipsychotics. J Clin Psychopharmacol 2006; 26:462–466.
- Alvarez-Jimenez M et al. Attenuation of antipsychotic-induced weight gain with early behavioral intervention in drug-naive first-episode psychosis patients: A randomized controlled trial. J Clin Psychiatry 2006; 67:1253–1260.
- Stroup TS et al. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: Comparison of Antipsychotics for Metabolic Problems (CAMP). Am J Psychiatry 2011; 168:947–956.
- Casey DE et al. Switching patients to aripiprazole from other antipsychotic agents: a multicenter randomized study. Psychopharmacology (Berl) 2003; 166:391–399.
- De Hert M et al. A case series: evaluation of the metabolic safety of aripiprazole. Schizophr Bull 2007; 33:823–830.
- Lin SK et al. Reversal of antipsychotic-induced hyperprolactinemia, weight gain, and dyslipidemia by aripiprazole: A case report. J Clin Psychiatry 2006; 67:1307.
- Newcomer JW et al. A multicenter, randomized, double-blind study of the effects of aripiprazole in overweight subjects with schizophrenia or schizoaffective disorder switched from olanzapine. J Clin Psychiatry 2008; 69:1046–1056.
- Kim SH et al. Metabolic impact of switching antipsychotic therapy to aripiprazole after weight gain: a pilot study. J Clin Psychopharmacol 2007; 27:365–368.
- Mukundan A et al. Antipsychotic switching for people with schizophrenia who have neuroleptic-induced weight or metabolic problems. The Cochrane database of systematic reviews 2010: CD006629.
- Weiden PJ et al. Improvement in indices of health status in outpatients with schizophrenia switched to ziprasidone. J Clin Psychopharmacol 2003; 23:595–600.
- Montes JM et al. Improvement in antipsychotic-related metabolic disturbances in patients with schizophrenia switched to ziprasidone. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31:383–388.
- Karayal ON et al. Switching from quetiapine to ziprasidone: a sixteen-week, open-label, multicenter study evaluating the effectiveness and safety of ziprasidone in outpatient subjects with schizophrenia or schizoaffective disorder. J Psychiatr Pract 2011; 17:100–109.
- McEvoy JP et al. Effectiveness of lurasidone in patients with schizophrenia or schizoaffective disorder switched from other antipsychotics: a randomized, 6-week, open-label study. J Clin Psychiatry 2013; 74:170–179.
- Stahl SM et al. Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: a 6-month, open-label, extension study. J Clin Psychiatry 2013; 74:507–515.
- Gupta S et al. Weight decline in patients switching from olanzapine to quetiapine. Schizophr Res 2004; 70:57–62.
- Ried LD et al. Weight change after an atypical antipsychotic switch. Ann Pharmacother 2003; 37:1381–1386.
- Englisch S et al. Combined antipsychotic treatment involving clozapine and aripiprazole. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:1386–1392.
- Fleischhacker WW et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol 2010; 13:1115–1125.
- Schorr SG et al. A 12-month follow-up study of treating overweight schizophrenic patients with aripiprazole. Acta Psychiatr Scand 2008; 118:246–250.
- Henderson DC et al. Aripiprazole added to overweight and obese olanzapine-treated schizophrenia patients. J Clin Psychopharmacol 2009; 29:165–169.
- de Kuijper G et al. Effects of controlled discontinuation of long-term used antipsychotics on weight and metabolic parameters in individuals with intellectual disability. J Clin Psychopharmacol 2013; 33:520–524.
- Werneke U et al. Behavioural management of antipsychotic-induced weight gain: a review. Acta Psychiatr Scand 2003; 108:252–259.
- Cohen S et al. Weight gain with risperidone among patients with mental retardation: effect of calorie restriction. J Clin Psychiatry 2001; 62:114–116.
- Smith H et al. Low glycaemic index diet in patients prescribed clozapine: pilot study. Psychiatr Bull 2004; 28:292–294.
- Ball MP et al. A program for treating olanzapine-related weight gain. Psychiatr Serv 2001; 52:967–969.
- Pendlebury J et al. Evaluation of a behavioural weight management programme for patients with severe mental illness: 3 year results. Hum Psychopharmacol 2005; 20:447–448.
- Vreeland B et al. A program for managing weight gain associated with atypical antipsychotics. Psychiatr Serv 2003; 54:1155–1157.
- Ohlsen RI et al. A dedicated nurse-led service for antipsychotic-induced weight gain: An evaluation. Psychiatr Bull 2004; 28:164–166.
- Chen CK et al. Effects of a 10-week weight control program on obese patients with schizophrenia or schizoaffective disorder: a 12-month follow up. Psychiatry Clin Neurosci 2009; 63:17–22.
- Caemmerer J et al. Acute and maintenance effects of non-pharmacologic interventions for antipsychotic associated weight gain and metabolic abnormalities: a meta-analytic comparison of randomized controlled trials. Schizophr Res 2012; 140:159–168.
- Floris M et al. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001; 11:181–182.
- Gracious BL et al. Amantadine treatment of psychotropic-induced weight gain in children and adolescents: case series. J Child Adolesc Psychopharmacol 2002; 12:249–257.
- Bahk WM et al. Open label study of the effect of amantadine on weight gain induced by olanzapine. Psychiatry Clin Neurosci 2004; 58:163–167.
- Deberdt W et al. Amantadine for weight gain associated with olanzapine treatment. Eur Neuropsychopharmacol 2005; 15:13–21.
- Maayan L et al. Effectiveness of medications used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology 2010; 35:1520–1530.
- Gadde KM et al. Bupropion for weight loss: an investigation of efficacy and tolerability in overweight and obese women. Obes Res 2001; 9:544–551.
- Jain, AK et al. Bupropion SR vs. placebo for weight loss in obese patients with depressive symptoms. Obes Res 2002; 10:1049–1056.
- Poyurovsky M et al. Olanzapine-induced weight gain in patients with first-episode schizophrenia: a double-blind, placebo-controlled study of fluoxetine addition. Am J Psychiatry 2002; 159:1058–1060.
- Bustillo JR et al. Treatment of weight gain with fluoxetine in olanzapine-treated schizophrenic outpatients. Neuropsychopharmacology 2003; 28:527–529.
- Cavazzoni P et al. Nizatidine for prevention of weight gain with olanzapine: a double-blind placebo-controlled trial. Eur Neuropsychopharmacol 2003; 13:81–85.
- Pae CU et al. Effect of nizatidine on olanzapine-associated weight gain in schizophrenic patients in Korea: a pilot study. Hum Psychopharmaco 2003; 18:453–456.
- Poyurovsky M et al. The effect of famotidine addition on olanzapine-induced weight gain in first-episode schizophrenia patients: a doubleblind placebo-controlled pilot study. Eur Neuropsychopharmacol 2004; 14:332–336.
- Atmaca M et al. Nizatidine for the treatment of patients with quetiapine-induced weight gain. Hum Psychopharmacol 2004; 19:37–40.
- Ranjbar F et al. The effect of ranitidine on olanzapine-induced weight gain. Biomed Res Int 2013; 2013:639391
- Praharaj SK et al. Metformin for olanzapine-induced weight gain: a systematic review and meta-analysis. Br J Clin Pharmacol 2011; 71:377–382.
- Ehret M et al. The effect of metformin on anthropometrics and insulin resistance in patients receiving atypical antipsychotic agents: a metaanalysis. J Clin Psychiatry 2010; 71:1286–1292.
- Wu RR et al. Metformin for treatment of antipsychotic-induced amenorrhea and weight gain in women with first-episode schizophrenia: a double-blind, randomized, placebo-controlled study. Am J Psychiatry 2012; 169:813–821.
- Jarskog LF et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry 2013; 170:1032–1040.
- Chen CH et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine-treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2013; 74:e424–e430.
- de Jager J et al. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ 2010; 340:c2181
- Modabbernia A et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res 2014; 53:133–140.
- Sjostrom L et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet 1998; 352:167–172.
- Hilger E et al. The effect of orlistat on plasma levels of psychotropic drugs in patients with long-term psychopharmacotherapy. J Clin Psychopharmacol 2002; 22:68–70.
- Werneke U et al. Options for pharmacological management of obesity in patients treated with atypical antipsychotics. Int Clin Psychopharmacol 2002; 17:145–160.
- Pavlovic ZM. Orlistat in the treatment of clozapine-induced hyperglycemia and weight gain. Eur Psychiatry 2005; 20:520
- Carpenter LL et al. A case series describing orlistat use in patients on psychotropic medications. Med Health R I 2004; 87:375–377.
- Joffe G et al. Orlistat in clozapineor olanzapine-treated patients with overweight or obesity: a 16-week randomized, double-blind, placebocontrolled trial. J Clin Psychiatry 2008; 69:706–711.
- Tchoukhine E et al. Orlistat in clozapineor olanzapine-treated patients with overweight or obesity: a 16-week open-label extension phase and both phases of a randomized controlled trial. J Clin Psychiatry 2011; 72:326–330.
- Chukhin E et al. In a randomized placebo-controlled add-on study orlistat significantly reduced clozapine-induced constipation. Int Clin Psychopharmacol 2013; 28:67–70.
- Poyurovsky M et al. Attenuation of olanzapine-induced weight gain with reboxetine in patients with schizophrenia: a double-blind, placebocontrolled study. Am J Psychiatry 2003; 160:297–302.
- Poyurovsky M et al. Attenuating effect of reboxetine on appetite and weight gain in olanzapine-treated schizophrenia patients: a double-blind placebo-controlled study. Psychopharmacology (Berl) 2007; 192:441–448.
- Amrami-Weizman A et al. The effect of reboxetine co-administration with olanzapine on metabolic and endocrine profile in schizophrenia patients. Psychopharmacology (Berl) 2013; 230:23–27.
- Poyurovsky M et al. Reducing antipsychotic-induced weight gain in schizophrenia: a double-blind placebo-controlled study of reboxetine-betahistine combination. Psychopharmacology (Berl) 2013; 226:615–622.
- Binkley K et al. Sibutramine and panic attacks. Am J Psychiatry 2002; 159:1793–1794.
- Taflinski T et al. Sibutramine-associated psychotic episode. Am J Psychiatry 2000; 157:2057–2058.
- Dursun SM et al. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000; 45:198
- Levy E et al. Topiramate produced weight loss following olanzapine-induced weight gain in schizophrenia. J Clin Psychiatry 2002; 63:1045
- Van Ameringen M et al. Topiramate treatment for SSRI-induced weight gain in anxiety disorders. J Clin Psychiatry 2002; 63:981–984.
- Appolinario JC et al. Topiramate use in obese patients with binge eating disorder: an open study. Can J Psychiatry 2002; 47:271–273.
- Chengappa KN et al. Changes in body weight and body mass index among psychiatric patients receiving lithium, valproate, or topiramate: an open-label, nonrandomized chart review. Clin Ther 2002; 24:1576–1584.
- Pavuluri MN et al. Topiramate plus risperidone for controlling weight gain and symptoms in preschool mania. J Child Adolesc Psychopharmacol 2002; 12:271–273.
- Cates ME et al. Efficacy of add-on topiramate therapy in psychiatric patients with weight gain. Ann Pharmacother 2008; 42:505–510.
- Egger C et al. Influence of topiramate on olanzapine-related weight gain in women: an 18-month follow-up observation. J Clin Psychopharmacol 2007; 27:475–478 .
- Hahn MK et al. Topiramate augmentation in clozapine-treated patients with schizophrenia: clinical and metabolic effects. J Clin Psychopharmacol 2010; 30:706–710.
- Afshar H et al. Topiramate add-on treatment in schizophrenia: a randomised, double-blind, placebo-controlled clinical trial. J Psychopharmacol 2009; 23:157–162.
- Gadde KM et al. Zonisamide for weight loss in obese adults: a randomized controlled trial. JAMA 2003; 289:1820–1825.
- Ghanizadeh A et al. The effect of zonisamide on antipsychotic-associated weight gain in patients with schizophrenia: a randomized, doubleblind, placebo-controlled clinical trial. Schizophr Res 2013; 147:110–115.
Tardive dyskinesia
Tardive dyskinesia (TD) is now a somewhat less commonly encountered problem than in previous decades,1 probably because of the introduction and widespread use of SGAs.2–5 Treatment of established TD is often unsuccessful, so prevention, early detection and early treatment are essential. TD is associated with greater cognitive impairment,6 more severe psychopathology7 and higher mortality.8
There is fairly good evidence that SGAs are less likely to cause TD9–13 although TD certainly does occur with these drugs albeit at quite different rates.14–18 The observation that SGAs produce less TD than typical drugs is consistent with the long-held belief that early acute movement disorders and akathisia predict later TD.19–21 Note, also, that TD can occur after minuscule doses of conventional drugs (and in the absence of portentous acute movement disorder22) and following the use of other dopamine antagonists such as metoclopramide.23 It can also occur in never-medicated patients with both firstepisode24 and established25 schizophrenia. FGA depot treatments may be particularly likely to bring about TD.18 Risk of TD may be related to the extent of D2 receptor occupancy (higher occupancy, higher risk).26 It follows that the lower doses of FGAs that are now becoming routine in clinical practice may be associated with a lower risk of TD, perhaps approaching that seen with SGAs, but this has not yet been systematically explored.
Treatment—first steps
Most authorities recommend the withdrawal of any anticholinergic drugs and a reduction in the dose of antipsychotic as initial steps in those with early signs of TD27,28 (dose reduction may initially worsen TD). Cochrane, however found little support for this approach29 and the American Academy of Neurology does not recommend it.30 It has now become common practice to withdraw the antipsychotic prescribed when TD was first observed and to substitute another drug. The use of clozapine27 is probably best supported in this regard, but quetiapine, another weak striatal dopamine antagonist, is also effective.31–37 Olanzapine and aripiprazole are also options.38,39,39–42 There are a few supporting data for risperidone40 but this might not be considered a logical choice in a patient with established TD, given that risperidone is more likely than clozapine, olanzapine and quetiapine to be associated with movement disorders in its own right. Again, the evidence for benefit in switching to particular SGAs is considered weak.30
Treatment—additional agents
Switching or withdrawing antipsychotics is not always effective or advisable and so additional agents are often used. Table 2.19 below describes the most frequently prescribed add-on drugs for TD, in order of preference.
Treatment—other possible options
The large number of proposed treatments for TD undoubtedly reflects the somewhat limited effectiveness of standard remedies. Table 2.20 lists some of these putative treatments in alphabetical order.
Table 2.19 Most frequently prescribed additional drugs for the treatment of tardive dyskinesia
|
Drug
|
Comments
|
|
Tetrabenazine41,42
|
Only licensed treatment for TD in UK. Has antipsychotic properties but reported to be depressogenic. Drowsiness, parkinsonism and akathisia also occur.43,44 Dose is 25-200 mg/day. Reserpine (similar mode of action) also effective but rarely, if ever, used
|
|
Benzodiazepines27,28
|
Widely used and considered effective but Cochrane review suggests benzodiazepines are 'experimental'.45 Intermittant use may be necessary to avoid tolerance to effects. Most used are clonazepam 1-4 mg/day and diazepam 6-25 mg/day. Better supporting evidence for clonazepam30,44
|
|
Vitamin E46,47
|
Numerous studies but efficacy remains to be conclusively established. Cochrane suggest there evidence only for slowing deterioration of TD.48 Dose is in the range 400–600 lU/day
|
|
Ginkgo biloba49
|
One good RCT showing significant benefit over placebo. Well tolerated
|
|
Propranolol50,51
|
Open label studies only but formerly a widely used treatment. Dose is 40-120 mg/day. Beware contraindications (asthma, bradycardia, hypotension)
|
|
IU, international units; RCT, randomised controlled trial; TD, tardive dyskinesia.
|
Table 2.20 Less commonly prescribed additional drugs for the treatment of tardive dyskinesia
|
Drug
|
Comments
|
|
Amantadine52,53
|
Rarely used but apparently effective at 100-300 mg a day
|
|
Amino acids54
|
Use is supported by a small randomised, placebo-controlled trial. Low risk of toxicity
|
|
Botulinum toxin55-58
|
Case reports of success for localised dyskinesia. Probably now treatment of choice for disabling or distressing focal symptoms
|
|
Calcium antagonists59
|
A few published studies but not widely used. Cochrane is dismissive
|
|
Donepezil60-62
|
Supported by a single open study and case series. One negative RCT (n=12). Dose is 10mg/day
|
|
Fish oils63,64
|
Very limited support for use of EPA at dose of 2 g/day
|
|
Fluvoxamine65
|
Three case reports. Dose is 100 mg/day. Beware interactions
|
|
Gabapentin66
|
Adds weight to theory that GABAergic mechansims improve TD. Dose is 900-1200 mg/day
|
|
Levetiracetam67-70
|
Three published case studies. One RCT. Dose up to 3000 mg/day
|
|
Melatonin71
|
Use is supported by a well-conducted trial. Usually well tolerated. Dose is 10 mg/day. Some evidence that melatonin receptor genotype determines risk of TD72
|
|
Naltrexone73
|
May be effective when added to benzodiazepines. Well tolerated. Dose is 200 mg/day
|
|
Ondansetron74,75
|
Limited evidence but low toxicity. Dose is up to 12 mg/day
|
|
Pyridoxine76
|
Supported by a well conducted trial. Dose is up to 400 mg/day
|
|
Quercetin77
|
Plant compound which is thought to be an antioxidant. No human studies in TD but widely used in other conditions
|
|
Sodium oxybate78
|
One case report. Dose was 8 g/day
|
|
Transcranial magnetic stimulation79 (rTMS)
|
Single case report
|
|
Zolpidem80
|
Three case reports. Dose 10-30 mg a day
|
|
EPA, eicosapentanoic acid; GABA, gamma-aminobutyric acid; RCT, randomised controlled trial; TD, tardive dyskinesia.
|
References
- Merrill RM et al. Tardive and spontaneous dyskinesia incidence in the general population. BMC Psychiatry 2013; 13:152.
- de Leon J. The effect of atypical versus typical antipsychotics on tardive dyskinesia : A naturalistic study. Eur Arch Psychiatry Clin Neurosci 2007; 257:169–172.
- Halliday J et al. Nithsdale Schizophrenia Surveys 23: movement disorders. 20-year review. Br J Psychiatry 2002; 181:422–427.
- Kane JM. Tardive dyskinesia circa 2006. Am J Psychiatry 2006; 163:1316–1318.
- Eberhard J et al. Tardive dyskinesia and antipsychotics: a 5-year longitudinal study of frequency, correlates and course. Int Clin Psychopharmacol 2006; 21:35–42.
- Wu JQ et al. Tardive dyskinesia is associated with greater cognitive impairment in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2013; 46:71–77.
- Ascher-Svanum H et al. Tardive dyskinesia and the 3-year course of schizophrenia: results from a large, prospective, naturalistic study. J Clin Psychiatry 2008; 69:1580–1588.
- Chong SA et al. Mortality rates among patients with schizophrenia and tardive dyskinesia. J Clin Psychopharmacol 2009; 29:5–8.
- Beasley C et al. Randomised double-blind comparison of the incidence of tardive dyskinesia in patients with schizophrenia during long-term treatment with olanzapine or haloperidol. Br J Psychiatry 1999; 174:23–30.
- Glazer WM. Expected incidence of tardive dyskinesia associated with atypical antipsychotics. J Clin Psychiatry 2000; 61 Suppl 4:21–26.
- Correll CU et al. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry 2004; 161:414–425.
- Dolder CR et al. Incidence of tardive dyskinesia with typical versus atypical antipsychotics in very high risk patients. Biol Psychiatry 2003; 53:1142–1145.
- Correll CU et al. Tardive dyskinesia and new antipsychotics. Curr Opin Psychiatry 2008; 21:151–156.
- Gafoor R et al. Three case reports of emergent dyskinesia with clozapine. Eur Psychiatry 2003; 18:260–261.
- Keck ME et al. Ziprasidone-related tardive dyskinesia (Letter). Am J Psychiatry 2004; 161:175–176.
- Maytal G et al. Aripiprazole-related tardive dyskinesia. CNS Spectr 2006; 11:435–439.
- Fountoulakis KN et al. Amisulpride-induced tardive dyskinesia. Schizophr Res 2006; 88:232–234.
- Novick D et al. Incidence of extrapyramidal symptoms and tardive dyskinesia in schizophrenia: thirty-six-month results from the European schizophrenia outpatient health outcomes study. J Clin Psychopharmacol 2010; 30:531–540.
- Sachdev P. Early extrapyramidal side-effects as risk factors for later tardive dyskinesia: a prospective study. Aust N Z J Psychiatry 2004; 38:445–449.
- Miller DD et al. Clinical correlates of tardive dyskinesia in schizophrenia: baseline data from the CATIE schizophrenia trial. Schizophr Res 2005; 80:33–43.
- Tenback DE et al. Evidence that early extrapyramidal symptoms predict later tardive dyskinesia: a prospective analysis of 10,000 patients in the European Schizophrenia Outpatient Health Outcomes (SOHO) Study. Am J Psychiatry 2006; 163:1438–1440.
- Oosthuizen PP et al. Incidence of tardive dyskinesia in first-episode psychosis patients treated with low-dose haloperidol. J Clin Psychiatry 2003; 64:1075–1080.
- Kenney C et al. Metoclopramide, an increasingly recognized cause of tardive dyskinesia. J Clin Pharmacol 2008; 48:379–384.
- Puri BK et al. Spontaneous dyskinesia in first episode schizophrenia. J Neurol Neurosurg Psychiatry 1999; 66:76–78.
- McCreadie RG et al. Spontaneous dyskinesia and parkinsonism in never-medicated, chronically ill patients with schizophrenia: 18-month follow-up. Br J Psychiatry 2002; 181:135–137.
- Yoshida K et al. Tardive dyskinesia in relation to estimated dopamine D2 receptor occupancy in patients with schizophrenia: analysis of the CATIE data. Schizophr Res 2014; 153:184–188.
- Duncan D et al. Tardive dyskinesia: how is it prevented and treated? Psychiatric Bulletin 1997; 21:422–425.
- Simpson GM. The treatment of tardive dyskinesia and tardive dystonia. J Clin Psychiatry 2000; 61 Suppl 4:39–44.
- Soares-Weiser K et al. Neuroleptic reduction and/or cessation and neuroleptics as specific treatments for tardive dyskinesia. The Cochrane database of systematic reviews 2006: CD000459.
- Bhidayasiri R et al. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2013; 81:463–469.
- Vesely C et al. Remission of severe tardive dyskinesia in a schizophrenic patient treated with the atypical antipsychotic substance quetiapine. Int Clin Psychopharmacol 2000; 15:57–60.
- Alptekin K et al. Quetiapine-induced improvement of tardive dyskinesia in three patients with schizophrenia. Int Clin Psychopharmacol 2002; 17:263–264.
- Nelson MW et al. Adjunctive quetiapine decreases symptoms of tardive dyskinesia in a patient taking risperidone. Clin Neuropharmacol 2003; 26:297–298.
- Emsley R et al. A single-blind, randomized trial comparing quetiapine and haloperidol in the treatment of tardive dyskinesia. J Clin Psychiatry 2004; 65:696–701.
- Bressan RA et al. Atypical antipsychotic drugs and tardive dyskinesia: relevance of D2 receptor affinity. J Psychopharmacol 2004; 18:124–127.
- Sacchetti E et al. Quetiapine, clozapine, and olanzapine in the treatment of tardive dyskinesia induced by first-generation antipsychotics: a 124-week case report. Int Clin Psychopharmacol 2003; 18:357–359.
- Gourzis P et al. Quetiapine in the treatment of focal tardive dystonia induced by other atypical antipsychotics: a report of 2 cases. Clin Neuropharmacol 2005; 28:195–196.
- Soutullo CA et al. Olanzapine in the treatment of tardive dyskinesia: a report of two cases. J Clin Psychopharmacol 1999; 19:100–101.
- Kinon BJ et al. Olanzapine treatment for tardive dyskinesia in schizophrenia patients: a prospective clinical trial with patients randomized to blinded dose reduction periods. Prog Neuropsychopharmacol Biol Psychiatry 2004; 28:985–996.
- Bai YM et al. Risperidone for severe tardive dyskinesia: a 12-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2003; 64:1342–1348.
- Jankovic J et al. Long-term effects of tetrabenazine in hyperkinetic movement disorders. Neurology 1997; 48:358–362.
- Leung JG et al. Tetrabenazine for the treatment of tardive dyskinesia. Ann Pharmacother 2011; 45:525–531.
- Kenney C et al. Long-term tolerability of tetrabenazine in the treatment of hyperkinetic movement disorders. Mov Disord 2007; 22:193–197.
- Rana AQ et al. New and emerging treatments for symptomatic tardive dyskinesia. Drug Des Devel Ther 2013; 7:1329–1340.
- Bhoopathi PS et al. Benzodiazepines for neuroleptic-induced tardive dyskinesia. The Cochrane database of systematic reviews 2006; 3: CD000205.
- Adler LA et al. Vitamin E treatment for tardive dyskinesia. Arch Gen Psychiatry 1999; 56:836–841.
- Zhang XY et al. The effect of vitamin E treatment on tardive dyskinesia and blood superoxide dismutase: a double-blind placebo-controlled trial. J Clin Psychopharmacol 2004; 24:83–86.
- Soares-Weiser K et al. Vitamin E for neuroleptic-induced tardive dyskinesia. The Cochrane database of systematic reviews 2011: CD000209.
- Zhang WF et al. Extract of Ginkgo biloba treatment for tardive dyskinesia in schizophrenia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2011; 72:615–621.
- Perenyi A et al. Propranolol in the treatment of tardive dyskinesia. Biol Psychiatry 1983; 18:391–394.
- Pitts FN, Jr. Treatment of tardive dyskinesia with propranolol. J Clin Psychiatry 1982; 43:304.
- Angus S et al. A controlled trial of amantadine hydrochloride and neuroleptics in the treatment of tardive dyskinesia. J Clin Psychopharmacol 1997; 17:88–91.
- Pappa S et al. Effects of amantadine on tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol 2010; 33:271–275.
- Richardson MA et al. Efficacy of the branched-chain amino acids in the treatment of tardive dyskinesia in men. Am J Psychiatry 2003; 160:1117–1124.
- Tarsy D et al. An open-label study of botulinum toxin A for treatment of tardive dystonia. Clin Neuropharmacol 1997; 20:90–93.
- Brashear A et al. Comparison of treatment of tardive dystonia and idiopathic cervical dystonia with botulinum toxin type A. Mov Disord 1998; 13:158–161.
- Hennings JM et al. Successful treatment of tardive lingual dystonia with botulinum toxin: case report and review of the literature. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:1167–1171.
- Beckmann YY et al. Treatment of intractable tardive lingual dyskinesia with botulinum toxin. J Clin Psychopharmacol 2011; 31:250–251.
- Essali A et al. Calcium channel blockers for neuroleptic-induced tardive dyskinesia. The Cochrane database of systematic reviews 2011: CD000206.
- Caroff SN et al. Treatment of tardive dyskinesia with donepezil. J Clin Psychiatry 2001; 62:128–129.
- Bergman J et al. Beneficial effect of donepezil in the treatment of elderly patients with tardive movement disorders. J Clin Psychiatry 2005; 66:107–110.
- Ogunmefun A et al. Effect of donepezil on tardive dyskinesia. J Clin Psychopharmacol 2009; 29:102–104.
- Emsley R et al. The effects of eicosapentaenoic acid in tardive dyskinesia: a randomized, placebo-controlled trial. Schizophr Res 2006; 84:112–120.
- Vaddadi K et al. Tardive dyskinesia and essential fatty acids. Int Rev Psychiatry 2006; 18:133–143.
- Albayrak Y et al. Benefical effects of sigma-1 agonist fluvoxamine for tardive dyskinesia and tardive akathisia in patients with schizophrenia: report of three cases. Psychiatry Investig 2013; 10:417–420.
- Hardoy MC et al. Gabapentin in antipsychotic-induced tardive dyskinesia: results of 1-year follow-up. J Affect Disord 2003; 75:125–130.
- McGavin CL et al. Levetiracetam as a treatment for tardive dyskinesia: a case report. Neurology 2003; 61:419.
- Meco G et al. Levetiracetam in tardive dyskinesia. Clin Neuropharmacol 2006; 29:265–268.
- Woods SW et al. Effects of levetiracetam on tardive dyskinesia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2008; 69:546–554.
- Chen PH et al. Rapid improvement of neuroleptic-induced tardive dyskinesia with levetiracetam in an interictal psychotic patient. J Clin Psychopharmacol 2010; 30:205–207.
- Shamir E et al. Melatonin treatment for tardive dyskinesia: a double-blind, placebo-controlled, crossover study. Arch Gen Psychiatry 2001; 58:1049–1052.
- Lai IC et al. Analysis of genetic variations in the human melatonin receptor (MTNR1A, MTNR1B) genes and antipsychotics-induced tardive dyskinesia in schizophrenia. World J Biol Psychiatry 2011; 12:143–148.
- Wonodi I et al. Naltrexone treatment of tardive dyskinesia in patients with schizophrenia. J Clin Psychopharmacol 2004; 24:441–445.
- Sirota P et al. Use of the selective serotonin 3 receptor antagonist ondansetron in the treatment of neuroleptic-induced tardive dyskinesia. Am J Psychiatry 2000; 157:287–289.
- Naidu PS et al. Reversal of neuroleptic-induced orofacial dyskinesia by 5-HT3 receptor antagonists. Eur J Pharmacol 2001; 420:113–117.
- Lerner V et al. Vitamin B(6) in the treatment of tardive dyskinesia: a double-blind, placebo-controlled, crossover study. Am J Psychiatry 2001; 158:1511–1514.
- Naidu PS et al. Reversal of haloperidol-induced orofacial dyskinesia by quercetin, a bioflavonoid. Psychopharmacology (Berl) 2003; 167:418–423.
- Berner JE. A case of sodium oxybate treatment of tardive dyskinesia and bipolar disorder. J Clin Psychiatry 2008; 69:862.
- Brambilla P et al. Transient improvement of tardive dyskinesia induced with rTMS. Neurology 2003; 61:1155.
- Waln O et al. Zolpidem improves tardive dyskinesia and akathisia. Mov Disord 2013; 28:1748–1749.
Further reading
Aia PG et al. Tardive dyskinesia. Curr Treat Options Neurol 2011; 13:231–241.
Paleacu D et al. Tetrabenazine treatment in movement disorders. Clin Neuropharmacol 2004; 27: 230–233.
Neuroleptic malignant syndrome
Neuroleptic malignant syndrome (NMS) is a rare, but potentially serious or even fatal, adverse effect of all antipsychotics. It is a syndrome essentially of muscular rigidity and sympathetic hyperactivity occurring as a result of dopaminergic antagonism in the context of psychological stressors and genetic predisposition.1 Although widely seen as an acute, severe syndrome, NMS may, in many cases, have few signs and symptoms; 'full-blown' NMS may thus represent the extreme of a range of non-malignant related symptoms.2 Certainly, asymptomatic rises in plasma creatine kinase (CK) are fairly common.3
The incidence and mortality rate of NMS are difficult to establish and probably vary as drug use changes and recognition of NMS increases. It has been estimated that fewer than 1% of all patients treated with conventional antipsychotics will experience NMS.4 Incidence figures for SGA drugs are not available, but all have been reported to be associated with the syndrome,5–12 even newer drugs like ziprasidone,13,14 iloperidone,15 aripiprazole,34–37 paliperidone,38 asenapine39 and risperidone injection.40 Mortality is probably lower with SGAs,41–43 but symptoms are the same as those seen with FGAs44 except that rigidity is less common.42 NMS is also sometimes seen with other drugs such as antidepressants45–48 and lithium.49 Combinations of antipsychotics with SSRIs50 or cholinesterase inhibitors51,52 may increase the risk of NMS. NMS-type syndromes induced by SGA/SSRI combinations may share their symptoms and pathogenesis with serotonin syndrome.53 The use of benzodiazepines has been linked to an important increase in the risk of NMS.22,23
Table 2.21 Diagnosis and management of neuroleptic malignant syndrome
Signs and symptoms1,4,16,17
(presentation varies considerably)18
|
Fever, diaphoresis, rigidity, confusion, fluctuating consciousness
Fluctuating blood pressure, tachycardia
Elevated creatine kinase, leukocytosis, altered liver function tests
|
|
Risk factors16,17,19-23
|
High potency typical drugs, recent or rapid dose increase, rapid dose reduction, abrupt withdrawal of anticholinergics, antipsychotic polypharmacy
Psychosis, organic brain disease, alcoholism, Parkinson's disease, hyperthyroidism, psychomotor agitation, mental retardation
Agitation, dehydration
|
|
Treatments4,16,24-27
|
In the psychiatric unit:
Withdraw antipsychotics, monitor temperature, pulse, blood pressure. Consider benzodiazepines if not already prescribed—IM lorazepam has been used28
In the medical/A&E unit:
Rehydration, bromocriptine + dantrolene, sedation with benzodiazepines, artificial ventilation if required
L-dopa, apomorphine, and carbamazepine have also been used, among many other drugs. Consider ECT for treatment of psychosis
|
|
Restarting antipsychotics16,24,29
|
Antipsychotic treatment will be required in most instances and re-challenge is associated with acceptable risk
Stop antipsychotics for at least 5 days, preferably longer. Allow time for symptoms and signs of NMS to resolve completely
Begin with very small dose and increase very slowly with close monitoring of temperature-pulse and blood pressure. Creatine kinase monitoring may be used, but is controversial.17-30 Close monitoring of physical and biochemical parameters is effective in reducing progression to 'full-blown' NMS31-32
Consider using an antipsychotic structurally unrelated to that previously associated with NMS-or a drug with low dopamine affinity (quetiapine or clozapine). Aripiprazole may also be considered33 but it has a long plasma half-life and has been linked to an increased risk of NMS22 Avoid depots (of any kind) and high potency conventional antipsychotics
|
|
A&E, accident and emergency; ECT, electroconvulsive therapy; IM, intramuscular; NMS, neuroleptic malignant syndrome.
|
The characteristics of NMS and its management are summarised in Table 2.21.
References
- Gurrera RJ. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry 1999; 156:169–180.
- Bristow MF et al. How "malignant" is the neuroleptic malignant syndrome? BMJ 1993; 307:1223–1224.
- Meltzer HY et al. Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment. Neuropsychopharmacology 1996; 15:395–405.
- Guze BH et al. Current concepts. Neuroleptic malignant syndrome. N Engl J Med 1985; 313:163–166.
- Sing KJ et al. Neuroleptic malignant syndrome and quetiapine (Letter). Am J Psychiatry 2002; 159:149–150.
- Suh H et al. Neuroleptic malignant syndrome and low-dose olanzapine (Letter). Am J Psychiatry 2003; 160:796.
- Gallarda T et al. Neuroleptic malignant syndrome in an 72-year-old-man with Alzheimer's disease: A case report and review of the literature. Eur Neuropsychopharmacol 2000; 10 (Suppl 3):357.
- Stanley AK et al. Possible neuroleptic malignant syndrome with quetiapine. Br J Psychiatry 2000; 176:497.
- Sierra-Biddle D et al. Neuropletic malignant syndrome and olanzapine. J Clin Psychopharmacol 2000; 20:704–705.
- Hasan S et al. Novel antipsychotics and the neuroleptic malignant syndrome: a review and critique. Am J Psychiatry 1998; 155:1113–1116.
- Tsai JH et al. Zotepine-induced catatonia as a precursor in the progression to neuroleptic malignant syndrome. Pharmacotherapy 2005; 25:1156–1159.
- Gortney JS et al. Neuroleptic malignant syndrome secondary to quetiapine. Ann Pharmacother 2009; 43:785–791.
- Leibold J et al. Neuroleptic malignant syndrome associated with ziprasidone in an adolescent. Clin Ther 2004; 26:1105–1108.
- Borovicka MC et al. Ziprasidoneand lithium-induced neuroleptic malignant syndrome. Ann Pharmacother 2006; 40:139–142.
- Guanci N et al. Atypical neuroleptic malignant syndrome associated with iloperidone administration. Psychosomatics 2012; 53:603–605.
- Levenson JL. Neuroleptic malignant syndrome. Am J Psychiatry 1985; 142:1137–1145.
- Hermesh H et al. High serum creatinine kinase level: possible risk factor for neuroleptic malignant syndrome. J Clin Psychopharmacol 2002; 22:252–256.
- Picard LS et al. Atypical neuroleptic malignant syndrome: diagnostic controversies and considerations. Pharmacotherapy 2008; 28:530–535.
- Viejo LF et al. Risk factors in neuroleptic malignant syndrome. A case-control study. Acta Psychiatr Scand 2003; 107:45–49.
- Spivak B et al. Neuroleptic malignant syndrome during abrupt reduction of neuroleptic treatment. Acta Psychiatr Scand 1990; 81:168–169.
- Spivak B et al. Neuroleptic malignant syndrome associated with abrupt withdrawal of anticholinergic agents. Int Clin Psychopharmacol 1996; 11:207–209.
- Su YP et al. Retrospective chart review on exposure to psychotropic medications associated with neuroleptic malignant syndrome. Acta Psychiatr Scand 2014; 130:52–60.
- Nielsen RE et al. Neuroleptic malignant syndrome-an 11-year longitudinal case-control study. Can J Psychiatry 2012; 57:512–518.
- Olmsted TR. Neuroleptic malignant syndrome: guidelines for treatment and reinstitution of neuroleptics. South Med J 1988; 81:888–891.
- Shoop SA et al. Carbidopa/levodopa in the treatment of neuroleptic malignant syndrome (Letter). Ann Pharmacother 1997; 31:119.
- Terao T. Carbamazepine in the treatment of neuroleptic malignant syndrome (Letter). Biol Psychiatry 1999; 45:381–382.
- Lattanzi L et al. Subcutaneous apomorphine for neuroleptic malignant syndrome. Am J Psychiatry 2006; 163:1450–1451.
- Francis A et al. Is lorazepam a treatment for neuroleptic malignant syndrome? CNS Spectr 2000; 5:54–57.
- Wells AJ et al. Neuroleptic rechallenge after neuroleptic malignant syndrome: case report and literature review. Drug Intell Clin Pharm 1988; 22:475–480.
- Klein JP et al. Massive creatine kinase elevations with quetiapine: report of two cases. Pharmacopsychiatry 2006; 39:39–40.
- Shiloh R et al. Precautionary measures reduce risk of definite neuroleptic malignant syndrome in newly typical neuroleptic-treated schizophrenia inpatients. Int Clin Psychopharmacol 2003; 18:147–149.
- Hatch CD et al. Failed challenge with quetiapine after neuroleptic malignant syndrome with conventional antipsychotics. Pharmacotherapy 2001; 21:1003–1006.
- Trutia A et al. Neuroleptic rechallenge with aripiprazole in a patient with previously documented neuroleptic malignant syndrome. J Psychiatr Pract 2008; 14:398–402.
- Spalding S et al. Aripiprazole and atypical neuroleptic malignant syndrome. J Am Acad Child Adolesc Psychiatry 2004; 43:1457–1458.
- Chakraborty N et al. Aripiprazole and neuroleptic malignant syndrome. Int Clin Psychopharmacol 2004; 19:351–353.
- Rodriguez OP et al. A case report of neuroleptic malignant syndrome without fever in a patient given aripiprazole. J Okla State Med Assoc 2006; 99:435–438.
- Srephichit S et al. Neuroleptic malignant syndrome and aripiprazole in an antipsychotic-naive patient. J Clin Psychopharmacol 2006; 26:94–95.
- Duggal HS. Possible neuroleptic malignant syndrome associated with paliperidone. J Neuropsychiatry Clin Neurosci 2007; 19:477–478.
- Singh N et al. Neuroleptic malignant syndrome after exposure to asenapine: a case report. Prim Care Companion J Clin Psychiatry 2010; 12:e1.
- Mall GD et al. Catatonia and mild neuroleptic malignant syndrome after initiation of long-acting injectable risperidone: case report. J Clin Psychopharmacol 2008; 28:572–573.
- Ananth J et al. Neuroleptic malignant syndrome and atypical antipsychotic drugs. J Clin Psychiatry 2004; 65:464–470.
- Trollor JN et al. Comparison of neuroleptic malignant syndrome induced by firstand second-generation antipsychotics. Br J Psychiatry 2012; 201:52–56.
- Nakamura M et al. Mortality of neuroleptic malignant syndrome induced by typical and atypical antipsychotic drugs: a propensity-matched analysis from the Japanese Diagnosis Procedure Combination database. J Clin Psychiatry 2012; 73:427–430.
- Trollor JN et al. Neuroleptic malignant syndrome associated with atypical antipsychotic drugs. CNS Drugs 2009; 23:477–492.
- Kontaxakis VP et al. Neuroleptic malignant syndrome after addition of paroxetine to olanzapine. J Clin Psychopharmacol 2003; 23:671–672.
- Young C. A case of neuroleptic malignant syndrome and serotonin disturbance. J Clin Psychopharmacol 1997; 17:65–66.
- June R et al. Neuroleptic malignant syndrome associated with nortriptyline. Am J Emerg Med 1999; 17:736–737.
- Lu TC et al. Neuroleptic malignant syndrome after the use of venlafaxine in a patient with generalized anxiety disorder. J Formos Med Assoc 2006; 105:90–93.
- Gill J et al. Acute lithium intoxication and neuroleptic malignant syndrome. Pharmacotherapy 2003; 23:811–815.
- Stevens DL. Association between selective serotonin-reuptake inhibitors, second-generation antipsychotics, and neuroleptic malignant syndrome. Ann Pharmacother 2008; 42:1290–1297.
- Stevens DL et al. Olanzapine-associated neuroleptic malignant syndrome in a patient receiving concomitant rivastigmine therapy. Pharmacotherapy 2008; 28:403–405.
- Warwick TC et al. Neuroleptic malignant syndrome variant in a patient receiving donepezil and olanzapine. Nat Clin Pract Neurol 2008; 4:170–174.
- Odagaki Y. Atypical neuroleptic malignant syndrome or serotonin toxicity associated with atypical antipsychotics? Curr Drug Saf 2009; 4:84–93.
Catatonia
Catatonia is a word usually used to describe a state of stupor occurring in the context of a psychotic illness. There are two problems with this. First, catatonic schizophrenia describes either immobile stupor or a state of chaotic physical and psychological agitation.1 Second, stupor is seen in many other non-organic conditions such as depression, mania and conversion disorder.2–6
Catatonia is thus one type of stupor, a condition characterised by at least two of the following symptoms:
- marked psychomotor retardation sometimes with complete immobility
- mutism
- waxy flexibility (no resistance from a patient to an attempt to move a limb into the most awkward position and maintenance of its position)
- negativism (strong opposite direction movement responses to an attempt to move a patients limb) or automatic obedience
- peculiar voluntary movements, e.g. posturing, mannerisms, stereotyped movements and grimacing
- echolalia, echopraxia
- refusal to eat and/or drink.
If psychiatric stupor is left untreated, physical health complications are unavoidable and develop rapidly. Prompt treatment is crucial as it may prevent complications, which include dehydration, venous thrombosis, pulmonary embolism, pneumonia, and ultimately death.7
There are three psychiatric illnesses which can present with stupor. Amongst them, stupor is mostly seen in psychotic illness. As outlined above, catatonic schizophrenia presents not only with an immobile mute picture of stupor, but also with a catatonic excitement, when a patient experiences the opposite to stupor—a chaotic psychomotor agitation and pronouncedly increased volume of speech, most of which is incoherent. The second psychiatric cause of stupor is affective illness, where an immobile mute clinical picture can occur in both depressive and manic states.2,4,8–11 The third cause is one of the most intriguing and rare psychiatric conditions—conversion disorder stupor, which sometimes is referred to as psychosomatic or hysterical catatonia.12–15
There are also developmental disorders such as autism, as well as neurodegenerative16,17 and organic disorders which can present with a catatonia-like picture of a mute and immobile patient. These include a number of medical disorders such as:
- subarachnoid haemorrhages
- basal ganglia disorders
- non-convulsive status epilepticus
- locked-in and akinetic mutism states
- endocrine and metabolic disorders, e.g. Wilson's disease18
- Prader-Willi syndrome
- antiphospholidid syndrome19
- systemic lupus erythematosus (SLE)20
- infections
- dementia
- and drug withdrawal and toxic drug states can precipitate catatonic symptoms, e.g. after abrupt withdrawal of clozapine and withdrawal of zolpidem, temazepam and many non-psychotropics including the medicines used in oncology.
The treatment of stupor is dependent on its cause. Benzodiazepines are the drugs of choice for stupor occurring in the context of affective and conversion disorders.8,9,21 It is postulated that benzodiazepines may act by increasing GABAergic transmission or reducing levels of brain-derived neurotropic factor.22 There is most experience with lorazepam. Many patients will respond to standard doses (up to 4 mg per day), but repeated and higher doses (between 8 and 24 mg per day) may be needed.23 One observational study of 9 years duration in patients with stupor of a mood disorder causality8–either major depressive episodes or bipolar I—reported an 83.3% response to intramuscular lorazepam 2 mg administered within first 2 hours of presentation, and a 100% response if 10 mg diazepam IV in 500 mL normal saline was added in cases of IM treatment failure. Where benzodiazepines are effective, their benefit is seen very quickly.
Catatonia in schizophrenia is somewhat less likely to respond to benzodiazepines, with a response in the range of 40–50%.24 A double-blind, placebo-controlled, crossover trial with lorazepam up to 6 mg per day demonstrated no effect on catatonic symptoms in patients with chronic schizophrenia,25 similar to the poor effect of lorazepam in a non-randomised trial.26 A further complication of schizophrenia is that of differential diagnosis. Debate continues on the similarities and differences between catatonic stupor in psychosis and NMS.27,28 Two terms have been coined—lethal catatonia and malignant catatonia29 to describe stupor which is accompanied by autonomic instability or hyperthermia. This potentially fatal condition cannot be distinguished either clinically or by laboratory testing from NMS, leading to a suggestion that NMS is a variant form of malignant catatonia.30 However, the absence of any prior administration of dopamine antagonist can help rule out NMS.
In stupor associated with schizophrenia, electroconvulsive therapy (ECT) and benzodiazepines remain first-choice treatments (Figure 2.5). The vast majority of current published evidence and evidence published over previous decades suggests that prompt ECT remains the most successful treatment.26,31–45 As with benzodiazepines, response to ECT may be lower in patients with schizophrenia (or who have been treated with antipsychotics) than in patients with mood disorders.46 In malignant catatonia, every effort should be made to maximise the effect of ECT by using liberalstimulus dosing to induce well-generalised seizures.47 Physical health needs should be also priorities and in-patient medical care obtained when necessary, especially for those showing autonomic imbalance and those whose dietary intake cannot be managed in psychiatric care.
The use of antipsychotics should be carefully considered (Table 2.22). Some authors recommend that antipsychotics should be avoided altogether in catatonic patients, although there are case reports of successful treatment with aripiprazole, risperidone, olanzapine, ziprasidone and clozapine.48–53 There is probably most evidence supporting clozapine and olanzapine.

Figure 2.5 Algorithm for treating stupor54
|
* Lorazepam is absorbed sublingually and is tasteless. This route may be preferred in non co-operative patients or those who cannot swallow.
† Intravenous diazepam may be considered here.
‡ Do not wait to give ECT if there is significant danger to life.
|
Table 2.22 Alternative treatments for catatonia/stupor. Listed in alphabetical order—no preference implied by order
|
Antipsychotics48-53,55-58
|
- aripiprazole
- clozapine
- olanzapine
|
|
|
Experimental treatments* 9,10,41,59-64
|
- amantadine
- amitriptyline
- carbamazepine
- fluoxetine
- fluvoxamine
- lithium
|
- memantine
- methyphenidate
- mirtazapine
- tramadol
- valproate
- zolpidem
|
|
* Always read the primary literature before using anything in this section.
|
Simple guidance on the usage of antipsychotics is to consider the history of a patient, their previous diagnosis and previous response to antipsychotic treatment, and the likelihood that non-compliance precipitated stupor. It needs to be noted that physical health problems, as in the examples listed above, can present as a catatonia-like clinical picture warranting treatment of the underlying medical condition. Avoid antipsychotics where there are clear signs of NMS, especially where stupor develops during treatment with antipsychotics and muscle rigidity is accompanied by autonomic instability. Where NMS can be ruled out and stupor occurs in the context of non-compliance with antipsychotic treatment, early re-establishment of antipsychotics is recommended. This is particularly important where stupor represents a withdrawal syndrome (as commonly seen with clozapine).
References
- Walther S. Dysfunctions of the motor system in schizophrenia. Eur Arch Psychiatry Clin Neurosci 2013; 263 Suppl 1:S16.
- Takacs R et al. Catatonia in Affective Disorders. Curr Psychiatry Rev 2013; 9:101–105.
- Fink M. Rediscovering catatonia: the biography of a treatable syndrome. Acta Psychiatr Scand Suppl 2013;1–47.
- Campos Mangas.M.C. et al. P-167 - Catatonia in bipolar disorder. Eur Psychiatry 2012; 27 Suppl 1:1.
- Bartolommei N et al. Catatonia: a critical review and therapeutic recommendation. J Psychopathol 2012; 18:234–246.
- Lee J. Dissociative catatonia: Dissociative-catatonic reactions, clinical presentations and responses to benzodiazepines. Aust N Z J Psychiatry 2011; 45:A42
- Petrides G et al. Synergism of lorazepam and electroconvulsive therapy in the treatment of catatonia. Biol Psychiatry 1997; 42:375–381.
- Huang YC et al. Rapid relief of catatonia in mood disorder by lorazepam and diazepam. Biomed J 2013; 36:35–39.
- Vasudev K et al. What works for delirious catatonic mania? BMJ Case Rep 2010; 2010.
- Neuhut R et al. Resolution of catatonia after treatment with stimulant medication in a patient with bipolar disorder. Psychosomatics 2012; 53:482–484.
- Ghaffarinejad AR et al. Periodic catatonia. Challenging diagnosis for psychiatrists. Neurosciences (Riyadh ) 2012; 17:156–158.
- Suzuki K et al. Hysteria presenting as a prodrome to catatonic stupor in a depressive patient resolved with electroconvulsive therapy. J ECT 2006; 22:276.
- Alwaki A et al. Catatonia: an elusive diagnosis. Neurology 2013; 80 Suppl 1.
- Dhadphale M. Eye gaze diagnostic sign in hysterical stupor. Lancet 1980; 2:374–375.
- Gomez J. Hysterical stupor and death. Br J Psychiatry 1980; 136:105–106.
- Mazzone L et al. Catatonia in patients with autism: prevalence and management. CNS Drugs 2014; 28:205–215.
- Dhossche DM, Wachtel LE. Catatonia in Psychiatric Illnesses. In: Fatemi SH, Clayton PJ, eds. The Medical Basis of Psychiatry. Totowa, NJ: Humana Press; 2008. pp. 471–486.
- Shetageri VN et al. Case report: catatonia as a presenting symptom of Wilsons disease. Indian J Psychiatry 2011; 53 Suppl 5:S93–S94.
- Cardinal RN et al. Psychosis and catatonia as a first presentation of antiphospholipid syndrome. Br J Psychiatry 2009; 195:272.
- Pustilnik S et al. Catatonia as the presenting symptom in systemic lupus erythematosus. J Psychiatr Pract 2011; 17:217–221.
- Sienaert P et al. Adult catatonia: Etiopathogenesis, diagnosis and treatment. Neuropsychiatry (London) 2013; 3:391–399.
- Huang TL et al. Lorazepam reduces the serum brain-derived neurotrophic factor level in schizophrenia patients with catatonia. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33:158–159.
- Fink M et al. Neuroleptic malignant syndrome is malignant catatonia, warranting treatments efficacious for catatonia. Prog Neuropsychopharmacol Biol Psychiatry 2006; 30:1182–1183.
- Rosebush PI et al. Catatonia: re-awakening to a forgotten disorder. Mov Disord 1999; 14:395–397.
- Ungvari GS et al. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology 1999; 142:393–398.
- Dutt A et al. Phenomenology and treatment of catatonia: A descriptive study from north India. Indian J Psychiatry 2011; 53:36–40.
- Luchini F et al. Catatonia and neuroleptic malignant syndrome: two disorders on a same spectrum? Four case reports. J Nerv Ment Dis 2013; 201:36–42.
- Mishima T et al. [Diazepam-responsive malignant catatonia in a patient with an initial clinical diagnosis of neuroleptic malignant syndrome: a case report]. Brain Nerve 2011; 63:503–507.
- Mann SC et al. Catatonia, malignant catatonia, and neuroleptic malignant syndrome. Curr Psychiatry Rev 2013; 9:111–119.
- Taylor MA et al. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry 2003; 160:1233–1241.
- Bush G et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand 1996; 93:137–143.
- Cristancho P et al. Successful use of right unilateral ECT for catatonia: a case series. J ECT 2014; 30:69–72.
- Philbin D et al. Catatonic schizophrenia: therapeutic challenges and potentially a new role for electroconvulsive therapy? BMJ Case Rep 2013; 2013.
- Takebayashi M. [Electroconvulsive therapy in schizophrenia]. Nihon Rinsho 2013; 71:694–700.
- Oviedo G et al. Trends in the administration of electroconvulsive therapy for schizophrenia in Colombia. Descriptive study and literature review. Eur Arch Psychiatry Clin Neurosci 2013; 263 Suppl 1:S98
- Pompili M et al. Indications for electroconvulsive treatment in schizophrenia: a systematic review. Schizophr Res 2013; 146:1–9.
- Ogando Portilla N et al. Electroconvulsive therapy as an effective treatment in neuroleptic malignant syndrome: purposely a case. Eur Psychiatry 2013; 28 Suppl 1:1.
- Unal A et al. Effective treatment of catatonia by combination of benzodiazepine and electroconvulsive therapy. J ECT 2013; 29:206–209.
- Kaliora SC et al. The practice of electroconvulsive therapy in Greece. J ECT 2013; 29:219–224.
- Girardi P et al. Life-saving electroconvulsive therapy in a patient with near-lethal catatonia. Riv Psichiatr 2012; 47:535–537.
- Kumar V et al. Electroconvulsive therapy in pregnancy. Indian J Psychiatry 2011; 53 Suppl 5:S100–S101.
- Weiss M et al. Treatment of catatonia with electroconvulsive therapy in adolescents. J Child Adolesc Psychopharmacol 2012; 22:96–100.
- Bauer J et al. Should the term catatonia be explicitly included in the ICD-10 description of acute transient psychotic disorder F23.0? Nord J Psychiatry 2012; 66:68–69.
- Mohammadbeigi H et al. Electroconvulsive therapy in single manic episodes: a case series. Afr J Psychiatry (Johannesbg ) 2011; 14:56–59.
- Dragasek J. [Utilisation of electroconvulsive therapy in treatment of depression disorders]. Psychiatrie 2011; 15:1211–9.
- van Waarde JA et al. Electroconvulsive therapy for catatonia: treatment characteristics and outcomes in 27 patients. J ECT 2010; 26:248–252.
- Kellner CH et al. Electroconvulsive therapy for catatonia. Am J Psychiatry 2010; 167:1127–1128.
- Van Den EF et al. The use of atypical antipsychotics in the treatment of catatonia. Eur Psychiatry 2005; 20:422–429.
- Caroff SN et al. Movement disorders associated with atypical antipsychotic drugs. J Clin Psychiatry 2002; 63 Suppl 4:12–19.
- Guzman CS et al. Treatment of periodic catatonia with atypical antipsychotic, olanzapine. Psychiatry Clin Neurosci 2008; 62:482.
- Babington PW et al. Treatment of catatonia with olanzapine and amantadine. Psychosomatics 2007; 48:534–536.
- Bastiampillai T et al. Catatonia resolution and aripiprazole. Aust N Z J Psychiatry 2008; 42:907.
- Strawn JR et al. Successful treatment of catatonia with aripiprazole in an adolescent with psychosis. J Child Adolesc Psychopharmacol 2007; 17:733–735.
- Rosebush PI et al. Catatonia and its treatment. Schizophr Bull 2010; 36:239–242.
- Voros V et al. [Use of aripiprazole in the treatment of catatonia]. Neuropsychopharmacol Hung 2010; 12:373–376.
- Yoshimura B et al. Is quetiapine suitable for treatment of acute schizophrenia with catatonic stupor? A case series of 39 patients. Neuropsychiatr Dis Treat 2013; 9:1565–1571.
- Todorova K. Olanzapine in the treatment of catatonic stupor - two case reports and discussion. Eur Neuropsychopharmacol 2012; 22 Suppl 2:S326.
- Prakash O et al. Catatonia and mania in patient with AIDS: treatment with lorazepam and risperidone. Gen Hosp Psychiatry 2012; 34:321–326.
- Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci 2009; 21:371–380.
- Obregon DF et al. Memantine and catatonia: a case report and literature review. J Psychiatr Pract 2011; 17:292–299.
- Hervey WM et al. Treatment of catatonia with amantadine. Clin Neuropharmacol 2012; 35:86–87.
- Consoli A et al. Lorazepam, fluoxetine and packing therapy in an adolescent with pervasive developmental disorder and catatonia. J Physiol Paris 2010; 104:309–314.
- Lewis AL et al. Malignant catatonia in a patient with bipolar disorder, B12 deficiency, and neuroleptic malignant syndrome: one cause or three? J Psychiatr Pract 2009; 15:415–422.
- Padhy SK et al. The catatonia conundrum: controversies and contradictions. Asian J Psychiatr 2014; 7:6–9.
QT prolongation
Introduction
Many psychotropic drugs are associated with ECG changes and some are causally linked to serious ventricular arrhythmia and sudden cardiac death. Specifically, some antipsychotics block cardiac potassium channels and are linked to prolongation of the cardiac QT interval, a risk factor for the ventricular arrhythmia torsade de pointes, which is often fatal. Case-control studies have suggested that the use of most antipsychotics is associated with an increase in the rate of sudden cardiac death.1–7 This risk is probably a result of the arrhythmogenic potential of antipsychotics8,9 although schizophrenia itself may be associated with QT prolongation.10 Overall risk is probably dose-related and, although the absolute risk is low, it is substantially higher than the, say, risk of fatal agranulocytosis with clozapine.8
ECG monitoring of drug-induced changes in mental health settings is complicated by a number of factors. Psychiatrists may have limited expertise in ECG interpretation, for example, and still less expertise in manually measuring QT intervals. Even cardiologists show an interrater reliability in QT measurement of up to 20 msec.11 Self-reading, computerised ECG devices are available and to some extent compensate for some lack of expertise, but different models use different algorithms and different correction formulae.12 In addition, ECG machines may not be as readily available in all clinical areas as they are in general medicine. Also, time for ECG determination may not be available in many areas (e.g. out-patients). Lastly, ECG determination may be difficult to perform in acutely disturbed, physically uncooperative patients.
ECG monitoring is essential for all patients prescribed antipsychotics. An estimate of QTC interval should be made on admission to in-patient units (note that this is recommended in the NICE schizophrenia guideline13) and at least yearly thereafter.
QT prolongation
- The cardiac QT interval (usually cited as QTc—QT corrected for heart rate) is a useful, but imprecise indicator of risk of torsade de pointes and of increased cardiac mortality14. Different correction factors and methods may give markedly different values.15
- The QT interval broadly reflects the duration of cardiac repolarisation. Lengthening of repolarisation duration induces heterogeneity of electrical phasing in different ventricular structures (dispersion), which in turn allows the emergence of early after depolarisations (EADs) which may provoke ventricular extrasystole and torsade de pointes.
- There is some controversy over the exact association between QTc and risk of arrhythmia. Very limited evidence suggests that risk is exponentially related to the extent of prolongation beyond normal limits (440 msec for men; 470 msec for women), although there are well-known exceptions which appear to disprove this theory16 (some drugs prolong QT without increasing dispersion). Rather stronger evidence links QTc values over 500 msec to a clearly increased risk of arrhythmia.17 QT intervals of > 650 msec may be more likely than not to induce torsades.18 Despite some uncertainties, QTc determination remains an important measure in estimating risk of arrhythmia and sudden death.
- QTc measurements and evaluation are complicated by:
- difficulty in determining the end of the T wave, particularly where U waves are present (this applies both to manual and self-reading ECG machines)
- normal physiological variation in QTc interval: QT varies with gender, time of day, food intake, alcohol intake, menstrual cycle, ECG lead, etc15,16
- variation in the extent of drug-induced prolongation of QTc because of changes in plasma levels. QTc prolongation is most prominent at peak drug plasma levels and least obvious at trough levels.15,16
Other Ecg changes
Other reported antipsychotic-induced changes include atrial fibrillation, giant P waves, T-wave changes and heart block.16
Quantifying risk
Drugs are categorised in Table 2.23 according to data available on their effects on the cardiac QTc interval (as reported; mostly using Bazett's correction formula). 'No-effect' drugs are those with which QTc prolongation has not been reported either at therapeutic doses or in overdose. 'Low-effect' drugs are those for which severe QTc prolongation has been reported only following overdose or where only small average increases (< 10 msec) have been observed at clinical doses.'Moderate-effect' drugs are those which have been observed to prolong QTc by > 10 msec on average when given at normal clinical doses or where ECG monitoring is officially recommended in some circumstances. 'High-effect' drugs are those for which extensive average QTc prolongation (usually > 20 msec at normal clinical doses).
Table 2.23 Effects of antipsychotics on QTc15,16,22–45
|
No effect
|
Low effect
|
Moderate effect
|
High effect
|
Unknown effect
|
Aripiprazole*
Lurasidone
|
Asenapine
Clozapine
Flupentixol
Fluphenazine
Perphenazine
Prochlorperazine
Olanzapine†
Paliperidone
Risperidone
Sulpiride
|
Amisulpride‡
Chlorpromazine
Haloperidol
Iloperidone
Levomepromazine
Melperone
Quetiapine
Ziprasidone
|
Any intravenous antipsychotic
Pimozide
Sertindole
Any drug or combination of drugs used in doses exceeding recommended maximum
|
Loxapine
Pipotiazine
Trifluoperazine
Zuclopenthixol
|
|
* One case of torsades de pointes reported.46
† Isolated cases of QTc prolongation26,47 and has effects on cardiac ion channel, IKr',48 other data suggest no effect on QTC.16,24,25,49
‡ Torsades de pointes common in overdose.18
|
Note that, as outlined above, effect on QTc may not necessarily equate directly to risk of torsade de pointes or sudden death,19 although this is often assumed. (A good example here is ziprasidone—a drug with a moderate effect on QTc but with no evidence of cardiac toxicity.20) Note also that categorisation is inevitably approximate given the problems associated with QTc measurements. Lastly, keep in mind that differences in the effects of different antipsychotics on the QT interval rarely reach statistical significance even in meta-analyses.21
Other risk factors
A number of physiological/pathological factors are associated with an increased risk of QT changes and of arrhythmia (Table 2.24) and many non-psychotropic drugs are linked to QT prolongation (Table 2.25). These additional risk factors seem almost always to be present in cases of antipsychotic-induced torsades de pointes.50
Table 2.24 Physiological risk factors for QTc prolongation and arrhythmia
|
Factor
|
Symptom
|
|
Cardiac
|
Long QT syndrome
Bradycardia
Ischaemic heart disease
Myocarditis
Myocardial infarction
Left ventricular hypertrophy
|
|
Metabolic
|
Hypokalaemia
Hypomagnesaemia
Hypocalcaemia
|
|
Others
|
Extreme physical exertion
Stress or shock Anorexia nervosa
Extremes of age—children and elderly may be more susceptible to QT changes
Female gender
|
|
Hypokalaemia-related QTc prolongation is more commonly observed in acute psychotic admissions.51 Also, be aware that there are number of physical and genetic factors which may not be discovered on routine examination but which probably predispose patients to arrhythmia.52,53
|
Table 2.25 Non-psychotropics associated with QT prolongation
|
Drug class
|
Drug
|
|
Antibiotics
|
Erythromycin
Clarithromycin
Ampicillin
Co-trimoxazole
Pentamidine
(Some 4 quinolones affect QTc—see manufacturers' literature)
|
|
Antimalarials
|
Chloroquine
Mefloquine
Quinine
|
|
Antiarrhythmics
|
Quinidine
Disopyramide
Procainamide
Sotalol
Amiodarone
Bretylium
|
|
Others
|
Amantadine
Cyclosporin
Diphenhydramine
Hydroxyzine
Methadone
Nicardipine
Tamoxifen
|
|
Beta-2 agonists and sympathomimetics may provoke torsade de pointes in patients with prolonged QTc.
|
ECG monitoring
Measure QTC in all patients prescribed antipsychotics:
- on admission
- if previous abnormality or known additional risk factor, at annual physical health check.
Consider measuring QTc within a week of achieving a therapeutic dose of a newly prescribed antipsychotic that is associated with a moderate or high risk of QTc prolongation or of newly prescribed combined antipsychotics. See Table 2.26 for the management of QT prolongation in patients receiving antipsychotic drugs.
Metabolic inhibition
The effect of drugs on the QTc interval is usually plasma level-dependent. Drug interactions are therefore important, especially when metabolic inhibition results in increased plasma levels of the drug affecting QTc. Commonly used metabolic inhibitors include fluvoxamine, fluoxetine, paroxetine and valproate.
Table 2.26 Management of QT prolongation in patients receiving antipsychotic drugs
|
QTc
|
Action
|
Refer to cardiologist
|
< 440 msec (men) or
< 470 msec (women)
|
None unless abnormal T-wave morphology
|
Consider if in doubt
|
> 440 msec (men) or
> 470 msec (women) but < 500 msec
|
Consider reducing dose or switching to drug of lower effect; repeat ECG
|
Consider
|
|
> 500 msec
|
Repeat ECG. Stop suspected causative drug(s) and switch to drug of lower effect
|
Immediately
|
|
Abnormal T-wave morphology
|
Review treatment. Consider reducing dose or switching to drug of lower effect
|
Immediately
|
Other cardiovascular risk factors
The risk of drug-induced arrhythmia and sudden cardiac death with psychotropics is an important consideration. With respect to cardiovascular disease, note that other risk factors such as smoking, obesity and impaired glucose tolerance, present a much greater risk to patient morbidity and mortality than the uncertain outcome of QT changes. See relevant sections for discussion of these problems.
Summary
- In the absence of conclusive data, assume all antipsychotics are linked to sudden cardiac death.
- Prescribe the lowest dose possible and avoid polypharmacy/metabolic interactions.
- Perform ECG on admission, and, if previous abnormality or additional risk factor, at yearly check-up.
- Consider measuring QTc within a week of achieving a therapeutic dose of a moderate/high risk antipsychotic.
References
- Reilly JG et al. Thioridazine and sudden unexplained death in psychiatric in-patients. Br J Psychiatry 2002; 180:515–522.
- Murray-Thomas T et al. Risk of mortality (including sudden cardiac death) and major cardiovascular events in atypical and typical antipsychotic users: a study with the general practice research database. Cardiovasc Psychiatry Neurol 2013; 2013:247486.
- Ray WA et al. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry 2001; 58:1161–1167.
- Hennessy S et al. Cardiac arrest and ventricular arrhythmia in patients taking antipsychotic drugs: cohort study using administrative data. BMJ 2002; 325:1070.
- Straus SM et al. Antipsychotics and the risk of sudden cardiac death. Arch Intern Med 2004; 164:1293–1297.
- Liperoti R et al. Conventional and atypical antipsychotics and the risk of hospitalization for ventricular arrhythmias or cardiac arrest. Arch Intern Med 2005; 165:696–701.
- Ray WA et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009; 360:225–235.
- Schneeweiss S et al. Antipsychotic agents and sudden cardiac death—how should we manage the risk? N Engl J Med 2009; 360:294–296.
- Nakagawa S et al. Antipsychotics and risk of first-time hospitalization for myocardial infarction: a population-based case-control study. J Intern Med 2006; 260:451–458.
- Fujii K et al. QT is longer in drug-free patients with schizophrenia compared with age-matched healthy subjects. PLoS One 2014; 9:e98555.
- Goldenberg I et al. QT interval: how to measure it and what is "normal". J Cardiovasc Electrophysiol 2006; 17:333–336.
- Nielsen J et al. Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs 2011; 25:473–490.
- National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: treatment and management. Clinical Guideline 178, 2014. http://www.nice.org.uk/Guidance/CG178
- Malik M et al. Evaluation of drug-induced QT interval prolongation: implications for drug approval and labelling. Drug Saf 2001; 24:323–351.
- Haddad PM et al. Antipsychotic-related QTc prolongation, torsade de pointes and sudden death. Drugs 2002; 62:1649–1671.
- Taylor DM. Antipsychotics and QT prolongation. Acta Psychiatr Scand 2003; 107:85–95.
- Botstein P. Is QT interval prolongation harmful? A regulatory perspective. Am J Cardiol 1993; 72:50B–52B.
- Joy JP et al. Prediction of torsade de pointes from the QT Interval: analysis of a case series of amisulpride overdoses. Clin Pharmacol Ther 2011; 90:243–245.
- Witchel HJ et al. Psychotropic drugs, cardiac arrhythmia, and sudden death. J Clin Psychopharmacol 2003; 23:58–77.
- Strom BL et al. Comparative mortality associated with ziprasidone and olanzapine in real-world use among 18,154 patients with schizophrenia: the Ziprasidone Observational Study of Cardiac Outcomes (ZODIAC). Am J Psychiatry 2011; 168:193–201.
- Chung AK et al. Effects on prolongation of Bazett's corrected QT interval of seven second-generation antipsychotics in the treatment of schizophrenia: a meta-analysis. J Psychopharmacol 2011; 25:646–666.
- Glassman AH et al. Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death. Am J Psychiatry 2001; 158:1774–1782.
- Warner B et al. Investigation of the potential of clozapine to cause torsade de pointes. Adverse Drug React Toxicol Rev 2002; 21:189–203.
- Harrigan EP et al. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol 2004; 24:62–69.
- Lindborg SR et al. Effects of intramuscular olanzapine vs. haloperidol and placebo on QTc intervals in acutely agitated patients. Psychiatry Res 2003; 119:113–123.
- Dineen S et al. QTc prolongation and high-dose olanzapine (Letter). Psychosomatics 2003; 44:174–175.
- Gupta S et al. Quetiapine and QTc issues: a case report (Letter). J Clin Psychiatry 2003; 64:612–613.
- Su KP et al. A pilot cross-over design study on QTc interval prolongation associated with sulpiride and haloperidol. Schizophr Res 2003; 59:93–94.
- Chong SA et al. Prolonged QTc intervals in medicated patients with schizophrenia. Hum Psychopharmacol 2003; 18:647–649.
- Stollberger C et al. Antipsychotic drugs and QT prolongation. Int Clin Psychopharmacol 2005; 20:243–251.
- Isbister GK et al. Amisulpride deliberate self-poisoning causing severe cardiac toxicity including QT prolongation and torsades de pointes. Med J Aust 2006; 184:354–356.
- Ward DI. Two cases of amisulpride overdose: a cause for prolonged QT syndrome. Emerg Med Australas 2005; 17:274–276.
- Vieweg WV et al. Torsade de pointes in a patient with complex medical and psychiatric conditions receiving low-dose quetiapine. Acta Psychiatr Scand 2005; 112:318–322.
- Huang BH et al. Sulpiride induced torsade de pointes. Int J Cardiol 2007; 118:e100–e102.
- Kane JM et al. Long-term efficacy and safety of iloperidone: results from 3 clinical trials for the treatment of schizophrenia. J Clin Psychopharmacol 2008; 28:S29–S35.
- Kim MD et al. Blockade of HERG human K+ channel and IKr of guinea pig cardiomyocytes by prochlorperazine. Eur J Pharmacol 2006; 544:82–90.
- Meltzer H et al. Efficacy and tolerability of oral paliperidone extended-release tablets in the treatment of acute schizophrenia: pooled data from three 6-week placebo-controlled studies. J Clin Psychiatry 2006; 69:817–829.
- Chapel S et al. Exposure-response analysis in patients with schizophrenia to assess the effect of asenapine on QTc prolongation. J Clin Pharmacol 2009; 49:1297–1308.
- Ozeki Y et al. QTc prolongation and antipsychotic medications in a sample of 1017 patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34:401–405.
- Girardin FR et al. Drug-induced long QT in adult psychiatric inpatients: the 5-year cross-sectional ECG Screening Outcome in Psychiatry study. Am J Psychiatry 2013; 170:1468–1476.
- Leucht S et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013; 382:951–962.
- Grande I et al. QTc prolongation: is clozapine safe? Study of 82 cases before and after clozapine treatment. Hum Psychopharmacol 2011.
- Hong HK et al. Block of hERG K+ channel and prolongation of action potential duration by fluphenazine at submicromolar concentration. Eur J Pharmacol 2013; 702:165–173.
- Vieweg WV et al. Risperidone, QTc interval prolongation, and torsade de pointes: a systematic review of case reports. Psychopharmacology (Berl) 2013; 228:515–524.
- Suzuki Y et al. QT prolongation of the antipsychotic risperidone is predominantly related to its 9-hydroxy metabolite paliperidone. Hum Psychopharmacol 2012; 27:39–42.
- Nelson S et al. Torsades de pointes after administration of low-dose aripiprazole. Ann Pharmacother 2013; 47:e11.
- Su KP et al. Olanzapine-induced QTc prolongation in a patient with Wolff-Parkinson-White syndrome. Schizophr Res 2004; 66:191–192.
- Morissette P et al. Olanzapine prolongs cardiac repolarization by blocking the rapid component of the delayed rectifier potassium current. J Psychopharmacol 2007; 21:735–741.
- Bar KJ et al. Influence of olanzapine on QT variability and complexity measures of heart rate in patients with schizophrenia. J Clin Psychopharmacol 2008; 28:694–698.
- Hasnain M et al. Quetiapine, QTc interval prolongation, and torsade de pointes: a review of case reports. Ther Adv Psychopharmacol 2014; 4:130–138.
- Hatta K et al. Prolonged QT interval in acute psychotic patients. Psychiatry Res 2000; 94:279–285.
- Priori SG et al. Low penetrance in the long-QT syndrome: clinical impact. Circulation 1999; 99:529–533.
- Frassati D et al. Hidden cardiac lesions and psychotropic drugs as a possible cause of sudden death in psychiatric patients: a report of 14 cases and review of the literature. Can J Psychiatry 2004; 49:100–105.
Further reading
Leonard CE et al. Antipsychotics and the risks of sudden cardiac death and all-cause death: cohort studies in Medicaid and dually-eligible Medicaid-Medicare beneficiaries of five states. J Clin Exp Cardiolog 2013; Suppl 10:1–9.
Poluzzi E et al. Antipsychotics and torsadogenic risk: signals emerging from the US FDA Adverse Event Reporting System database. Drug Saf 2013; 36:467–479.
Dyslipidaemia
Morbidity and mortality from cardiovascular disease are higher in people with schizophrenia than in the general population.1 Dyslipidaemia is an established risk factor for cardiovascular disease along with obesity, hypertension, smoking, diabetes and sedentary lifestyle. The majority of patients with schizophrenia have several of these risk factors and can be considered at 'high risk' of developing cardiovascular disease. Dyslipidaemia is treatable and intervention is known to reduce morbidity and mortality.2 Aggressive treatment is particularly important in people with diabetes, the prevalence of which is increased twoto three-fold over population norms in people with schizophrenia (see section on 'Diabetes and impaired glucose tolerance' in this chapter).
Effect of antipsychotic drugs on lipids
First-generation antipsychotics
Phenothiazines are known to be associated with increases in triglycerides and lowdensity lipoprotein (LDL) cholesterol and decreases in high-density lipoprotein (HDL)3 cholesterol, but the magnitude of these effects is poorly quantified.4 Haloperidol seems to have minimal effect on lipid profiles.3
Second-generation antipsychotics
Although there are relatively more data pertaining to some atypicals, they are derived from a variety of sources and are reported in different ways, making it difficult to compare drugs directly. While cholesterol levels can rise, the most profound effect of these drugs seems to be on triglycerides. Raised triglycerides are in general, associated with obesity and diabetes. From the available data, olanzapine5 would seem to have the greatest propensity to increase lipids, and quetiapine and risperidone moderate propensity.6,7 Aripiprazole, lurasidone and ziprasidone have minimal adverse effect on blood lipids5,8–12 and may even modestly reverse dyslipidaemias associated with previous antipsychotics.12–14 Iloperidone causes some weight gain but may not adversely affect cholesterol or triglycerides.15
Olanzapine has been shown to increase triglyceride levels by 40% over the short (12 weeks) and medium (16 months) term.16,17 Levels may continue to rise for up to a year.18 Up to two-thirds of olanzapine-treated patients have raised triglycerides19 and just under 10% may develop severe hypertriglyceridaemia.20 While weight gain with olanzapine is generally associated with both increases in cholesterol17,21 and triglycerides,20 severe hypertriglyceridaemia can occur independently of weight gain.20 In one study, patients treated with olanzapine and risperidone gained a similar amount of weight, but in olanzapine patients serum triglyceride levels increased by four times as much (80 mg/dL) as in risperidone patients (20 mg/dL).20 Quetiapine22 seems to have more modest effects than olanzapine, although data are conflicting.23
A case-control study conducted in the UK found that patients with schizophrenia who were treated with olanzapine were five times more likely to develop hyperlipidaemia than controls and three times more likely to develop hyperlipidaemia than patients receiving typical antipsychotics.24 Risperidone-treated patients could not be distinguished from controls.
Clozapine
Mean triglyceride levels have been shown to double and cholesterol levels to increase by at least 10% after 5 years' treatment with clozapine.25 Patients treated with clozapine have triglyceride levels that are almost double those of patients who are treated with FGA drugs.26,27 Cholesterol levels are also increased.5
Particular care should be taken before prescribing clozapine or olanzapine for patients who are obese, diabetic or known to have pre-existing hyperlipidaemia.28
Screening
All patients should have their lipids measured at baseline, 3 months after starting treatment with a new antipsychotic, and then annually. Those prescribed clozapine and olanzapine should ideally have their serum lipids measured every 3 months for the first year of treatment, and then annually. Clinically significant changes in cholesterol are unlikely over the short term but triglycerides can increase dramatically.29 In practice, dyslipidaemia is widespread in patients taking long-term antipsychotics irrespective of drug prescribed or of diagnosis.30–32 Screening for this potentially serious side-effect of antipsychotics is not yet routine in clinical practice,33 but is strongly recommended by NICE.34
Severe hypertriglyceridemia (fasting level of > 5 mmol/L) is a risk factor for pancreatitis. Note that antipsychotic-induced dyslipidaemia can occur independent of weight gain.35
Treatment of dyslipidaemia
If moderate to severe hyperlipidaemia develops during antipsychotic treatment, a switch to another antipsychotic less likely to cause this problem should be considered in the first instance. Although not recommended as a strategy in patients with treatmentresistant illness, clozapine-induced hypertriglyceridaemia has been shown to reverse after a switch to risperidone.36 This may hold true with other switching regimens but data are scarce.37 Aripiprazole (or ziprasidone outside the UK) seems at present to be the treatment of choice in those with prior antipsychotic-induced dyslipidaemia.14,38
Patients with raised cholesterol may benefit from dietary advice, lifestyle changes and/or treatment with statins.39 Statins seem to be effective in this patient group but interactions are possible.40 Risk tables and treatment guidelines can be found in the British National Formulary (BNF). Evidence supports the treatment of cholesterol concentrations as low as 4 mmol/L in high-risk patients41 and this is the highest level recommended by NICE for secondary prevention of cardiovascular events.42 NICE makes no recommendations on target levels for primary prevention but recent advice promotes the use of statins for anyone with a > 10% ten year risk of cardiovascular disease.42 Coronary heart disease and stroke risk can be reduced by one-third by reducing cholesterol to as low as 3.5 mmol/L.2 When triglycerides alone are raised, diets low in saturated fats, and the taking of fish oil and fibrates are effective treatments18,43 although there is no proof that mortality is reduced. Such patients should be screened for impaired glucose tolerance and diabetes.
Table 2.27 Monitoring lipid concentrations in patients on antipsychotic drugs
|
Drug
|
Suggested monitoring
|
Clozapine
Olanzapine
|
Fasting lipids at baseline then every 3 months for a year, then annually
|
|
Other antipsychotics
|
Fasting lipids at baseline and at 3 months, and then annually
|
The recommended procedure for monitoring lipid levels in patients on antipsychotics is summarised in Table 2.27.
References
- Brown S et al. Causes of the excess mortality of schizophrenia. Br J Psychiatry 2000; 177:212–217.
- Durrington P. Dyslipidaemia. Lancet 2003; 362:717–731.
- Sasaki J et al. Lipids and apolipoproteins in patients treated with major tranquilizers. Clin Pharmacol Ther 1985; 37:684–687.
- Henkin Y et al. Secondary dyslipidemia. Inadvertent effects of drugs in clinical practice. JAMA 1992; 267:961–968.
- Chaggar PS et al. Effect of antipsychotic medications on glucose and lipid levels. J Clin Pharmacol 2011; 51:631–638.
- Smith RC et al. Effects of olanzapine and risperidone on lipid metabolism in chronic schizophrenic patients with long-term antipsychotic treatment: a randomized five month study. Schizophr Res 2010; 120:204–209.
- Perez-Iglesias R et al. Glucose and lipid disturbances after 1 year of antipsychotic treatment in a drug-naive population. Schizophr Res 2009; 107:115–121.
- Olfson M et al. Hyperlipidemia following treatment with antipsychotic medications. Am J Psychiatry 2006; 163:1821–1825.
- L'Italien GJ et al. Comparison of metabolic syndrome incidence among schizophrenia patients treated with aripiprazole versus olanzapine or placebo. J Clin Psychiatry 2007; 68:1510–1516.
- Fenton WS et al. Medication-induced weight gain and dyslipidemia in patients with schizophrenia. Am J Psychiatry 2006; 163:1697–1704.
- Meyer JM et al. Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: prospective data from phase 1. Schizophr Res 2008; 101:273–286.
- Potkin SG et al. Double-blind comparison of the safety and efficacy of lurasidone and ziprasidone in clinically stable outpatients with schizophrenia or schizoaffective disorder. Schizophr Res 2011; 132:101–117.
- Fleischhacker WW et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol 2010; 13:1115–1125.
- Chen Y et al. Comparative effectiveness of switching antipsychotic drug treatment to aripiprazole or ziprasidone for improving metabolic profile and atherogenic dyslipidemia: a 12-month, prospective, open-label study. J Psychopharmacol 2012; 26:1201–1210.
- Citrome L. Iloperidone: chemistry, pharmacodynamics, pharmacokinetics and metabolism, clinical efficacy, safety and tolerability, regulatory affairs, and an opinion. Expert Opin Drug Metab Toxicol 2010; 6:1551–1564.
- Sheitman BB et al. Olanzapine-induced elevation of plasma triglyceride levels. Am J Psychiatry 1999; 156:1471–1472.
- Osser DN et al. Olanzapine increases weight and serum triglyceride levels. J Clin Psychiatry 1999; 60:767–770.
- Meyer JM. Effects of atypical antipsychotics on weight and serum lipid levels. J Clin Psychiatry 2001; 62 Suppl 27:27–34.
- Melkersson KI et al. Elevated levels of insulin, leptin, and blood lipids in olanzapine-treated patients with schizophrenia or related psychoses. J Clin Psychiatry 2000; 61:742–749.
- Meyer JM. Novel antipsychotics and severe hyperlipidemia. J Clin Psychopharmacol 2001; 21:369–374.
- Kinon BJ et al. Long-term olanzapine treatment: weight change and weight-related health factors in schizophrenia. J Clin Psychiatry 2001; 62:92–100.
- Atmaca M et al. Serum leptin and triglyceride levels in patients on treatment with atypical antipsychotics. J Clin Psychiatry 2003; 64:598–604.
- de Leon J et al. A clinical study of the association of antipsychotics with hyperlipidemia. Schizophr Res 2007; 92:95–102.
- Koro CE et al. An assessment of the independent effects of olanzapine and risperidone exposure on the risk of hyperlipidemia in schizophrenic patients. Arch Gen Psychiatry 2002; 59:1021–1026.
- Henderson DC et al. Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: A five-year naturalistic study. Am J Psychiatry 2000; 157:975–981.
- Ghaeli P et al. Serum triglyceride levels in patients treated with clozapine. Am J Health Syst Pharm 1996; 53:2079–2081.
- Spivak B et al. Diminished suicidal and aggressive behavior, high plasma norepinephrine levels, and serum triglyceride levels in chronic neuroleptic-resistant schizophrenic patients maintained on clozapine. Clin Neuropharmacol 1998; 21:245–250.
- Baptista T et al. Novel antipsychotics and severe hyperlipidemia: comments on the Meyer paper. J Clin Psychopharmacol 2002; 22:536–537.
- Meyer JM et al. The effects of antipsychotic therapy on serum lipids: a comprehensive review. Schizophr Res 2004; 70:1–17.
- Paton C et al. Obesity, dyslipidaemias and smoking in an inpatient population treated with antipsychotic drugs. Acta Psychiatr Scand 2004; 110:299–305.
- De Hert M et al. The METEOR study of diabetes and other metabolic disorders in patients with schizophrenia treated with antipsychotic drugs. I. Methodology. Int J Methods Psychiatr Res 2010; 19:195–210.
- Jin H et al. Comparison of longer-term safety and effectiveness of 4 atypical antipsychotics in patients over age 40: a trial using equipoisestratified randomization. J Clin Psychiatry 2013; 74:10–18.
- Barnes TR et al. Screening for the metabolic syndrome in community psychiatric patients prescribed antipsychotics: a quality improvement programme. Acta Psychiatr Scand 2008; 118:26–33.
- National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: treatment and management. Clinical Guideline 178, 2014. http://www.nice.org.uk/Guidance/CG178
- Birkenaes AB et al. Dyslipidemia independent of body mass in antipsychotic-treated patients under real-life conditions. J Clin Psychopharmacol 2008; 28:132–137.
- Ghaeli P et al. Elevated serum triglycerides in clozapine resolve with risperidone. Pharmacotherapy 1995; 15:382–385.
- Weiden PJ. Switching antipsychotics as a treatment strategy for antipsychotic-induced weight gain and dyslipidemia. J Clin Psychiatry 2007; 68 Suppl 4:34–39.
- Newcomer JW et al. A multicenter, randomized, double-blind study of the effects of aripiprazole in overweight subjects with schizophrenia or schizoaffective disorder switched from olanzapine. J Clin Psychiatry 2008; 69:1046–1056.
- Ojala K et al. Statins are effective in treating dyslipidemia among psychiatric patients using second-generation antipsychotic agents. J Psychopharmacol 2008; 22:33–38.
- Tse L et al. Pharmacological treatment of antipsychotic-induced dyslipidemia and hypertension. Int Clin Psychopharmacol 2014; 29:125–137.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- National Institute for Health and Care Excellence. Lipid modification: cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. Clinical Guideline 181, 2014. http://www.nice.org.uk/Guidance/CG181
- Caniato RN et al. Effect of omega-3 fatty acids on the lipid profile of patients taking clozapine. Aust N Z J Psychiatry 2006; 40:691–697.
Diabetes and impaired glucose tolerance
Schizophrenia
Schizophrenia is associated with relatively high rates of insulin resistance and diabetes1,2–an observation that predates the discovery and widespread use of antipsychotics.3–5 Lifestyle interventions (lower weight, more activity) are effective in preventing diabetes6 and should be considered for all people with a diagnosis of schizophrenia.
Antipsychotics
Data relating to diabetes and antipsychotic use are numerous but less than perfect.7–10 The main problem is that incidence and prevalence studies assume full or uniform screening for diabetes. Neither assumption is likely to be correct.7 Many studies do not account for other factors affecting risk of diabetes.10 Small differences between drugs are therefore difficult to substantiate but may in any case be ultimately unimportant: risk is probably increased for all those with schizophrenia receiving any antipsychotic. The mechanisms involved in the development of antipsychotic-related diabetes are unclear, but may include 5HT2A/5HT2C antagonism, increased plasma lipids, weight gain and leptin resistance.11 Insulin resistance may occur in the absence of weight gain.12
First-generation antipsychotics
Phenothiazine derivatives have long been associated with impaired glucose tolerance and diabetes.13 Diabetes prevalence rates were reported to have substantially increased following the introduction and widespread use of FGA drugs.14 The prevalence of impaired glucose tolerance seems to be higher with aliphatic phenothiazines than with fluphenazine or haloperidol.15 Hyperglycaemia has also been reported with other conventional drugs, such as loxapine,16 and other data confirm an association with haloperidol.17 Some studies even suggest that FGAs are no different from SGAs in their propensity to cause diabetes,18,19 whereas others suggest a modest but statistically significant excess incidence of diabetes with SGAs.20
Second-generation antipsychotics
Clozapine
Clozapine is strongly linked to hyperglycaemia, impaired glucose tolerance and diabetic ketoacidosis.21 The risk of diabetes appears to be higher with clozapine than with other SGAs and conventional drugs, especially in younger patients,22–25 although this is not a consistent finding.26,27
As many as one-third of patients might develop diabetes after 5 years of treatment.28 Many cases of diabetes are noted in the first 6 months of treatment and some occur within 1 month,29 some only after many years.27 Death from ketoacidosis has also been reported.29 Diabetes associated with clozapine is not necessarily linked to obesity or to family history of diabetes,21,30 although these factors greatly increase the risk of developing diabetes on clozapine.31
Clozapine appears to increase plasma levels of insulin in a clozapine level-dependent fashion.32,33 It has been shown to be more likely than FGAs to increase plasma glucose and insulin following oral glucose challenge.34 Testing for diabetes is essential given the high prevalence of diabetes in people receiving clozapine.35
Olanzapine
As with clozapine, olanzapine has been strongly linked to impaired glucose tolerance, diabetes and diabetic ketoacidosis.36 Olanzapine and clozapine appear to directly induce insulin resistance.37,38 Risk of diabetes has also been reported to be higher with olanzapine than with FGA drugs,39 again with a particular risk in younger patients.23 The time course of development of diabetes has not been established but impaired glucose tolerance seems to occur even in the absence of obesity and family history of diabetes.21,30 Olanzapine is probably more diabetogenic than risperidone.40–44 Olanzapine is also associated with plasma levels of glucose and insulin higher than those seen with FGAs (after oral glucose load).34,45
Risperidone
Risperidone has been linked, mainly in case reports, to impaired glucose tolerance,46 diabetes47 and ketoacidosis.48 The number of reports of such adverse effects is substantially smaller than with either clozapine or olanzapine.49 At least one study has suggested that changes in fasting glucose are significantly less common with risperidone than with olanzapine40 but other studies have detected no difference.50
Risperidone seems no more likely than FGA drugs to be associated with diabetes,23,39,41 although there may be an increased risk in patients under 40 years of age.23 Risperidone has, however, been observed adversely to affect fasting glucose and plasma glucose (following glucose challenge) compared with levels seen in healthy volunteers (but not compared with patients taking conventional drugs).34
Quetiapine
Like risperidone, quetiapine has been linked to cases of new-onset diabetes and ketoacidosis.51,52 Again, the number of reports is much lower than with olanzapine or clozapine. Quetiapine appears to be more likely than FGA drugs to be associated with diabetes.23,53 Two studies showed quetiapine to be equal to olanzapine in incidence of diabetes.50,54 Risk with quetiapine may be dose-related, with daily doses of 400 mg or more being clearly linked to changes in HbA1C.55
Other SGAs
Amisulpride appears not to elevate plasma glucose56 and seems not to be associated with diabetes.57 There is one reported case of ketoacidosis occurring in a patient given the closely related sulpiride.58 Data for aripiprazole59–62 and ziprasidone63,64 suggest that neither drug alters glucose homeostasis. Aripiprazole may even reverse diabetes caused by other drugs65 (although ketoacidosis has been reported with aripiprazole66–68). A large case-control study has confirmed that neither amisulpride nor aripiprazole increase the risk of diabetes.69 These three drugs (amisulpride, aripiprazole and ziprasidone) are cautiously recommended for those with a history of or predisposition to diabetes mellitus or as an alternative to other antipsychotics known to be diabetogenic. Limited data suggest neither lurasidone70 nor asenapine71,72 has any effect on glucose homeostasis.
Predicting antipsychotic-related diabetes
Risk of diabetes is increased to a much greater extent in younger adults than in the elderly73 (in which antipsychotics may show no increased risk74). First-episode patients seem particularly prone to the development of diabetes when given a variety of antipsychotics.75–77 During treatment, rapid weight gain and a rise in plasma triglycerides seem to predict the development of diabetes.78
Monitoring
Diabetes is a growing problem in western society and has a strong association with obesity, (older) age, (lower) educational achievement and certain racial groups.79,80 Diabetes markedly increases cardiovascular mortality, largely as a consequence of atherosclerosis.81 Likewise, the use of antipsychotics also increases cardiovascular mortality.82–84 Intervention to reduce plasma glucose levels and minimise other risk factors (obesity, hypercholesterolaemia) is therefore essential.85
There is no clear consensus on diabetes-monitoring practice for those receiving antipsychotics86 and recommendations in formal guidelines vary considerably.87 Given the previous known parlous state of testing for diabetes in the UK7,88,89 and elsewhere90,91 arguments over precisely which tests are done and when seem to miss the point. There is an overwhelming need to improve monitoring by any means and so any tests for diabetes are supported—urine glucose and random plasma glucose included (Table 2.28).
Ideally, though, all patients should have oral glucose tolerance tests (OGTT) performed as this is the most sensitive method of detection.92 Fasting plasma glucose (FPG) tests are less sensitive but recommended.93 Any abnormality in FPG should provoke an OGTT.
Table 2.28 Recommended monitoring for diabetes in patients receiving antipsychotic drugs
|
Time
|
ideally
|
Minimum
|
|
Baseline
|
OGTT or FPG
HbA1C if fasting not possible
|
Urine glucose
RPG
|
|
Continuation
|
All drugs: OGTT or FPG + HbA1C every 12 months For clozapine and olanzapine or if other risk factors present: OGTT or FPG after one month, then every 4-6 months
For on-going regular screening, HbA1C is a suitable test. Note that this test is not suitable for detecting short-term change
|
Urine glucose or RPG every 12 months, with symptom monitoring
|
|
FPG, fasting plasma glucose; OGTT, oral glucose tolerance tests; RPG, random plasma glucose.
|
Table 2.29 Antipsychotics—risk of diabetes and impaired glucose tolerance
|
Degree of risk
|
Antipsychotic drug
|
|
High risk
|
Clozapine, olanzapine
|
|
Moderate risk
|
Quetiapine, risperidone, phenothiazines
|
|
Low risk
|
High potency FGAs (e.g. haloperidol)
|
|
Minimal risk
|
Aripiprazole, amisulpride, asenapine, lurasidone, ziprasidone
|
|
FGA, first-generation antipsychotic.
|
Fasting tests are often difficult to obtain in acutely ill, disorganised patients so measurement of random plasma glucose or glycosylated haemoglobin (HbA1C) may also be used (fasting not required). HbA1C is now recognised as a useful tool in detecting and monitoring diabetes.94 Frequency of monitoring should then be determined by physical factors (e.g. weight gain) and known risk factors (e.g. family history of diabetes, lipid abnormalities, smoking status). The absolute minimum is yearly testing for diabetes for all patients. In addition, all patients should be asked to look out for and report signs and symptoms of diabetes (fatigue, candida infection, thirst, polyuria).
Treatment of antipsychotic-related diabetes
Switching to a drug of low or minimal risk of diabetes is often effective in reversing changes in glucose tolerance. In this respect the most compelling evidence is for switching to aripiprazole95,96 but also to ziprasidone.96 Standard anti-diabetic treatments are otherwise recommended. Pioglitazone97 may have particular benefit but note the hepatotoxic potential of this drug.
The overall risk of impaired glucose tolerance and diabetes for different antipsychotics is summarised in Table 2.29.
References
- Schimmelbusch WH et al. The positive correlation between insulin resistance and duration of hospitalization in untreated schizophrenia. Br J Psychiatry 1971; 118:429–436.
- Waitzkin L. A survey for unknown diabetics in a mental hospital. I. Men under age fifty. Diabetes 1966; 15:97–104.
- Kasanin J. The blood sugar curve in mental disease. II. The schizophrenia (dementia praecox) groups. Arch Neurol Psychiatry 1926; 16:414–419.
- Braceland FJ et al. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945; 102:108–110.
- Kohen D. Diabetes mellitus and schizophrenia: historical perspective. Br J Psychiatry Suppl 2004; 47:S64–S66.
- Knowler WC et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009; 374:1677–1686.
- Taylor D et al. Testing for diabetes in hospitalised patients prescribed antipsychotic drugs. Br J Psychiatry 2004; 185:152–156.
- Haddad PM. Antipsychotics and diabetes: review of non-prospective data. Br J Psychiatry Suppl 2004; 47:S80–S86.
- Bushe C et al. Association between atypical antipsychotic agents and type 2 diabetes: review of prospective clinical data. Br J Psychiatry 2004; 184:S87–S93.
- Gianfrancesco F et al. The influence of study design on the results of pharmacoepidemiologic studies of diabetes risk with antipsychotic therapy. Ann Clin Psychiatry 2006; 18:9–17.
- Buchholz S et al. Atypical antipsychotic-induced diabetes mellitus: an update on epidemiology and postulated mechanisms. Intern Med J 2008; 38:602–606.
- Teff KL et al. Antipsychotic-induced insulin resistance and postprandial hormonal dysregulation independent of weight gain or psychiatric disease. Diabetes 2013; 62:3232–3240.
- Arneson GA. Phenothiazine derivatives and glucose metabolism. J Neuropsychiatr 1964; 5:181.
- Lindenmayer JP et al. Hyperglycemia associated with the use of atypical antipsychotics. J Clin Psychiatry 2001; 62 Suppl 23:30–38.
- Keskiner A et al. Psychotropic drugs, diabetes and chronic mental patients. Psychosomatics 1973; 14:176–181.
- Tollefson G et al. Nonketotic hyperglycemia associated with loxapine and amoxapine: case report. J Clin Psychiatry 1983; 44:347–348.
- Lindenmayer JP et al. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. Am J Psychiatry 2003; 160:290–296.
- Carlson C et al. Diabetes mellitus and antipsychotic treatment in the United Kingdom. Eur Neuropsychopharmacol 2006; 16:366–375.
- Ostbye T et al. Atypical antipsychotic drugs and diabetes mellitus in a large outpatient population: a retrospective cohort study. Pharmacoepidemiol Drug Saf 2005; 14:407–415.
- Smith M et al. Firstv. second-generation antipsychotics and risk for diabetes in schizophrenia: systematic review and meta-analysis. Br J Psychiatry 2008; 192:406–411.
- Mir S et al. Atypical antipsychotics and hyperglycaemia. Int Clin Psychopharmacol 2001; 16:63–74.
- Lund BC et al. Clozapine use in patients with schizophrenia and the risk of diabetes, hyperlipidemia, and hypertension: a claims-based approach. Arch Gen Psychiatry 2001; 58:1172–1176.
- Sernyak MJ et al. Association of diabetes mellitus with use of atypical neuroleptics in the treatment of schizophrenia. Am J Psychiatry 2002; 159:561–566.
- Gianfrancesco FD et al. Differential effects of risperidone, olanzapine, clozapine, and conventional antipsychotics on type 2 diabetes: findings from a large health plan database. J Clin Psychiatry 2002; 63:920–930.
- Guo JJ et al. Risk of diabetes mellitus associated with atypical antipsychotic use among patients with bipolar disorder: A retrospective, population-based, case-control study. J Clin Psychiatry 2006; 67:1055–1061.
- Wang PS et al. Clozapine use and risk of diabetes mellitus. J Clin Psychopharmacol 2002; 22:236–243.
- Sumiyoshi T et al. A comparison of incidence of diabetes mellitus between atypical antipsychotic drugs: a survey for clozapine, risperidone, olanzapine, and quetiapine (Letter). J Clin Psychopharmacol 2004; 24:345–348.
- Henderson DC et al. Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: A five-year naturalistic study. Am J Psychiatry 2000; 157:975–981.
- Koller E et al. Clozapine-associated diabetes. Am J Med 2001; 111:716–723.
- Sumiyoshi T et al. The effect of hypertension and obesity on the development of diabetes mellitus in patients treated with atypical antipsychotic drugs (Letter). J Clin Psychopharmacol 2004; 24:452–454.
- Zhang R et al. The prevalence and clinical-demographic correlates of diabetes mellitus in chronic schizophrenic patients receiving clozapine. Hum Psychopharmacol 2011; 26:392–396.
- Melkersson KI et al. Different influences of classical antipsychotics and clozapine on glucose-insulin homeostasis in patients with schizophrenia or related psychoses. J Clin Psychiatry 1999; 60:783–791.
- Melkersson K. Clozapine and olanzapine, but not conventional antipsychotics, increase insulin release in vitro. Eur Neuropsychopharmacol 2004; 14:115–119.
- Newcomer JW et al. Abnormalities in glucose regulation during antipsychotic treatment of schizophrenia. Arch Gen Psychiatry 2002; 59:337–345.
- Lamberti JS et al. Diabetes mellitus among outpatients receiving clozapine: prevalence and clinical-demographic correlates. J Clin Psychiatry 2005; 66:900–906.
- Wirshing DA et al. Novel antipsychotics and new onset diabetes. Biol Psychiatry 1998; 44:778–783.
- Engl J et al. Olanzapine impairs glycogen synthesis and insulin signaling in L6 skeletal muscle cells. Mol Psychiatry 2005; 10:1089–1096.
- Vestri HS et al. Atypical antipsychotic drugs directly impair insulin action in adipocytes: effects on glucose transport, lipogenesis, and antilipolysis. Neuropsychopharmacology 2007; 32:765–772.
- Koro CE et al. Assessment of independent effect of olanzapine and risperidone on risk of diabetes among patients with schizophrenia: population based nested case-control study. BMJ 2002; 325:243.
- Meyer JM. A retrospective comparison of weight, lipid, and glucose changes between risperidoneand olanzapine-treated inpatients: metabolic outcomes after 1 year. J Clin Psychiatry 2002; 63:425–433.
- Gianfrancesco F et al. Antipsychotic-induced type 2 diabetes: evidence from a large health plan database. J Clin Psychopharmacol 2003; 23:328–335.
- Leslie DL et al. Incidence of newly diagnosed diabetes attributable to atypical antipsychotic medications. Am J Psychiatry 2004; 161:1709–1711.
- Duncan E et al. Relative risk of glucose elevation during antipsychotic exposure in a Veterans Administration population. Int Clin Psychopharmacol 2007; 22:1–11.
- Meyer JM et al. Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: prospective data from phase 1. Schizophr Res 2008; 101:273–286.
- Ebenbichler CF et al. Olanzapine induces insulin resistance: results from a prospective study. J Clin Psychiatry 2003; 64:1436–1439.
- Mallya A et al. Resolution of hyperglycemia on risperidone discontinuation: a case report. J Clin Psychiatry 2002; 63:453–454.
- Wirshing DA et al. Risperidone-associated new-onset diabetes. Biol Psychiatry 2001; 50:148–149.
- Croarkin PE et al. Diabetic ketoacidosis associated with risperidone treatment? Psychosomatics 2000; 41:369–370.
- Koller EA et al. Risperidone-associated diabetes mellitus: a pharmacovigilance study. Pharmacotherapy 2003; 23:735–744.
- Lambert BL et al. Diabetes risk associated with use of olanzapine, quetiapine, and risperidone in veterans health administration patients with schizophrenia. Am J Epidemiol 2006; 164:672–681.
- Henderson DC. Atypical antipsychotic-induced diabetes mellitus: how strong is the evidence? CNS Drugs 2002; 16:77–89.
- Koller EA et al. A survey of reports of quetiapine-associated hyperglycemia and diabetes mellitus. J Clin Psychiatry 2004; 65:857–863.
- Citrome L et al. Relationship between antipsychotic medication treatment and new cases of diabetes among psychiatric inpatients. Psychiatr Serv 2004; 55:1006–1013.
- Bushe C et al. Comparison of metabolic and prolactin variables from a six-month randomised trial of olanzapine and quetiapine in schizophrenia. J Psychopharmacol 2010; 24:1001–1009.
- Guo Z et al. A real-world data analysis of dose effect of second-generation antipsychotic therapy on hemoglobin A1C level. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35:1326–1332.
- Vanelle JM et al. A double-blind randomised comparative trial of amisulpride versus olanzapine for 2 months in the treatment of subjects with schizophrenia and comorbid depression. Eur Psychiatry 2006; 21:523–530.
- De Hert MA et al. Prevalence of the metabolic syndrome in patients with schizophrenia treated with antipsychotic medication. Schizophr Res 2006; 83:87–93.
- Toprak O et al. New-onset type II diabetes mellitus, hyperosmolar non-ketotic coma, rhabdomyolysis and acute renal failure in a patient treated with sulpiride. Nephrol Dial Transplant 2005; 20:662–663.
- Keck PE, Jr. et al. Aripiprazole: a partial dopamine D2 receptor agonist antipsychotic. Expert Opin Investig Drugs 2003; 12:655–662.
- Pigott TA et al. Aripiprazole for the prevention of relapse in stabilized patients with chronic schizophrenia: a placebo-controlled 26-week study. J Clin Psychiatry 2003; 64:1048–1056.
- van WR et al. Major changes in glucose metabolism, including new-onset diabetes, within 3 months after initiation of or switch to atypical antipsychotic medication in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry 2008; 69:472–479.
- Baker RA et al. Atypical antipsychotic drugs and diabetes mellitus in the US Food and Drug Administration Adverse Event Database: A Systematic Bayesian Signal Detection Analysis. Psychopharmacol Bull 2009; 42:11–31.
- Simpson GM et al. Randomized, controlled, double-blind multicenter comparison of the efficacy and tolerability of ziprasidone and olanzapine in acutely ill inpatients with schizophrenia or schizoaffective disorder. Am J Psychiatry 2004; 161:1837–1847.
- Sacher J et al. Effects of olanzapine and ziprasidone on glucose tolerance in healthy volunteers. Neuropsychopharmacology 2008; 33:1633–1641.
- De Hert M et al. A case series: evaluation of the metabolic safety of aripiprazole. Schizophr Bull 2007; 33:823–830.
- Church CO et al. Diabetic ketoacidosis associated with aripiprazole. Diabet Med 2005; 22:1440–1443.
- Reddymasu S et al. Elevated lipase and diabetic ketoacidosis associated with aripiprazole. JOP 2006; 7:303–305.
- Campanella LM et al. Severe hyperglycemic hyperosmolar nonketotic coma in a nondiabetic patient receiving aripiprazole. Ann Emerg Med 2009; 53:264–266.
- Kessing LV et al. Treatment with antipsychotics and the risk of diabetes in clinical practice. Br J Psychiatry 2010; 197:266–271.
- McEvoy JP et al. Effectiveness of lurasidone in patients with schizophrenia or schizoaffective disorder switched from other antipsychotics: a randomized, 6-week, open-label study. J Clin Psychiatry 2013; 74:170–179.
- McIntyre RS et al. Asenapine for long-term treatment of bipolar disorder: a double-blind 40-week extension study. J Affect Disord 2010; 126:358–365.
- McIntyre RS et al. Asenapine versus olanzapine in acute mania: a double-blind extension study. Bipolar Disord 2009; 11:815–826.
- Hammerman A et al. Antipsychotics and diabetes: an age-related association. Ann Pharmacother 2008; 42:1316–1322.
- Albert SG et al. Atypical antipsychotics and the risk of diabetes in an elderly population in long-term care: a retrospective nursing home chart review study. J Am Med Dir Assoc 2009; 10:115–119.
- De HM et al. Typical and atypical antipsychotics differentially affect long-term incidence rates of the metabolic syndrome in first-episode patients with schizophrenia: a retrospective chart review. Schizophr Res 2008; 101:295–303.
- Saddichha S et al. Metabolic syndrome in first episode schizophrenia - a randomized double-blind controlled, short-term prospective study. Schizophr Res 2008; 101:266–272.
- Saddichha S et al. Diabetes and schizophrenia - effect of disease or drug? Results from a randomized, double-blind, controlled prospective study in first-episode schizophrenia. Acta Psychiatr Scand 2008; 117:342–347.
- Reaven GM et al. In search of moderators and mediators of hyperglycemia with atypical antipsychotic treatment. J Psychiatr Res 2009; 43:997–1002.
- Mokdad AH et al. The continuing increase of diabetes in the US. Diabetes Care 2001; 24:412.
- Mokdad AH et al. Diabetes trends in the US: 1990-1998. Diabetes Care 2000; 23:1278–1283.
- Beckman JA et al. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 2002; 287:2570–2581.
- Henderson DC et al. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10-year naturalistic study. J Clin Psychiatry 2005; 66:1116–1121.
- Lamberti JS et al. Prevalence of the metabolic syndrome among patients receiving clozapine. Am J Psychiatry 2006; 163:1273–1276.
- Goff DC et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res 2005; 80:45–53.
- Haupt DW et al. Hyperglycemia and antipsychotic medications. J Clin Psychiatry 2001; 62 Suppl 27:15–26.
- Cohn TA et al. Metabolic monitoring for patients treated with antipsychotic medications. Can J Psychiatry 2006; 51:492–501.
- De HM et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry 2011; 199:99–105.
- Barnes TR et al. A UK audit of screening for the metabolic side effects of antipsychotics in community patients. Schizophr Bull 2007; 33:1397–1403.
- Barnes TR et al. Screening for the metabolic syndrome in community psychiatric patients prescribed antipsychotics: a quality improvement programme. Acta Psychiatr Scand 2008; 118:26–33.
- Barnes TR et al. Screening for the metabolic syndrome in community psychiatric patients prescribed antipsychotics: a quality improvement programme. Acta Psychiatr Scand 2008; 118:26–33.
- Morrato EH et al. Metabolic screening after the ADA's Consensus Statement on Antipsychotic Drugs and Diabetes. Diabetes Care 2009; 32:1037–1042.
- De Hert M et al. Oral glucose tolerance tests in treated patients with schizophrenia. Data to support an adaptation of the proposed guidelines for monitoring of patients on second generation antipsychotics? Eur Psychiatry 2006; 21:224–226.
- Marder SR et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry 2004; 161:1334–1349.
- National Institute for Health and Clinical Excellence. Type 2 diabetes - newer agents (a partial update of CG66). Clinical Guideline 87, 2009. http://guidance.nice.org.uk/CG87
- Stroup TS et al. Effects of switching from olanzapine, quetiapine, and risperidone to aripiprazole on 10-year coronary heart disease risk and metabolic syndrome status: results from a randomized controlled trial. Schizophr Res 2013; 146:190–195.
- Chen Y et al. Comparative effectiveness of switching antipsychotic drug treatment to aripiprazole or ziprasidone for improving metabolic profile and atherogenic dyslipidemia: a 12-month, prospective, open-label study. J Psychopharmacol 2012; 26:1201–1210.
- Smith RC et al. Effects of pioglitazone on metabolic abnormalities, psychopathology, and cognitive function in schizophrenic patients treated with antipsychotic medication: a randomized double-blind study. Schizophr Res 2013; 143:18–24.
Hypertension
There are two ways in which antipsychotic drugs may be associated with the development or worsening of hypertension.
- Slow steady rise in blood pressure over time. This may be linked to weight gain. Being overweight increases the risk of developing hypertension. The magnitude of the effect has been modelled using the Framingham data; for every 30 people who gain 4 kg, one will develop hypertension over the next 10 years.1 Note that this is a very modest weight gain, the majority of patients treated with some antipsychotics gain more than this, increasing further the risk of developing hypertension.
- Unpredictable rapid sharp increase in blood pressure on starting a new drug or increasing the dose. Increases in blood pressure occur shortly after starting, ranging from within hours of the first dose to a month. The information below relates to the pharmacological mechanism behind this and the antipsychotic drugs that are most implicated.
Postural hypotension is commonly associated with antipsychotic drugs that are antagonists at postsynaptic α1-adrenergic receptors. Examples include clozapine, chlorpromazine, quetiapine and risperidone. Some antipsychotics are also antagonists at pre-synaptic α2-adrenergic receptors; this can lead to increased release of norepinephrine, increased vagal activity and vasoconstriction. As all antipsychotics that are antagonists at α2- receptors are also antagonists at α1-receptors, the end result for any given patient can be difficult to predict, but for a very small number the result can be hypertension. Some antipsychotics are more commonly implicated than others, but individual patient factors are undoubtedly also important.
Receptor binding studies have demonstrated that clozapine, olanzapine and risperidone have the highest affinity for α2-adrenergic receptors2 so it might be predicted that these drugs would be most likely to cause hypertension. Most case reports implicate clozapine3–9 with some clearly describing normal blood pressure before clozapine was introduced, a sharp rise during treatment and return to normal when clozapine was discontinued. Blood pressure has also been reported to rise again on re-challenge and increased plasma catecholamines have been noted in some cases. Case reports also implicate aripiprazole,10–13 sulpiride,14 risperidone,8 quetiapine8 and ziprasidone.15
Data available through the Medical and Healthcare Products Regulatory Agency (MHRA) yellow card system indicate that clozapine is the antipsychotic drug most associated with hypertension. There are a very small number of reports with aripiprazole, olanzapine, quetiapine and risperidone.16 The timing of the onset of hypertension in these reports with respect to antipsychotic initiation is unknown, and likely to be variable.
In long-term treatment, hypertension is seen in around 30–40% of patients regardless of antipsychotic prescribed.17 A recent cross-sectional study found an increased risk of hypertension only for perphenazine,18 a finding not readily explained by its pharmacology.
No antipsychotic is contraindicated in essential hypertension but extreme care is needed when clozapine is prescribed. Concomitant treatment with SSRIs may increase risk of hypertension, possibly via inhibition of the metabolism of the antipsychotic.8 It is also (theoretically) possible that α2 antagonism may be at least partially responsible for clozapine-induced tachycardia and nausea.19
Treatment of antipsychotic-associated hypertension should follow standard protocols. There is specific evidence for the efficacy of valsartan and telmisartan in antipsychoticrelated hypertension.20
References
- Fontaine KR et al. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001; 101:277–288.
- Abi-Dargham A et al. Mechanisms of action of second generation antipsychotic drugs in schizophrenia: insights from brain imaging studies. Eur Psychiatry 2005; 20:15–27.
- Gupta S et al. Paradoxical hypertension associated with clozapine. Am J Psychiatry 1994; 151:148.
- Krentz AJ et al. Drug Points: Pseudophaeochromocytoma syndrome associated with clozapine. BMJ 2001; 322:1213.
- George TP et al. Hypertension after initiation of clozapine. Am J Psychiatry 1996; 153:1368–1369.
- Prasad SE et al. Pseudophaeochromocytoma associated with clozapine treatment. Ir J Psychol Med 2003; 20:132–134.
- Shiwach RS. Treatment of clozapine induced hypertension and possible mechanisms. Clin Neuropharmacol 1998; 21:139–140.
- Coulter D. Atypical antipsychotics may cause hypertension. Prescriber Update 2003; 24:4.
- Li JK et al. Clozapine: a mimicry of phaeochromocytoma. Aust N Z J Psychiatry 1997; 31:889–891.
- Borras L et al. Hypertension and aripiprazole. Am J Psychiatry 2005; 162:2392.
- Hsiao YL et al. Aripiprazole augmentation induced hypertension in major depressive disorder: a case report. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35:305–306.
- Pitchot W et al. Aripiprazole, hypertension, and confusion. J Neuropsychiatry Clin Neurosci 2010; 22:123.
- Yasui-Furukori N et al. Worsened hypertension control induced by aripiprazole. Neuropsychiatr Dis Treat 2013; 9:505–507.
- Mayer RD et al. Acute hypertensive episode induced by sulpiride - a case report. Hum Psychopharmacol 1989; 4:149–150.
- Villanueva N et al. Probable association between ziprasidone and worsening hypertension. Pharmacotherapy 2006; 26:1352–1357.
- Medicines and Healthcare Products Regulatory Agency. Drug Analysis Prints (DAPs). http://www.mhra.gov.uk/, 2014.
- Kelly AC et al. A naturalistic comparison of the long-term metabolic adverse effects of clozapine versus other antipsychotics for patients with psychotic illnesses. J Clin Psychopharmacol 2014; 34:441–445.
- Boden R et al. A comparison of cardiovascular risk factors for ten antipsychotic drugs in clinical practice. Neuropsychiatr Dis Treat 2013; 9:371–377.
- Pandharipande P et al. Alpha-2 agonists: can they modify the outcomes in the Postanesthesia Care Unit? Curr Drug Targets 2005; 6:749–754.
- Tse L et al. Pharmacological treatment of antipsychotic-induced dyslipidemia and hypertension. Int Clin Psychopharmacol 2014; 29:125–137.
Hyponatraemia
Hyponatraemia can occur in the context of the following.
- Water intoxication where water consumption exceeds the maximal renal clearance capacity. Serum and urine osmolality are low. Cross-sectional studies of chronically ill, hospitalised, psychiatric patients have found the prevalence of water intoxication to be approximately 5%.1,2 A longitudinal study found that 10% of severely ill patients with a diagnosis of schizophrenia had episodic hyponatraemia secondary to fluid overload.3 The primary aetiology is poorly understood. It has been postulated that it may be driven, at least in part, by an extreme compensatory response to the anticholinergic side-effects of antipsychotic drugs.4
- Drug-induced syndrome of inappropriate antidiuretic hormone (SIADH) where the kidney retains an excessive quantity of solute-free water. Serum osmolality is low and urine osmolality relatively high. The prevalence of SIADH has been estimated to be as high as 11% in acutely ill psychiatric patients.5 Risk factors for antidepressantinduced SIADH (increasing age, female gender, medical co-morbidity and polypharmacy) seem to be less relevant in the population of patients treated with antipsychotic drugs.6 SIADH usually develops in the first few weeks of treatment with the offending drug. Case reports and case series implicate phenothiazines, haloperidol, pimozide, risperidone, quetiapine, olanzapine, aripiprazole and clozapine.6–9 A systematic review10 and a case-control study11 each suggested a clear increase in risk of hyponatraemia with antipsychotics. Another review12 confirmed that drug-induced hyponatraemia is associated with concentrated urine and suggested that an antipsychotic was five times more likely than water intoxication to be the cause of hyponatraemia. Overall prevalence of antipsychotic-induced hyponatraemia has been estimated at 0.004%13 and 26.1%14 of patients. It is assumed the true figure is somewhere between these two extremes. Desmopressin use (for clozapine-induced enuresis) can also result in hyponatraemia.15
- Severe hyperlipidaemia and/or hyperglycaemia lead to secondary increases in plasma volume and 'pseudohyponatraemia'.4 Both are more common in people treated with antipsychotic drugs than in the general population and should be excluded as causes.
Mild to moderate hyponatraemia presents as confusion, nausea, headache and lethargy. As the plasma sodium falls, these symptoms become increasingly severe and seizures and coma can develop.
Monitoring of plasma sodium is desirable for all those receiving antipsychotics. Signs of confusion or lethargy should provoke thorough diagnostic analysis, including plasma sodium determination and urine osmolality.
Standard treatments for antipsychotic-induced hyponatraemia are summarised in Table 2.30. Recently introduced drugs such as tolvaptan,23 a so-called vaptan (nonpeptide arginine-vasopression antagonist—also known as aquaretics because they induce a highly hypotonic diuresis24), show promise in the treatment of hyponatraemia of varying aetiology, including that caused by drug-related SIADH.
Table 2.30 Treatment of antipsychotic-induced hyponatraemia
|
Cause of hyponatraemia
|
Antipsychotic drugs implicated
|
Treatment4,5
|
Water intoxication
(serum and urine osmolality
low)
|
Only very speculative evidence to support drugs as a cause Core part of illness in a minority of patients (e.g. psychotic polydipsia)
|
- Fluid restriction with careful monitoring of serum sodium, particularly diurnal variation (Na drops as the day progresses). Refer to specialist medical care if Na < 125 mmol/L. Note that the use of IV saline to correct hyponatraemia has been reported to precipitate rhabdomyolysis16
- Consider treatment with clozapine: shown to increase plasma osmolality into the normal range and increase urine osmolality (not usually reaching the normal range).17 These effects are consistent with reduced fluid intake.
This effect is not clearly related to improvements in mental state18
- There are both6 positive and negative reports for olanzapine19 and risperidone20 and one positive case report for quetiapine.21 Compared with clozapine, the evidence base is weak
- There is no evidence that either reducing or increasing the dose of an antipsychotic results in improvements in serum sodium in water-intoxicated patients22
- Demeclocycline should not be used (exerts its effect by interfering with ADH and increasing water excretion, already at capacity in these patients)
|
SIADH
(serum osmolality low; urine osmolality relatively high)
|
All antipsychotic drugs
|
- If mild, fluid restriction with careful monitoring of serum sodium. Refer to specialist medical care if Na < 125 mmol/L
- Switching to a different antipsychotic drug. There are insufficient data available to guide choice. Be aware that cross-sensitivity may occur (the individual may be predisposed and the choice of drug relatively less important)
- Consider demeclocycline (see formal prescribing instruction for details)
- Lithium may be effective6 but is a potentially toxic drug. Remember that hyponatraemia predisposes to lithium toxicity
|
|
ADH, antidiuretic hormone; IV, intravenous; SIADH, syndrome of inappropriate antidiuretic hormone.
|
References
- de Leon J et al. Polydipsia and water intoxication in psychiatric patients: a review of the epidemiological literature. Biol Psychiatry 1994; 35:408–419.
- Patel JK. Polydipsia, hyponatremia, and water intoxication among psychiatric patients. Hosp Community Psychiatry 1994; 45:1073–1074.
- de Leon J. Polydipsia—a study in a long-term psychiatric unit. Eur Arch Psychiatry Clin Neurosci 2003; 253:37–39.
- Siegel AJ et al. Primary and drug-induced disorders of water homeostasis in psychiatric patients: principles of diagnosis and management. Harv Rev Psychiatry 1998; 6:190–200.
- Siegler EL et al. Risk factors for the development of hyponatremia in psychiatric inpatients. Arch Intern Med 1995; 155:953–957.
- Madhusoodanan S et al. Hyponatraemia associated with psychotropic medications. A review of the literature and spontaneous reports. Adverse Drug React Toxicol Rev 2002; 21:17–29.
- Bachu K et al. Aripiprazole-induced syndrome of inappropriate antidiuretic hormone secretion (SIADH). Am J Ther 2006; 13:370–372.
- Dudeja SJ et al. Olanzapine induced hyponatraemia. Ulster Med J 2010; 79:104–105.
- Yam FK et al. Syndrome of inappropriate antidiuretic hormone associated with aripiprazole. Am J Health Syst Pharm 2013; 70:2110–2114.
- Meulendijks D et al. Antipsychotic-induced hyponatraemia: a systematic review of the published evidence. Drug Saf 2010; 33:101–114.
- Mannesse CK et al. Hyponatraemia as an adverse drug reaction of antipsychotic drugs: a case-control study in VigiBase. Drug Saf 2010; 33:569–578.
- Atsariyasing W et al. A systematic review of the ability of urine concentration to distinguish antipsychoticfrom psychosis-induced hyponatremia. Psychiatry Res 2014; 217:129–133.
- Letmaier M et al. Hyponatraemia during psychopharmacological treatment: results of a drug surveillance programme. Int J Neuropsychopharmacol 2012; 15:739–748.
- Serrano A et al. Safety of long-term clozapine administration. Frequency of cardiomyopathy and hyponatraemia: two cross-sectional, naturalistic studies. Aust N Z J Psychiatry 2014; 48:183–192.
- Sarma S et al. Severe hyponatraemia associated with desmopressin nasal spray to treat clozapine-induced nocturnal enuresis. Aust N Z J Psychiatry 2005; 39:949.
- Zaidi AN. Rhabdomyolysis after correction of hyponatremia in psychogenic polydipsia possibly complicated by ziprasidone. Ann Pharmacother 2005; 39:1726–1731.
- Canuso CM et al. Clozapine restores water balance in schizophrenic patients with polydipsia-hyponatremia syndrome. J Neuropsychiatry Clin Neurosci 1999; 11:86–90.
- Spears NM et al. Clozapine treatment in polydipsia and intermittent hyponatremia. J Clin Psychiatry 1996; 57:123–128.
- Littrell KH et al. Effects of olanzapine on polydipsia and intermittent hyponatremia. J Clin Psychiatry 1997; 58:549.
- Kawai N et al. Risperidone failed to improve polydipsia-hyponatremia of the schizophrenic patients. Psychiatry Clin Neurosci 2002; 56:107–110.
- Montgomery JH et al. Adjunctive quetiapine treatment of the polydipsia, intermittent hyponatremia, and psychosis syndrome: a case report. J Clin Psychiatry 2003; 64:339–341.
- Canuso CM et al. Does minimizing neuroleptic dosage influence hyponatremia? Psychiatry Res 1996; 63:227–229.
- Josiassen RC et al. Tolvaptan: a new tool for the effective treatment of hyponatremia in psychotic disorders. Expert Opin Pharmacother 2010; 11:637–648.
- Decaux G et al. Non-peptide arginine-vasopressin antagonists: the vaptans. Lancet 2008; 371:1624–1632.
Hyperprolactinaemia
Dopamine inhibits prolactin release and so dopamine antagonists can be expected to increase prolactin plasma levels. All antipsychotics cause measurable changes in prolactin but some do not increase prolactin above the normal range at standard doses. These drugs are asenapine, clozapine, olanzapine, quetiapine, lurasidone, aripiprazole and ziprasidone.1–6 Even with these drugs (particularly olanzapine and ziprasidone), raised prolactin and prolactin-related symptoms are occasionally reported.7–10 Aripiprazole usually decreases plasma prolactin.11 With all drugs, the degree of prolactin elevation is probably dose-related,12 and for most the threshold activity (D2 occupancy) for increased prolactin is very close to that of therapeutic efficacy.13
Hyperprolactinaemia is often superficially asymptomatic (that is, the patient does not spontaneously report problems) and there is some evidence that hyperprolactinaemia does not affect subjective quality of life.14 Nonetheless, persistent elevation of plasma prolactin is associated with a number of adverse consequences. These include sexual dysfunction15–18 (but note that other pharmacological activities also give rise to sexual dysfunction), reductions in bone mineral density,19–22 menstrual disturbances,2,23 breast growth and galactorrhoea,23 suppression of the hypothalamic-pituitary-gonadal axis24 and a possible increase in the risk of breast cancer.2,25–27
Monitoring
All patients should have a prolactin level measured before starting any antipsychotic known to be associated with raised prolactin. This gives a baseline measure against which any change can be gauged. At 3 months, all patients should be asked about prolactin-related symptoms (sexual dysfunction, amenorrhoea, etc.). If hyperprolactinaemia is suspected, another prolactin level should be obtained. Where prolactin is high and the patient is symptomatic, switching to an antipsychotic that is less likely to raise prolactin (see Box 2.1) should be considered. Where prolactin is high but the patient is not symptomatic, the clinical implications of the test results should be discussed with the patient and a joint decision taken on whether to continue current treatment with annual monitoring or switch to another antipsychotic.
Prolactin-elevating drugs (amisulpride, sulpiride, risperidone, paliperidone, FGAs) should, if possible, be avoided in the following patient groups:
- patients under 25 years of age (i.e. before peak bone mass)
- patients with osteoporosis
- patients with a history of hormone-dependent breast cancer.
|
Box 2.1 Established antipsychotics not usually associated with hyperprolactinaemia
|
- Aripiprazole
- Asenapine
- Clozapine
- Lurasidone
- Olanzapine
- Quetiapine
- Ziprasidone
|
Long-term use of prolactin-elevating drugs should probably be avoided in young women, given the possible increased risk of breast cancer and the known risk of decreased bone mineral density.
Prolactin concentration interpretation28
- Take blood sample at least 1 hour after waking or eating.
- Minimise stress during venepuncture (stress elevates plasma prolactin).
- Treatment of hyperprolactinaemia depends more on symptoms and long-term risk than on measured plasma level.
| Normal |
Women |
0–25 ng/mL |
~0–530 mIU/L |
|
Men |
0–20 ng/mL |
~0–424 mIU/L |
- Need systematic assessment of prolactin-related side-effects and discussion of clinical consequences of prolonged raised prolactin if prolactin concentration 25–118 ng/mL (530–2500 mIU/L)
- Need referral for tests to rule out prolactinoma if prolactin concentration > 118 ng/ mL (> 2500 mIU/L)
Symptoms (reduced libido, infertility) are usually seen with prolactin levels are above 31–50 ng/mL (~660–1060 mIU/L). When levels exceed 100 ng/mL (~2120 mIU/L) there is usually galactorrhoea and amenorrhoea.29
Treatment
For most patients with symptomatic hyperprolactinaemia, a switch to a non prolactinelevating drug (see Box 2.1) is the first choice.2,18,30,31 (Limited data32–34 suggest asenapine and lurasidone have minimal effects on prolactin.) An alternative is to add aripiprazole to existing treatment35–39–hyperprolactinaemia and related symptoms are reported to improve fairly promptly following the addition of aripiprazole. The effect of co-administered aripiprazole on prolactin is dose-dependent: 3 mg/day is effective but 6 mg/day more so. Higher doses appear unnecessary.40 When switching, symptoms tend to resolve slowly and symptom severity does not always reflect prolactin changes.30 Genetic differences may play a part.41 Where aripiprazole augmentation has been successful, consideration should be given to slowly reducing the dose of the antipsychotic responsible for raising prolactin, with the aim of maintaining the patient on aripiprazole as the sole antipsychotic. Only if this strategy fails should long-term combined antipsychotics be considered.
For patients who need to remain on a prolactin-elevating antipsychotic and who cannot tolerate aripiprazole, dopamine agonists may be effective.3,42,43 Amantadine, carbergoline and bromocriptine have all been used, but each has the potential to worsen psychosis (although this has not been reported in trials). A herbal remedy—Peony Glycyrrhiza Decoction—has also been shown to be effective.44
References
- David SR et al. The effects of olanzapine, risperidone, and haloperidol on plasma prolactin levels in patients with schizophrenia. Clin Ther 2000; 22:1085–1096.
- Haddad PM et al. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs 2004; 64:2291–2314.
- Hamner MB et al. Hyperprolactinaemia in antipsychotic-treated patients: guidelines for avoidance and management. CNS Drugs 1998; 10:209–222.
- Bushe C et al. Comparison of metabolic and prolactin variables from a six-month randomised trial of olanzapine and quetiapine in schizophrenia. J Psychopharmacol 2010; 24:1001–1009.
- Byerly MJ et al. Effects of aripiprazole on prolactin levels in subjects with schizophrenia during cross-titration with risperidone or olanzapine: Analysis of a randomized, open-label study. Schizophr Res 2009; 107:218–222.
- Leucht S et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013; 382:951–962.
- Melkersson K. Differences in prolactin elevation and related symptoms of atypical antipsychotics in schizophrenic patients. J Clin Psychiatry 2005; 66:761–767.
- Kopecek M et al. Ziprasidone-induced galactorrhea: a case report. Neuro Endocrinol Lett 2005; 26:69–70.
- Buhagiar K et al. Quetiapine-Induced Hyperprolactinemic Galactorrhea in an Adolescent Male. German J Psychiatry 2006; 9:118–120.
- Johnsen E et al. Antipsychotic-induced hyperprolactinemia: a cross-sectional survey. J Clin Psychopharmacol 2008; 28:686–690.
- Suzuki Y et al. Differences in plasma prolactin levels in patients with schizophrenia treated on monotherapy with five second-generation antipsychotics. Schizophr Res 2013; 145:116–119.
- Staller J. The effect of long-term antipsychotic treatment on prolactin. J Child Adolesc Psychopharmacol 2006; 16:317–326.
- Tsuboi T et al. Hyperprolactinemia and estimated dopamine D2 receptor occupancy in patients with schizophrenia: analysis of the CATIE data. Prog Neuropsychopharmacol Biol Psychiatry 2013; 45:178–182.
- Kaneda Y. The impact of prolactin elevation with antipsychotic medications on subjective quality of life in patients with schizophrenia. Clin Neuropharmacol 2003; 26:182–184.
- Bobes J et al. Frequency of sexual dysfunction and other reproductive side-effects in patients with schizophrenia treated with risperidone, olanzapine, quetiapine, or haloperidol: the results of the EIRE study. J Sex Marital Ther 2003; 29:125–147.
- Smith S. Effects of antipsychotics on sexual and endocrine function in women: implications for clinical practice. J Clin Psychopharmacol 2003; 23:S27–S32.
- Spollen JJ, III et al. Prolactin levels and erectile function in patients treated with risperidone. J Clin Psychopharmacol 2004; 24:161–166.
- Knegtering R et al. A randomized open-label study of the impact of quetiapine versus risperidone on sexual functioning. J Clin Psychopharmacol 2004; 24:56–61.
- Becker D et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry 2003; 64:761–766.
- Meaney AM et al. Reduced bone mineral density in patients with schizophrenia receiving prolactin raising anti-psychotic medication. J Psychopharmacol 2003; 17:455–458.
- Meaney AM et al. Effects of long-term prolactin-raising antipsychotic medication on bone mineral density in patients with schizophrenia. Br J Psychiatry 2004; 184:503–508.
- Kishimoto T et al. Antipsychotic-induced hyperprolactinemia inhibits the hypothalamo-pituitary-gonadal axis and reduces bone mineral density in male patients with schizophrenia. J Clin Psychiatry 2008; 69:385–391.
- Wieck A et al. Antipsychotic-induced hyperprolactinaemia in women: pathophysiology, severity and consequences. Selective literature review. Br J Psychiatry 2003; 182:199–204.
- Smith S et al. The effects of antipsychotic-induced hyperprolactinaemia on the hypothalamic-pituitary-gonadal axis. J Clin Psychopharmacol 2002; 22:109–114.
- Halbreich U et al. Are chronic psychiatric patients at increased risk for developing breast cancer? Am J Psychiatry 1996; 153:559–560.
- Wang PS et al. Dopamine antagonists and the development of breast cancer. Arch Gen Psychiatry 2002; 59:1147–1154.
- Harvey PW et al. Adverse effects of prolactin in rodents and humans: breast and prostate cancer. J Psychopharmacol 2008; 22:20–27.
- Walters J et al. Clinical questions and uncertainty—prolactin measurement in patients with schizophrenia and bipolar disorder. J Psychopharmacol 2008; 22:82–89.
- Serri O et al. Diagnosis and management of hyperprolactinemia. CMAJ 2003; 169:575–581.
- Duncan D et al. Treatment of psychotropic-induced hyperprolactinaemia. Psychiatr Bull 1995; 19:755–757.
- Anghelescu I et al. Successful switch to aripiprazole after induction of hyperprolactinemia by ziprasidone: a case report. J Clin Psychiatry 2004; 65:1286–1287.
- McIntyre RS et al. Asenapine in the treatment of acute mania in bipolar I disorder: a randomized, double-blind, placebo-controlled trial. J Affect Disord 2010; 122:27–38.
- Stahl SM et al. Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: a 6-month, open-label, extension study. J Clin Psychiatry 2013; 74:507–515.
- McEvoy JP et al. Effectiveness of lurasidone in patients with schizophrenia or schizoaffective disorder switched from other antipsychotics: a randomized, 6-week, open-label study. J Clin Psychiatry 2013; 74:170–179.
- Shim JC et al. Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebocontrolled trial. Am J Psychiatry 2007; 164:1404–1410.
- Lorenz RA et al. Resolution of haloperidol-induced hyperprolactinemia with aripiprazole. J Clin Psychopharmacol 2007; 27:524–525.
- Lu ML et al. Time course of the changes in antipsychotic-induced hyperprolactinemia following the switch to aripiprazole. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:1978–1981.
- Mir A et al. Change in sexual dysfunction with aripiprazole: a switching or add-on study. J Psychopharmacol 2008; 22:244–253.
- van KM et al. Preliminary report: a naturalistic study of the effect of aripiprazole addition on risperidone-related hyperprolactinemia in patients treated with risperidone long-acting injection. J Clin Psychopharmacol 2011; 31:126–128.
- Yasui-Furukori N et al. Dose-dependent effects of adjunctive treatment with aripiprazole on hyperprolactinemia induced by risperidone in female patients with schizophrenia. J Clin Psychopharmacol 2010; 30:596–599.
- Young RM et al. Prolactin levels in antipsychotic treatment of patients with schizophrenia carrying the DRD2*A1 allele. Br J Psychiatry 2004; 185:147–151.
- Duncan D et al. Treatment of psychotropic-induced hyperprolactinaemia. Psychiatr Bull 1995; 19:755–757.
- Cavallaro R et al. Cabergoline treatment of risperidone-induced hyperprolactinemia: a pilot study. J Clin Psychiatry 2004; 65:187–190.
- Yuan HN et al. A randomized, crossover comparison of herbal medicine and bromocriptine against risperidone-induced hyperprolactinemia in patients with schizophrenia. J Clin Psychopharmacol 2008; 28:264–370.
Sexual dysfunction
Primary sexual disorders are common, although reliable normative data are lacking.1 Physical illness, psychiatric illness, substance misuse and prescribed drug treatment can all cause sexual dysfunction.2 It has been estimated that 50–60% of people with schizophrenia have problems with sexual dysfunction compared with 30% of the general population,3 but note that in both groups reported prevalence rates vary depending on the method of data collection (low numbers with spontaneous reports, increasing with confidential questionnaires and further still with direct questioning2). In one study of patients with psychosis, 37% spontaneously reported sexual problems but 46% were found to be experiencing difficulties when directly questioned.4
Baseline sexual functioning should be determined if possible (questionnaires may be useful) because sexual function can affect quality of life5 and compliance with medication (sexual dysfunction is one of the major causes of treatment dropout).6 Complaints of sexual dysfunction may also indicate progression or inadequate treatment of underlying medical or psychiatric conditions.7,8 Sexual problems may also be caused by drug treatment where intervention may greatly improve quality of life.9
There are four phases of the human sexual response, as detailed in Table 2.31.2,10,11
Effects of psychosis
Sexual dysfunction is already known to be a problem in first-episode schizophrenia12 and up to 82% of men and 96% of women with established illness report problems, with associated reductions in quality of life.5 Men13 complain of reduced desire, inability to achieve an erection and premature ejaculation, whereas women complain more generally about reduced enjoyment.13,14 Women with psychosis are known to have reduced fertility.15 People with psychosis are less able to develop good psychosexual relationships and, for some, treatment with an antipsychotic can improve sexual functioning.16 Assessment of sexual functioning can clearly be difficult in someone who is psychotic. The Arizona Sexual Experience Scale (ASEX) may be useful in this respect.17
Table 2.31 The human sexual response
|
Desire
|
- Related to testosterone levels in men
- Possibly increased by dopamine and decreased by prolactin
- Psychosocial context and conditioning significantly affect desire
|
|
Arousal
|
- Influenced by testosterone in men and oestrogen in women
- Other potential mechanisms include: central dopamine stimulation, modulation of the cholinergic/adrenergic balance, peripheral a1 agonism and nitric oxide pathways
- Physical pathology such as hypertension or diabetes can have a significant effect
|
|
Orgasm
|
- May be related to oxytocin
- Inhibition of orgasm may be caused by an increase in serotonin activity and raised prolactin, as well as a1 blockade
|
|
Resolution
|
- Occurs passively after orgasm
|
|
Note: Many other hormones and neurotransmitters may interact in a complex way at each phase.
|
Effects of antipsychotic drugs
Sexual dysfunction has been reported as a side-effect of all antipsychotics, and up to 45% of people taking older or conventional antipsychotics experience sexual dysfunction.18 Individual susceptibility varies and all effects are reversible. Note though that physical illness and drugs other than antipsychotics can cause sexual dysfunction and many studies do not control for either, making the prevalence of sexual dysfunction with different antipsychotics difficult to compare.19
Antipsychotics decrease dopaminergic transmission, which in itself can decrease libido but may also increase prolactin levels via negative feedback. It has been estimated that prolactin elevation explains 40% of the sexual dysfunction that is associated with antipsychotic medication.3 Hyperprolactinaemia can also cause amenorrhoea in women, and breast enlargement and galactorrhoea in both men and women.20 Although it has been suggested that the overall propensity of an antipsychotic to cause sexual dysfunction is related to propensity to raise prolactin, i.e. risperidone > haloperidol > olanzapine > quetiapine > aripiprazole,7,19,21 it should be noted that in the CUtLASS-1 study, FGAs (primarily sulpiride, but also other FGAs known to be associated with prolactin elevation) did not fare any worse than SGAs (70% of patients in this arm were prescribed an antipsychotic not associated with prolactin elevation) with respect to worsening sexual dysfunction. In fact, sexual functioning improved in both arms over the one year duration of the study.16 Aripiprazole is relatively free of sexual side-effects when used as monotherapy22 and possibly also in combination with another antipsychotic.23,24
Anticholinergic effects can cause disorders of arousal25 and drugs that block periph-
eral α1-receptors cause particular problems with erection and ejaculation in men.9 Drugs that are antagonists at both peripheral α1-receptors and cholinergic receptors can cause priapism.26 Antipsychotic-induced sedation and weight gain may reduce sexual desire.26 These principles can be used to predict the sexual side-effects of different antipsychotic drugs (Table 2.32).
Treatment
Before attempting to treat sexual dysfunction, a thorough assessment is essential to determine the most likely cause. Assuming that physical pathology (diabetes, hypertension, cardiovascular disease, etc.) has been excluded, the following principles apply.
Spontaneous remission may occasionally occur.26 The most obvious first step is to decrease the dose or discontinue the offending drug where appropriate. The next step is to switch to a different drug that is less likely to cause the specific sexual problem experienced (see Table 2.32). Another option is to add 5–10 mg aripiprazole—this can normalise prolactin and improve sexual function.27–29 If this fails or is not practicable, 'antidote' drugs can be tried: for example, cyproheptadine (a 5HT2 antagonist at doses of 4–16 mg/day) has been used to treat SSRI-induced sexual dysfunction but sedation is a common side-effect. Amantadine, bupropion, buspirone, bethanechol and yohimbine have all been used with varying degrees of success but have a number of unwanted side-effects and interactions with other drugs (Table 2.33). Given that hyperprolactinaemia may contribute to sexual dysfunction, selegiline (enhances dopamine activity) has been tested in an RCT. This was negative.53 Testosterone patches have been shown to increase libido in women, although note though that breast cancer risk may be significantly increased.54,55 The evidence base supporting the use of 'antidotes' is poor.26
Table 2.32 Sexual adverse effects of antipsychotics
|
Drug
|
Type of problem
|
Phenothiazines
(e.g. chlorpromazine)
|
- Hyperprolactinaemia and anticholinergic effects. Reports of delayed orgasm at lower doses followed by normal orgasm but without ejaculation at higher doses14
- Most problems occur with thioridazine (which can also reduce testosterone levels)30
- Priapism has been reported with thioridazine, risperidone and chlorpromazine (probably due to α1 blockade)31-33
|
Thioxanthenes
(e.g. flupentixol)
|
- Arousal problems and anorgasmia34
|
|
Haloperidol
|
- Similar problems to the phenothiazines35 but anticholinergic effects reduced31
- Prevalence of sexual dysfunction reported to be up to 70%36
|
|
Olanzapine
|
- Possibly less sexual dysfunction due to relative lack of prolactin-related effects35
- Priapism reported rarely37,38
- Prevalence of sexual dysfunction reported to be > 50%36
|
|
Risperidone
|
- Potent elevator of serum prolactin
- Less anticholinergic
- Specific peripheral α1-adrenergic blockade leads to a moderately high reported incidence of ejaculatory problems such as retrograde ejaculation39,40
- Priapism reported rarely26
- Prevalence of sexual dysfunction reported to be 60-70%36
|
|
Sulpiride/amisulpride
|
- Potent elevators of serum prolactin18 but note that sulpiride (as the main FGA prescribed in the study) was not associated with greater sexual dysfunction than SGAs (with variable ability to raise prolactin) in the CUtLASS-1 study.16
|
|
Quetiapine
|
- No effect on serum prolactin41
- Possibly associated with low risk of sexual dysfunction,42-45 but studies are conflicting46,47
|
|
Clozapine
|
- Significant α1-adrenergic blockade and anticholinergic effects.48 No effect on prolactin49
- Probably fewer problems than with typical antipsychotics50
|
|
Aripiprazole
|
- No effect on prolactin or α1-receptors. No reported adverse effects on sexual function. Improves sexual function in those switched from other antipsychotics22,24,51 Case reports of aripiprazole-induced hypersexuality have been published52
|
|
FGA, first-generation antipsychotic; SGA, second-generation antipsychotic.
|
Table 2.33 Remedial treatments for psychotropic-induced sexual dysfunction
|
Drug
|
Pharmacology
|
potential treatment for
|
Side-effects
|
|
Alprostadil1,11
|
Prostaglandin
|
Erectile dysfunction
|
Pain, fibrosis, hypotension, priapism
|
|
Amantadine1,56
|
Dopamine agonist
|
Prolactin-induced reduction in desire and arousal (dopamine increases libido and facilitates ejaculation)
|
Return of psychotic symptoms, GI effects, nervousness, insomnia, rash
|
|
Bethanechol57
|
Cholinergic or cholinergic potentiation of adrenergic neurotransmission
|
Anticholinergic induced arousal problems and anorgasmia (from TCAs, antipsychotics, etc)
|
Nausea and vomiting, colic, bradycardia, blurred vision, sweating
|
|
Bromocriptine9
|
Dopamine agonist
|
Prolactin-induced reduction in desire and arousal
|
Return of psychotic symptoms, GI effects
|
|
Bupropion58
|
Noradrenaline and dopamine reuptake inhibitor
|
SSRI-induced sexual dysfunction (evidence poor)
|
Concentration problems, reduced sleep, tremor
|
|
Buspirone59
|
5HT1a partial agonist
|
SSRI-induced sexual dysfunction,
particularly decreased libido and anorgasmia
|
Nausea, dizziness, headache
|
|
Cyproheptadine1,59,60
|
5HT2 antagonist
|
Sexual dysfunction caused by increased serotonin transmission (e.g. SSRIs), particularly anorgasmia
|
Sedation and fatigue. Reversal of the therapeutic
effect of antidepressants
|
|
Sildenafil11,61-64
|
Phosphodiesterase inhibitor
|
Erectile dysfunction of any aetiology Anorgasmia in women. Effective when prolactin raised
|
Mild headaches, dizziness, nasal congestion
|
|
Yohimbine1,11,65-67
|
Central and peripheral α2 adrenoceptor antagonist
|
SSRI-induced sexual dysfunction, particularly erectile dysfunction, decreased libido and anorgasmia (evidence poor)
|
Anxiety, nausea, fine tremor, increased blood pressure, sweating, fatigue
|
|
Note: The use of the drugs listed above should ideally be under the care or supervision of a specialist in sexual dysfunction.
GI, gastrointestinal; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
|
Drugs such as sildenafil (Viagra) or alprostadil (Caverject) are effective only in the treatment of erectile dysfunction. Psychological approaches used by sexual dysfunction clinics may be difficult for clients with mental health problems to engage in.9
References
- Baldwin DS et al. Effects of antidepressant drugs on sexual function. Int J Psychiatry Clin Pract 1997; 1:47–58.
- Pollack MH et al. Genitourinary and sexual adverse effects of psychotropic medication. Int J Psychiatry Med 1992; 22:305–327.
- Nunes LV et al. Strategies for the treatment of antipsychotic-induced sexual dysfunction and/or hyperprolactinemia among patients of the schizophrenia spectrum: a review. J Sex Marital Ther 2012; 38:281–301.
- Montejo AL et al. Frequency of sexual dysfunction in patients with a psychotic disorder receiving antipsychotics. J Sex Med 2010; 7:3404–3413.
- Olfson M et al. Male sexual dysfunction and quality of life in schizophrenia. J Clin Psychiatry 2005; 66:331–338.
- Montejo AL et al. Incidence of sexual dysfunction associated with antidepressant agents: a prospective multicenter study of 1022 outpatients. Spanish Working Group for the Study of Psychotropic-Related Sexual Dysfunction. J Clin Psychiatry 2001; 62 Suppl 3:10–21.
- Baggaley M. Sexual dysfunction in schizophrenia: focus on recent evidence. Hum Psychopharmacol 2008; 23:201–209.
- Ucok A et al. Sexual dysfunction in patients with schizophrenia on antipsychotic medication. Eur Psychiatry 2007; 22:328–333.
- Segraves RT. Effects of psychotropic drugs on human erection and ejaculation. Arch Gen Psychiatry 1989; 46:275–284.
- Stahl SM. The psychopharmacology of sex, Part 1: Neurotransmitters and the 3 phases of the human sexual response. J Clin Psychiatry 2001; 62:80–81.
- Garcia-Reboll L et al. Drugs for the treatment of impotence. Drugs Aging 1997; 11:140–151.
- Bitter I et al. Antipsychotic treatment and sexual functioning in first-time neuroleptic-treated schizophrenic patients. Int Clin Psychopharmacol 2005; 20:19–21.
- Macdonald S et al. Nithsdale Schizophrenia Surveys 24: sexual dysfunction. Case-control study. Br J Psychiatry 2003; 182:50–56.
- Smith S. Effects of antipsychotics on sexual and endocrine function in women: implications for clinical practice. J Clin Psychopharmacol 2003; 23:S27–S32.
- Howard LM et al. The general fertility rate in women with psychotic disorders. Am J Psychiatry 2002; 159:991–997.
- Peluso MJ et al. Non-neurological and metabolic side effects in the Cost Utility of the Latest Antipsychotics in Schizophrenia Randomised Controlled Trial (CUtLASS-1). Schizophr Res 2013; 144:80–86.
- Byerly MJ et al. An empirical evaluation of the Arizona sexual experience scale and a simple one-item screening test for assessing antipsychotic-related sexual dysfunction in outpatients with schizophrenia and schizoaffective disorder. Schizophr Res 2006; 81:311–316.
- Smith SM et al. Sexual dysfunction in patients taking conventional antipsychotic medication. Br J Psychiatry 2002; 181:49–55.
- Serretti A et al. A meta-analysis of sexual dysfunction in psychiatric patients taking antipsychotics. Int Clin Psychopharmacol 2011; 26:130–140.
- Anon. Adverse effects of the atypical antipsychotics. Collaborative Working Group on Clinical Trial Evaluations. J Clin Psychiatry 1998; 59 Suppl 12:17–22.
- Knegtering H et al. Are sexual side effects of prolactin-raising antipsychotics reducible to serum prolactin? Psychoneuroendocrinology 2008; 33:711–717.
- Hanssens L et al. The effect of antipsychotic medication on sexual function and serum prolactin levels in community-treated schizophrenic patients: results from the Schizophrenia Trial of Aripiprazole (STAR) study (NCT00237913). BMC Psych 2008; 8:95.
- Mir A et al. Change in sexual dysfunction with aripiprazole: a switching or add-on study. J Psychopharmacol 2008; 22:244–253.
- Byerly MJ et al. Effects of aripiprazole on prolactin levels in subjects with schizophrenia during cross-titration with risperidone or olanzapine: Analysis of a randomized, open-label study. Schizophr Res 2009; 107:218–222.
- Aldridge SA. Drug-induced sexual dysfunction. Clin Pharm 1982; 1:141–147.
- Baldwin D et al. Sexual side-effects of antidepressant and antipsychotic drugs. Adv Psychiatr Treat 2003; 9:202–210.
- Shim JC et al. Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebocontrolled trial. Am J Psychiatry 2007; 164:1404–1410.
- Yasui-Furukori N et al. Dose-dependent effects of adjunctive treatment with aripiprazole on hyperprolactinemia induced by risperidone in female patients with schizophrenia. J Clin Psychopharmacol 2010; 30:596–599.
- Trives MZ et al. Effect of the addition of aripiprazole on hyperprolactinemia associated with risperidone long-acting injection. J Clin Psychopharmacol 2013; 33:538–541.
- Kotin J et al. Thioridazine and sexual dysfunction. Am J Psychiatry 1976; 133:82–85.
- Mitchell JE et al. Antipsychotic drug therapy and sexual dysfunction in men. Am J Psychiatry 1982; 139:633–637.
- Loh C et al. Risperidone-induced retrograde ejaculation: case report and review of the literature. Int Clin Psychopharmacol 2004; 19:111–112.
- Thompson JW, Jr. et al. Psychotropic medication and priapism: a comprehensive review. J Clin Psychiatry 1990; 51:430–433.
- Aizenberg D et al. Sexual dysfunction in male schizophrenic patients. J Clin Psychiatry 1995; 56:137–141.
- Crawford AM et al. The acute and long-term effect of olanzapine compared with placebo and haloperidol on serum prolactin concentrations. Schizophr Res 1997; 26:41–54.
- Serretti A et al. Sexual side effects of pharmacological treatment of psychiatric diseases. Clin Pharmacol Ther 2011; 89:142–147.
- Dossenbach M et al. Effects of atypical and typical antipsychotic treatments on sexual function in patients with schizophrenia: 12-month results from the Intercontinental Schizophrenia Outpatient Health Outcomes (IC-SOHO) study. Eur Psychiatry 2006; 21:251–258.
- Aurobindo Pharma - Milpharm Ltd. Summary of Product Characteristics. Olanzapine 10 mg tablets. 2013. http://www.medicines.org.uk/ emc/medicine/27661/SPC/Olanzapine++10+mg+tablets/
- Tran PV et al. Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. J Clin Psychopharmacol 1997; 17:407–418.
- Raja M. Risperidone-induced absence of ejaculation. Int Clin Psychopharmacol 1999; 14:317–319.
- Peuskens J et al. A comparison of quetiapine and chlorpromazine in the treatment of schizophrenia. Acta Psychiatr Scand 1997; 96:265–273.
- Bobes J et al. Frequency of sexual dysfunction and other reproductive side-effects in patients with schizophrenia treated with risperidone, olanzapine, quetiapine, or haloperidol: the results of the EIRE study. J Sex Marital Ther 2003; 29:125–147.
- Byerly MJ et al. An open-label trial of quetiapine for antipsychotic-induced sexual dysfunction. J Sex Marital Ther 2004; 30:325–332.
- Knegtering R et al. A randomized open-label study of the impact of quetiapine versus risperidone on sexual functioning. J Clin Psychopharmacol 2004; 24:56–61.
- Montejo Gonzalez AL et al. A 6-month prospective observational study on the effects of quetiapine on sexual functioning. J Clin Psychopharmacol 2005; 25:533–538.
- Atmaca M et al. A new atypical antipsychotic: quetiapine-induced sexual dysfunctions. Int J Impot Res 2005; 17:201–203.
- Kelly DL et al. A randomized double-blind 12-week study of quetiapine, risperidone or fluphenazine on sexual functioning in people with schizophrenia. Psychoneuroendocrinology 2006; 31:340–346.
- Coward DM. General pharmacology of clozapine. Br J Psychiatry 1992; 160:5–11.
- Meltzer HY et al. Effect of clozapine on human serum prolactin levels. Am J Psychiatry 1979; 136:1550–1555.
- Aizenberg D et al. Comparison of sexual dysfunction in male schizophrenic patients maintained on treatment with classical antipsychotics versus clozapine. J Clin Psychiatry 2001; 62:541–544.
- Rykmans V et al. A comparision of switching strategies from risperidone to aripiprazole in patients with schizophrenia with insufficient efficacy/tolerability on risperidone (cn138-169). Eur Psychiatry 2008; 23:S111.
- Chen CY et al. Improvement of serum prolactin and sexual function after switching to aripiprazole from risperidone in schizophrenia: a case series. Psychiatry Clin Neurosci 2011; 65:95–97.
- Kodesh A et al. Selegiline in the treatment of sexual dysfunction in schizophrenic patients maintained on neuroleptics: a pilot study. Clin Neuropharmacol 2003; 26:193–195.
- Davis SR et al. Testosterone for low libido in postmenopausal women not taking estrogen. N Engl J Med 2008; 359:2005–2017.
- Schover LR. Androgen therapy for loss of desire in women: is the benefit worth the breast cancer risk? Fertil Steril 2008; 90:129–140.
- Valevski A et al. Effect of amantadine on sexual dysfunction in neuroleptic-treated male schizophrenic patients. Clin Neuropharmacol 1998; 21:355–357.
- Gross MD. Reversal by bethanechol of sexual dysfunction caused by anticholinergic antidepressants. Am J Psychiatry 1982; 139:1193–1194.
- Masand PS et al. Sustained-release bupropion for selective serotonin reuptake inhibitor-induced sexual dysfunction: a randomized, doubleblind, placebo-controlled, parallel-group study. Am J Psychiatry 2001; 158:805–807.
- Rothschild AJ. Sexual side effects of antidepressants. J Clin Psychiatry 2000; 61 Suppl 11:28–36.
- Lauerma H. Successful treatment of citalopram-induced anorgasmia by cyproheptadine. Acta Psychiatr Scand 1996; 93:69–70.
- Nurnberg HG et al. Sildenafil for women patients with antidepressant-induced sexual dysfunction. Psychiatr Serv 1999; 50:1076–1078.
- Salerian AJ et al. Sildenafil for psychotropic-induced sexual dysfunction in 31 women and 61 men. J Sex Marital Ther 2000; 26:133–140.
- Nurnberg HG et al. Treatment of antidepressant-associated sexual dysfunction with sildenafil: a randomized controlled trial. JAMA 2003; 289:56–64.
- Gopalakrishnan R et al. Sildenafil in the treatment of antipsychotic-induced erectile dysfunction: a randomized, double-blind, placebo-controlled, flexible-dose, two-way crossover trial. Am J Psychiatry 2006; 163:494–499.
- Jacobsen FM. Fluoxetine-induced sexual dysfunction and an open trial of yohimbine. J Clin Psychiatry 1992; 53:119–122.
- Michelson D et al. Mirtazapine, yohimbine or olanzapine augmentation therapy for serotonin reuptake-associated female sexual dysfunction: a randomized, placebo controlled trial. J Psychiatr Res 2002; 36:147–152.
- Woodrum ST et al. Management of SSRI-induced sexual dysfunction. Ann Pharmacother 1998; 32:1209–1215.
Pneumonia
A Dutch database study published in 20081 found that current use of antipsychotics was associated with a 60% increased risk of pneumonia in an elderly population. Risk was highest in the first week of treatment and an increased risk was seen with SGAs but not FGAs. Another study2 found a higher rate of chest infection in people taking SGAs. Three further studies found a dose-related increased risk of pneumonia in older people taking both FGAs and SGAs.3–5 The risk was again noted to be highest in the first week(s) of treatment. More recently, a study of patients with bipolar affective disorder found that clozapine, olanzapine and haloperidol were linked to increased rates of pneumonia while lithium was protective.6 Another recent study suggests amisulpride is not linked to pneumonia.7 Schizophrenia itself seems to afford a higher risk of complications (e.g. admission to intensive care) in people diagnosed with pneumonia.8
The mechanism by which antipsychotics increase the risk of pneumonia is not known. Possibilities include sedation (risk seems to be highest with drugs that show greatest H1 antagonism3,7); dystonia or dyskinesia; dry mouth causing poor bolus transport and so increasing the risk of aspiration; general poor physical health;7 or perhaps some illdefined effect on immune response.1,3 With clozapine, pneumonia may also be secondary to constipation.9
An increased risk of pneumonia should be assumed for all patients (regardless of age) taking any antipsychotic for any period. All patients should be very carefully monitored for signs of chest infection and effective treatment started promptly. Early referral to general medical services should be considered where there is any doubt about the severity or type of chest infection.
Summary
- Assume the use of all antipsychotics increase the risk of pneumonia.
- Monitor all patients for signs of chest infection and treat promptly.
References
- Knol W et al. Antipsychotic drug use and risk of pneumonia in elderly people. J Am Geriatr Soc 2008; 56:661–666.
- Star K et al. Pneumonia following antipsychotic prescriptions in electronic health records: a patient safety concern? Br J Gen Pract 2010; 60:e385–e394.
- Trifiro G et al. Association of community-acquired pneumonia with antipsychotic drug use in elderly patients: a nested case-control study. Ann Intern Med 2010; 152:418–440.
- Pratt N et al. Risk of hospitalization for hip fracture and pneumonia associated with antipsychotic prescribing in the elderly: a self-controlled case-series analysis in an Australian health care claims database. Drug Saf 2011; 34:567–575.
- Aparasu RR et al. Risk of pneumonia in elderly nursing home residents using typical versus atypical antipsychotics. Ann Pharmacother 2013; 47:464–474.
- Yang SY et al. Antipsychotic drugs, mood stabilizers, and risk of pneumonia in bipolar disorder: a nationwide case-control study. J Clin Psychiatry 2013; 74:e79–e86.
- Kuo CJ et al. Second-generation antipsychotic medications and risk of pneumonia in schizophrenia. Schizophr Bull 2013; 39:648–657.
- Chen YH et al. Poor clinical outcomes among pneumonia patients with schizophrenia. Schizophr Bull 2011; 37:1088–1094.
- Galappathie N et al. Clozapine-associated pneumonia and respiratory arrest secondary to severe constipation. Med Sci Law 2014; 54:105–109.
Switching antipsychotics
General recommendations for switching antipsychotics because of poor tolerability are shown in Table 2.34.
Table 2.34 General recommendations for switching antipsychotic drugs
|
Adverse effect
|
Suggested drugs
|
Alternatives
|
|
Acute EPS1-6
|
Aripiprazole
Olanzapine
Quetiapine
|
Clozapine
Lurasidone
Ziprasidone
|
|
Dyslipidaemia7-12
|
Amisulpride
Aripiprazole42
Lurasidone
Ziprasidone
|
Asenapine
|
|
Impaired glucose tolerance11,13-16
|
Amisulpride
Aripiprazole42
Lurasidone
Ziprasidone
|
Risperidone
Haloperidol
|
|
Hyperprolactinaemia11,17-22
|
Aripiprazole*
Lurasidone
Quetiapine
|
Clozapine
Olanzapine
Ziprasidone
|
|
Postural hypotension11
|
Amisulpride
Aripiprazole
Lurasidone
|
Haloperidol
Sulpiride
Trifluoperazine
|
|
QT prolongation22-27
|
Aripiprazole
Lurasidone
Paliperidone
(with ECG monitoring)
|
Low dose monotherapy of any drug not formally contra-indicated in QT prolongation (with ECG monitoring)
|
|
Sedation22
|
Amisulpride
Aripiprazole
Risperidone
Sulpiride
|
Haloperidol
Trifuoperazine
Ziprasidone
|
|
Sexual dysfuction28-34
|
Aripiprazole
Quetiapine
|
Clozapine
Lurasidone
|
|
Tardive dyskinesia35-38
|
Clozapine
|
Aripiprazole
Olanzapine
Quetiapine
|
|
Weight gain12,39-45
|
Amisulpride
Aripiprazole42
Haloperidol
Lurasidone
Ziprasidone
|
Asenapine
Trifluoperazine
|
|
* There is evidence that both switching to and co-prescription of aripiprazole are effective in reducing weight, prolactin and dyslipidaemia and in reversing impaired glucose tolerance.46–48
ECG, electrocardiogram; EPS, extrapyramidal side-effects.
|
References
- Stanniland C et al. Tolerability of atypical antipsychotics. Drug Saf 2000; 22:195–214.
- Tarsy D et al. Effects of newer antipsychotics on extrapyramidal function. CNS Drugs 2002; 16:23–45.
- Caroff SN et al. Movement disorders associated with atypical antipsychotic drugs. J Clin Psychiatry 2002; 63 Suppl 4:12–19.
- Lemmens P et al. A combined analysis of double-blind studies with risperidone vs. placebo and other antipsychotic agents: factors associated with extrapyramidal symptoms. Acta Psychiatr Scand 1999; 99:160–170.
- Taylor DM. Aripiprazole: a review of its pharmacology and clinical use. Int J Clin Pract 2003; 57:49–54.
- Meltzer HY et al. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placeboand olanzapine-controlled study. Am J Psychiatry 2011; 168:957–967.
- Rettenbacher MA et al. Early changes of plasma lipids during treatment with atypical antipsychotics. Int Clin Psychopharmacol 2006; 21:369–372.
- Ball MP et al. Clozapine-induced hyperlipidemia resolved after switch to aripiprazole therapy. Ann Pharmacother 2005; 39:1570–1572.
- Chrzanowski WK et al. Effectiveness of long-term aripiprazole therapy in patients with acutely relapsing or chronic, stable schizophrenia: a 52-week, open-label comparison with olanzapine. Psychopharmacology 2006; 189:259–266.
- De Hert M et al. A case series: evaluation of the metabolic safety of aripiprazole. Schizophr Bull 2007; 33:823–830.
- Citrome L et al. Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int Clin Psychopharmacol 2012; 27:165–176.
- Kemp DE et al. Weight change and metabolic effects of asenapine in patients with schizophrenia and bipolar disorder. J Clin Psychiatry 2014; 75:238–245.
- Haddad PM. Antipsychotics and diabetes: review of non-prospective data. Br J Psychiatry Suppl 2004; 47:S80–S86.
- Berry S et al. Improvement of insulin indices after switch from olanzapine to risperidone. Eur Neuropsychopharmacol 2002; 12:316.
- Gianfrancesco FD et al. Differential effects of risperidone, olanzapine, clozapine, and conventional antipsychotics on type 2 diabetes: findings from a large health plan database. J Clin Psychiatry 2002; 63:920–930.
- Mir S et al. Atypical antipsychotics and hyperglycaemia. Int Clin Psychopharmacol 2001; 16:63–74.
- Turrone P et al. Elevation of prolactin levels by atypical antipsychotics. Am J Psychiatry 2002; 159:133–135.
- David SR et al. The effects of olanzapine, risperidone, and haloperidol on plasma prolactin levels in patients with schizophrenia. Clin Ther 2000; 22:1085–1096.
- Hamner MB et al. Hyperprolactinaemia in antipsychotic-treated patients: guidelines for avoidance and management. CNS Drugs 1998; 10:209–222.
- Trives MZ et al. Effect of the addition of aripiprazole on hyperprolactinemia associated with risperidone long-acting injection. J Clin Psychopharmacol 2013; 33:538–541.
- Suzuki Y et al. Differences in plasma prolactin levels in patients with schizophrenia treated on monotherapy with five second-generation antipsychotics. Schizophr Res 2013; 145:116–119.
- Leucht S et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013; 382:951–962.
- Glassman AH et al. Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death. Am J Psychiatry 2001; 158:1774–1782.
- Taylor D. Antipsychotics and QT prolongation. Acta Psychiatr Scand 2003; 107:85–95.
- Titier K et al. Atypical antipsychotics: from potassium channels to torsade de pointes and sudden death. Drug Saf 2005; 28:35–51.
- Ray WA et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009; 360:225–235.
- Loebel A et al. Efficacy and safety of lurasidone 80 mg/day and 160 mg/day in the treatment of schizophrenia: a randomized, double-blind, placeboand active-controlled trial. Schizophr Res 2013; 145:101–109.
- Byerly MJ et al. An open-label trial of quetiapine for antipsychotic-induced sexual dysfunction. J Sex Marital Ther 2004; 30:325–332.
- Byerly MJ et al. Sexual dysfunction associated with second-generation antipsychotics in outpatients with schizophrenia or schizoaffective disorder: an empirical evaluation of olanzapine, risperidone, and quetiapine. Schizophr Res 2006; 86:244–250.
- Montejo Gonzalez AL et al. A 6-month prospective observational study on the effects of quetiapine on sexual functioning. J Clin Psychopharmacol 2005; 25:533–538.
- Dossenbach M et al. Effects of atypical and typical antipsychotic treatments on sexual function in patients with schizophrenia: 12-month results from the Intercontinental Schizophrenia Outpatient Health Outcomes (IC-SOHO) study. Eur Psychiatry 2006; 21:251–258.
- Kerwin R et al. A multicentre, randomized, naturalistic, open-label study between aripiprazole and standard of care in the management of community-treated schizophrenic patients Schizophrenia Trial of Aripiprazole: (STAR) study. Eur Psychiatry 2007; 22:433–443.
- Hanssens L et al. The effect of antipsychotic medication on sexual function and serum prolactin levels in community-treated schizophrenic patients: results from the Schizophrenia Trial of Aripiprazole (STAR) study (NCT00237913). BMC Psych 2008; 8:95.
- Loebel A et al. Effectiveness of lurasidone vs. quetiapine XR for relapse prevention in schizophrenia: a 12-month, double-blind, noninferiority study. Schizophr Res 2013; 147:95–102.
- Lieberman J et al. Clozapine pharmacology and tardive dyskinesia. Psychopharmacology 1989; 99 Suppl 1:S54–S59.
- O'Brien J et al. Marked improvement in tardive dyskinesia following treatment with olanzapine in an elderly subject. Br J Psychiatry 1998; 172:186.
- Sacchetti E et al. Quetiapine, clozapine, and olanzapine in the treatment of tardive dyskinesia induced by first-generation antipsychotics: a 124-week case report. Int Clin Psychopharmacol 2003; 18:357–359.
- Witschy JK et al. Improvement in tardive dyskinesia with aripiprazole use. Can J Psychiatry 2005; 50:188.
- Taylor DM et al. Atypical antipsychotics and weight gain - a systematic review. Acta Psychiatr Scand 2000; 101:416–432.
- Allison D et al. Antipsychotic-induced weight gain: A comprehensive research synthesis. Am J Psychiatry 1999; 156:1686–1696.
- Brecher M et al. The long term effect of quetiapine (SeroquelTM) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000; 4:287–291.
- Casey DE et al. Switching patients to aripiprazole from other antipsychotic agents: a multicenter randomized study. Psychopharmacology 2003; 166:391–399.
- Newcomer JW et al. A multicenter, randomized, double-blind study of the effects of aripiprazole in overweight subjects with schizophrenia or schizoaffective disorder switched from olanzapine. J Clin Psychiatry 2008; 69:1046–1056.
- McEvoy JP et al. Effectiveness of lurasidone in patients with schizophrenia or schizoaffective disorder switched from other antipsychotics: a randomized, 6-week, open-label study. J Clin Psychiatry 2013; 74:170–179.
- McEvoy JP et al. Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: a randomized clinical trial. JAMA 2014; 311:1978–1987.
- Shim JC et al. Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebocontrolled trial. Am J Psychiatry 2007; 164:1404–1410.
- Fleischhacker WW et al. Weight change on aripiprazole-clozapine combination in schizophrenic patients with weight gain and suboptimal response on clozapine: 16-week double-blind study. Eur Psychiatry 2008; 23 Suppl 2:S114–S115.
- Henderson DC et al. Aripiprazole added to overweight and obese olanzapine-treated schizophrenia patients. J Clin Psychopharmacol 2009; 29:165–169.
REFRACTORY SCHIZOPHRENIA AND CLOZAPINE
Clozapine—dosing regimen
Many of the adverse effects of clozapine are dose-dependent and associated with speed of titration. Adverse effects also tend to be more common and severe at the beginning of therapy. Standard maintenance doses may even prove fatal in clozapine-naïve subjects.1 To minimise these problems, it is important to start treatment at a low dose and to increase dosage slowly.
Clozapine should normally be started at a dose of 12.5 mg once a day, at night. Blood pressure should be monitored hourly for 6 hours because of the hypotensive effect of clozapine. This monitoring is not usually necessary if the first dose is given at night. On day 2, the dose can be increased to 12.5 mg twice daily. If the patient is tolerating clozapine, the dose can be increased by 25–50 mg a day, until a dose of 300 mg a day is reached. This can usually be achieved in 2–3 weeks. Further dosage increases should be made slowly in increments of 50–100 mg each week. A plasma level of 350 μg/L should be aimed for to ensure an adequate trial, but response may occur at lower plasma level. The average (there is substantial variation) dose at which this plasma level is reached varies according to gender and smoking status. The range is approximately 250 mg/day (female non-smoker) to 550 mg/day (male smoker).2 The total clozapine dose should be divided (usually twice daily) and, if sedation is a problem, the larger portion of the dose can be given at night.
Table 2.35 Suggested starting regime for clozapine (in-patients)
| Day |
Morning dose (mg) |
Evening dose (mg) |
| 1 |
– |
12.5 |
| 2 |
12.5 |
12.5 |
| 3 |
25 |
25 |
| 4 |
25 |
25 |
| 5 |
25 |
50 |
| 6 |
25 |
50 |
| 7 |
50 |
50 |
| 8 |
50 |
75 |
| 9 |
75 |
75 |
| 10 |
75 |
100 |
| 11 |
100 |
100 |
| 12 |
100 |
125 |
| 13 |
125 |
125* |
| 14 |
125 |
150 |
| 15 |
150 |
150 |
| 18 |
150 |
200† |
| 21 |
200 |
200 |
| 28 |
200 |
250‡ |
|
* Target dose for female non smokers (250 mg/day)
† Target dose for male non smokers (350 mg/day)
‡ Target dose for female smokers (450 mg/day)
|
Table 2.34 shows a suggested starting regime for clozapine. This is a cautious regimen—more rapid increases have been used. Slower titration may be necessary where sedation or other dose-related side-effects are severe, in the elderly, the very young, those who are physically compromised or those who have poorly tolerated other antipsychotics. If the patient is not tolerating a particular dose, decrease to one that was previously tolerated. If the adverse effect resolves, increase the dose again but at a slower rate.
If for any reason a patient misses fewer than 2 days' clozapine, restart at the dose prescribed before the event. Do not administer extra tablets to catch up. If more than 2 days are missed, restart and increase slowly (but at a faster rate than in drug-naïve patients). See section on 'Restarting clozapine after a break in treatment' in this chapter.
References
- Stanworth D et al. Clozapine - a dangerous drug in a clozapine-naive subject. Forensic Sci Int 2011; 214:e23–e25.
- Rostami-Hodjegan A et al. Influence of dose, cigarette smoking, age, sex, and metabolic activity on plasma clozapine concentrations: a predictive model and nomograms to aid clozapine dose adjustment and to assess compliance in individual patients. J Clin Psychopharmacol 2004; 24:70–78.
Optimising clozapine treatment
Using clozapine alone
Target dose—note that dose is best adjusted according to patient tolerability and plasma level.
- Average dose in UK is around 450 mg/day.1
- Response usually seen in the range 150–900 mg/day.2
- Lower doses required in the elderly, females and non-smokers, and in those prescribed certain enzyme inhibitors3,4 (see Table 2.35).
Plasma levels
- Most studies indicate that threshold for response is in the range 350–420 μg/L.5,6 Threshold may be as high as 500 μg/L7 (see Chapter 1).
- In male smokers who cannot achieve therapeutic plasma levels, metabolic inhibitors (fluvoxamine or cimetidine for example8,9) can be co-prescribed but extreme caution is required.
- Importance of norclozapine levels not established but clozapine/norclozapine ratio may aid assessment of recent compliance.
Clozapine augmentation
Clozapine 'augmentation' has become common practice because inadequate response to clozapine alone is a frequent clinical event. The evidence base supporting augmentation strategies is growing but remains insufficient to allow the development of any algorithm or schedule of treatment options. In practice, the result of clozapine augmentation is often disappointing and substantial changes in symptom severity are rarely observed. This clinical impression is supported by the equivocal results of many studies, which suggests a small effect size at best. Meta-analyses of antipsychotic augmentation suggest no effect,10 a small effect in long-term studies11 or, in the largest meta-analysis, a very small effect overall.12 An update on this last study13 confirmed this small effect size. Recent investigations into dopaminergic activity in refractory schizophrenia suggest there is no overproduction of dopamine.14,15 Dopamine antagonists are thus unlikely to be effective.
It is recommended that all augmentation attempts are carefully monitored and, if no clear benefit is forthcoming, abandoned after 3–6 months. The addition of another drug to clozapine treatment must be expected to worsen overall adverse effect burden and so continued ineffective treatment is not appropriate. In some cases, the addition of an augmenting agent may reduce the severity of some adverse effects (e.g. weight gain, dyslipidaemia) or allow a reduction in clozapine dose. The addition of aripiprazole to clozapine may be particularly effective in reversing metabolic effects.16,17
Table 2.36 shows suggested treatment options (in alphabetical order) where 3–6 months of optimised clozapine alone has provided unsatisfactory benefit.
Table 2.36 Suggested options for augmenting clozapine
|
Option
|
Comment
|
Add amisulpride18-23
(400-800 mg/day)
|
- Some evidence and experience suggests amisulpride augmentation may be worthwhile. One small RCT. May allow clozapine dose reduction.24 Large study—AMICUS—in progress
|
Add aripiprazole16,25-27
(15-30 mg/day)
|
- Very limited evidence of therapeutic benefit. Improves metabolic parameters
|
Add haloperidol27-29
(2-3 mg/day)
|
- Modest evidence of benefit
|
Add lamotrigine30-32
(25-300 mg/day)
|
- May be useful in partial or non-responders. May reduce alcohol consumption.33 Several equivocal reports34-36 but meta-analysis suggests moderate effect size37
|
Add omega-3 triglycerides38,39
(2-3 g EPA daily)
|
- Modest, and somewhat contested, evidence to support efficacy in non, or partial responders to antipsychotics, including clozapine
|
Add risperidone40,41
(2-6 mg/day)
|
- Supported by an RCT but there are also two negative RCTs each with minuscule response rates42,43 Small number of reports of increases in clozapine plasma levels. Long-term injection also an option44
|
Add sulpiride45
(400 mg/day)
|
- May be useful in partial or non-responders. Supported by a single randomised trial in English and three in Chinese.46 Overall effect modest
|
Add topiramate47-51
(50-300 mg/day)
|
- Two positive RCTs, two negative. Can worsen psychosis in some.31,52 Causes weight loss but impairs cognitive function (especially at doses > 200 mg/day). Not recommended
|
Add ziprasidone53-56
(80-160 mg/day)
|
- Supported by two RCTs.56,57 Associated with QTc prolongation. Rarely used
|
|
Notes:
- Always consider the use of mood stabilisers and/or antidepressants especially where mood disturbance is thought to contribute to symptoms.58–60
- Other options include adding pimozide, olanzapine or sertindole. None is recommended: pimozide and sertindole have important cardiac toxicity and the addition of olanzapine is poorly supported61 and likely to exacerbate metabolic adverse effects. Several studies of pimozide62,63 and sertindole64 have shown no effect. One small RCT supports the use of Ginkgo biloba,65 another supports the use of memantine.66
|
References
- Taylor D et al. A prescription survey of the use of atypical antipsychotics for hospital patients in the UK. Int J Psychiatry Clin Pract 2000; 4:41–46.
- Murphy B et al. Maintenance doses for clozapine. Psychiatr Bull 1998; 22:12–14.
- Taylor D. Pharmacokinetic interactions involving clozapine. Br J Psychiatry 1997; 171:109–112.
- Lane HY et al. Effects of gender and age on plasma levels of clozapine and its metabolites: analyzed by critical statistics. J Clin Psychiatry 1999; 60:36–40.
- Taylor D et al. The use of clozapine plasma levels in optimising therapy. Psychiatr Bull 1995; 19:753–755.
- Spina E et al. Relationship between plasma concentrations of clozapine and norclozapine and therapeutic response in patients with schizophrenia resistant to conventional neuroleptics. Psychopharmacology 2000; 148:83–89.
- Perry PJ. Therapeutic drug monitoring of antipsychotics. Psychopharmacol Bull 2001; 35:19–29.
- Papetti F et al. [Clozapine-resistant schizophrenia related to an increased metabolism and benefit of fluvoxamine: four case reports]. Encephale 2007; 33:811–818.
- Watras M et al. A therapeutic interaction between cimetidine and clozapine: case study and review of the literature. Ther Adv Psychopharmacol 2013; 3:294–297.
- Barbui C et al. Does the addition of a second antipsychotic drug improve clozapine treatment? Schizophr Bull 2009; 35:458–468.
- Paton C et al. Augmentation with a second antipsychotic in patients with schizophrenia who partially respond to clozapine: a meta-analysis. J Clin Psychopharmacol 2007; 27:198–204.
- Taylor DM et al. Augmentation of clozapine with a second antipsychotic - a meta-analysis of randomized, placebo-controlled studies. Acta Psychiatr Scand 2009; 119:419–425.
- Taylor D et al. Augmentation of clozapine with a second antipsychotic. A meta analysis. Acta Psychiatr Scand 2012; 125:15–24.
- Demjaha A et al. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am J Psychiatry 2012; 169:1203–1210.
- Demjaha A et al. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol Psychiatry 2014; 75:e11–e13.
- Fleischhacker WW et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol 2010; 13:1115–1125.
- Correll CU et al. Selective effects of individual antipsychotic cotreatments on cardiometabolic and hormonal risk status: results from a systematic review and meta-analysis. Schizophr Bull 2013; 39 (Suppl 1):S29–S30.
- Matthiasson P et al. Relationship between dopamine D2 receptor occupancy and clinical response in amisulpride augmentation of clozapine non-response. J Psychopharmacol 2001; 15:S41.
- Munro J et al. Amisulpride augmentation of clozapine: an open non-randomized study in patients with schizophrenia partially responsive to clozapine. Acta Psychiatr Scand 2004; 110:292–298.
- Zink M et al. Combination of clozapine and amisulpride in treatment-resistant schizophrenia—case reports and review of the literature. Pharmacopsychiatry 2004; 37:26–31.
- Ziegenbein M et al. Augmentation of clozapine with amisulpride in patients with treatment-resistant schizophrenia. An open clinical study. German J Psychiatry 2006; 9:17–21.
- Kampf P et al. Augmentation of clozapine with amisulpride: a promising therapeutic approach to refractory schizophrenic symptoms. Pharmacopsychiatry 2005; 38:39–40.
- Assion HJ et al. Amisulpride augmentation in patients with schizophrenia partially responsive or unresponsive to clozapine. A randomized, double-blind, placebo-controlled trial. Pharmacopsychiatry 2008; 41:24–28.
- Croissant B et al. Reduction of side effects by combining clozapine with amisulpride: case report and short review of clozapine-induced hypersalivation-a case report. Pharmacopsychiatry 2005; 38:38–39.
- Chang JS et al. Aripiprazole augmentation in clozapine-treated patients with refractory schizophrenia: an 8-week, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2008; 69:720–731.
- Muscatello MR et al. Effect of aripiprazole augmentation of clozapine in schizophrenia: a double-blind, placebo-controlled study. Schizophr Res 2011; 127:93–99.
- Cipriani A et al. Aripiprazole versus haloperidol in combination with clozapine for treatment-resistant schizophrenia: a 12-month, randomized, naturalistic trial. J Clin Psychopharmacol 2013; 33:533–537.
- Rajarethinam R et al. Augmentation of clozapine partial responders with conventional antipsychotics. Schizophr Res 2003; 60:97–98.
- Barbui C et al. Aripiprazole versus haloperidol in combination with clozapine for treatment-resistant schizophrenia in routine clinical care: a randomized, controlled trial. J Clin Psychopharmacol 2011; 31:266–273.
- Dursun SM et al. Clozapine plus lamotrigine in treatment-resistant schizophrenia. Arch Gen Psychiatry 1999; 56:950.
- Dursun SM et al. Augmenting antipsychotic treatment with lamotrigine or topiramate in patients with treatment-resistant schizophrenia: a naturalistic case-series outcome study. J Psychopharmacol 2001; 15:297–301.
- Tiihonen J et al. Lamotrigine in treatment-resistant schizophrenia: a randomized placebo-controlled crossover trial. Biol Psychiatry 2003; 54:1241–1248.
- Kalyoncu A et al. Use of lamotrigine to augment clozapine in patients with resistant schizophrenia and comorbid alcohol dependence: a potent anti-craving effect? J Psychopharmacol 2005; 19:301–305.
- Goff DC et al. Lamotrigine as add-on therapy in schizophrenia: results of 2 placebo-controlled trials. J Clin Psychopharmacol 2007; 27:582–589.
- Heck AH et al. Addition of lamotrigine to clozapine in inpatients with chronic psychosis. J Clin Psychiatry 2005; 66:1333.
- Vayisoglu S et al. Lamotrigine augmentation in patients with schizophrenia who show partial response to clozapine treatment. Schizophr Res 2013; 143:207–214.
- Tiihonen J et al. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res 2009; 109:10–14.
- Peet M et al. Double-blind placebo controlled trial of N-3 polyunsaturated fatty acids as an adjunct to neuroleptics. Schizophr Res 1998; 29:160–161.
- Puri BK et al. Sustained remission of positive and negative symptoms of schizophrenia following treatment with eicosapentaenoic acid. Arch Gen Psychiatry 1998; 55:188–189.
- Josiassen RC et al. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry 2005; 162:130–136.
- Raskin S et al. Clozapine and risperidone: combination/augmentation treatment of refractory schizophrenia: a preliminary observation. Acta Psychiatr Scand 2000; 101:334–336.
- Anil Yagcioglu AE et al. A double-blind controlled study of adjunctive treatment with risperidone in schizophrenic patients partially responsive to clozapine: efficacy and safety. J Clin Psychiatry 2005; 66:63–72.
- Honer WG et al. Clozapine alone versus clozapine and risperidone with refractory schizophrenia. N Engl J Med 2006; 354:472–482.
- Se HK et al. The combined use of risperidone long-acting injection and clozapine in patients with schizophrenia non-adherent to clozapine: a case series. J Psychopharmacol 2010; 24:981–986.
- Shiloh R et al. Sulpiride augmentation in people with schizophrenia partially responsive to clozapine. A double-blind, placebo-controlled study. Br J Psychiatry 1997; 171:569–573.
- Wang J et al. Sulpiride augmentation for schizophrenia. Schizophr Bull 2010; 36:229–230.
- Tiihonen J et al. Topiramate add-on in treatment-resistant schizophrenia: a randomized, double-blind, placebo-controlled, crossover trial. J Clin Psychiatry 2005; 66:1012–1015.
- Afshar H et al. Topiramate add-on treatment in schizophrenia: a randomised, double-blind, placebo-controlled clinical trial. J Psychopharmacol 2009; 23:157–162.
- Muscatello MR et al. Topiramate augmentation of clozapine in schizophrenia: a double-blind, placebo-controlled study. J Psychopharmacol 2011; 25:667–674.
- Hahn MK et al. Topiramate augmentation in clozapine-treated patients with schizophrenia: clinical and metabolic effects. J Clin Psychopharmacol 2010; 30:706–710.
- Behdani F et al. Effect of topiramate augmentation in chronic schizophrenia: a placebo-controlled trial. Arch Iran Med 2011; 14:270–275.
- Millson RC et al. Topiramate for refractory schizophrenia. Am J Psychiatry 2002; 159:675.
- Zink M et al. Combination of ziprasidone and clozapine in treatment-resistant schizophrenia. Hum Psychopharmacol 2004; 19:271–273.
- Ziegenbein M et al. Clozapine and ziprasidone: a useful combination in patients with treatment-resistant schizophrenia. J Neuropsychiatry Clin Neurosci 2006; 18:246–247.
- Ziegenbein M et al. Combination of clozapine and ziprasidone in treatment-resistant schizophrenia: an open clinical study. Clin Neuropharmacol 2005; 28:220–224.
- Zink M et al. Efficacy and tolerability of ziprasidone versus risperidone as augmentation in patients partially responsive to clozapine: a randomised controlled clinical trial. J Psychopharmacol 2009; 23:305–314.
- Muscatello MR et al. Augmentation of clozapine with ziprasidone in refractory schizophrenia: a double-blind, placebo-controlled study. J Clin Psychopharmacol 2014; 34:129–133.
- Citrome L. Schizophrenia and valproate. Psychopharmacol Bull 2003; 37 Suppl 2:74–88.
- Tranulis C et al. Somatic augmentation strategies in clozapine resistance—what facts? Clin Neuropharmacol 2006; 29:34–44.
- Suzuki T et al. Augmentation of atypical antipsychotics with valproic acid. An open-label study for most difficult patients with schizophrenia. Hum Psychopharmacol 2009; 24:628–638.
- Gupta S et al. Olanzapine augmentation of clozapine. Ann Clin Psychiatry 1998; 10:113–115.
- Friedman JI et al. Pimozide augmentation of clozapine inpatients with schizophrenia and schizoaffective disorder unresponsive to clozapine monotherapy. Neuropsychopharmacology 2011; 36:1289–1295.
- Gunduz-Bruce H et al. Efficacy of pimozide augmentation for clozapine partial responders with schizophrenia. Schizophr Res 2013; 143:344–347.
- Nielsen J et al. Augmenting clozapine with sertindole: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol 2012; 32:173–178.
- Doruk A et al. A placebo-controlled study of extract of ginkgo biloba added to clozapine in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol 2008; 23:223–227.
- de Lucena D et al. Improvement of negative and positive symptoms in treatment-refractory schizophrenia: a double-blind, randomized, placebo-controlled trial with memantine as add-on therapy to clozapine. J Clin Psychiatry 2009; 70:1416–1423.
Further reading
Nielsen J et al. Optimizing clozapine treatment. Acta Psychiatr Scand 2011; 123:411–422.
Porcelli S et al. Clozapine resistance: augmentation strategies. Eur Neuropsychopharmacol 2012; 22:165–182.
Remington G et al. Augmenting strategies in clozapine-resistant schizophrenia. CNS Drugs 2006;20:171.
Sommer IE et al. Pharmacological augmentation strategies for schizophrenia patients with insufficient response to clozapine: a quantitative literature review. Schizophr Bull 2011; 38:1003–1111.
Alternatives to clozapine
Clozapine is the treatment of choice in refractory schizophrenia. Where treatment resistence is established, clozapine treatment should not normally be delayed or withheld. The practice of using successive antipsychotics (or the latest) instead of clozapine is widespread but not supported by any cogent research. Where clozapine cannot be used (because of toxicity or patient refusal) other drugs or drug combinations may be tried (Table 2.37) but outcome is usually disappointing. Available data do not allow the drawing of any distinction between treatment regimens, but it seems wise to use single drugs before trying multiple drug regimens. In practice, olanzapine is most often used, usually in above licensed doses. If this fails, then the addition of a second antipsychotic (amisulpride, for example) is a reasonable next step. Amongst unconventional agents, minocycline and ondansetron have the advantage of low toxcity and very good tolerability. Depot medication is an option where adherence is in doubt. Many of the treatments listed below are somewhat experimental and some of the compounds difficult to obtain (e.g. glycine, d-serine, sarcosine, etc). Before using any of the regimens outlined, readers should consult the primary literature cited. Particular care should be taken to inform patients where prescribing is off-label and to ensure they understand the potential side-effects of more experimental treatments.
Table 2.37 Alternatives to clozapine. Treatments are listed in alphabetical order: no preference is implied by position in table
|
Treatment
|
Comments
|
Allopurinol 300-600 mg/day
(+antipsychotic)1-4
|
Increases adenosinergic transmission which may reduce effects of dopamine. Three positive RCTs1,2,4
|
Amisulpride
(up to 1200 mg/day)
|
Single, small open study. Not usually a treatment option in practice
|
Aripiprazole6,7
(15-30 mg/day)
|
Single randomized controlled study indicating moderate effect in patients resistant to risperidone or olanzapine (+others). Higher doses (60 mg/day) have been used8
|
Bexarotene 75 mg/day9
(+antipsychotic)
|
Retinoid receptor agonist. One RCT (n = 90) in non-refractory but suboptimally treated patients suggest worthwhile effect on positive symptoms.
|
|
CBT10
|
Non-drug therapies should always be considered
|
Celexcoxib + risperidone11
(400 mg + 6 mg/day)
|
COX-2 inhibitors modulate immune response and may prevent glutamate-related cell death. One RCT showed useful activity in all main symptom domains. Associated with increased caardiovascular mortality
|
Donepezil 5-10 mg/day
(+antipsychotic)12-14
|
Three RCTs, one negative,13 two positive,12,14 suggesting a small effect on cognitive and negative symptoms
|
D-Alanine 100 mg/kg/day
(+antipsychotic)15
|
Glycine (NMDA) agonist. One positive RCT
|
D-Serine 30 mg/kg/day
(+olanzapine)16
|
Glycine (NMDA) agonist. One positive RCT
|
|
d-serine up to 3 g as monotherapy17
|
Improved negative symptoms in one RCT, but inferior to high dose olanzapine for treatment of positive symptoms
|
|
ECT18-21
|
Open studies suggest moderate effect. Often reserved for last-line treatment in practice but can be successful in the short22 and long23 term
|
|
Famotidine 100 mg bd + antipsychotic24
|
H2 antagonist. One short (4 week) RCT suggested some benefit in overall PANSS and CGI scores
|
Ginkgo biloba
(+antipsychotic)25,26
|
Possibly effective in combination with haloperidol. Unlikely to give rise to additional adverse effects but clinical experience limited
|
Memantine 20 mg/day
(+antipsychotic)27-29
|
Memantine is an NMDA antagonist. Two RCTs. The larger of the two (n = 138) was negative. In the smaller (n = 21), memantine improved positive and negative symptoms when added to clozapine. In another study in non-refractory schizophrenia, memantine improved negative symptoms when added to risperidone
|
Mianserin + FGA
30 mg/day30
|
5HT2 antagonist. One, small positive RCT
|
Minocycline 200 mg/day
(+antipsychotic)31,32
|
May be anti-inflammatory and neuroprotective. One open study (n = 22) and one RCT (n = 54) suggest good effect on negative and cognitive symptoms. Also two cases of augmentation of clozapine.33 RCT evidence of neuroprotective effect in early psychosis34
|
Mirtazapine 30 mg/day
(+antipsychotic)35-37
|
5HT2 antagonist. Two RCTs, one negative,36 one positive.35 Effect seems to be mainly on positive symptoms
|
N-acetylcysteine 2 g/day
(+antipsychotic)38
|
One RCT suggests small benefits in negative symptoms and rates of akathisia. Case study of successful use of 600 mg a day39
|
Olanzapine40-45
5-25 mg/day
|
Supported by some well conducted trials but clinical experience disappointing. Some patients show moderate response
|
Olanzapine46-52
30-60 mg/day
|
Contradictory findings in the literature but possibly effective. High dose olanzapine is not atypical53 and can be poorly tolerated54 with gross metabolic changes52
|
Olanzapine + amisulpride55
(up to 800 mg/day)
|
Small open study suggests benefit
|
|
Olanzapine + aripiprazole56
|
Single case report suggests benefit. Probably reduces metabolic toxicity
|
Olanzapine + glycine57
(0.8 g/kg/day)
|
Small, double-blind crossover trial suggests clinically relevant improvement in negative symptoms
|
Olanzapine + lamotrigine58,59
(up to 400 mg/day)
|
Reports contradictory and rather unconvincing. Reasonable theoretical basis for adding lamotrigine which is usually well tolerated
|
Olanzapine + risperidone60
(various doses)
|
Small study suggests some patients may benefit from combined therapy after sequential failure of each drug alone
|
Olanzapine + sulpiride61
(600 mg/day)
|
Some evidence that this combination improves mood symptoms
|
|
Omega-3-triglycerides62,63
|
Suggested efficacy but data very limited
|
Ondansetron 8 mg/day
(+antipsychotic)64,65
|
Two RCTs. Both show improvements in negative and cognitive symptoms
|
Propentofylline + risperidone66
(900 mg + 6 mg/day)
|
One RCT suggests some activity against postive symptoms
|
|
Quetiapine67-70
|
Very limited evidence and clinical experience not encouraging. High doses (> 1200 mg/day) have been used but are no more effective71
|
|
Quetiapine + amisulpride72
|
Single naturalistic observation of 19 patients suggested useful benefit. Doses averaged 700 mg quetiapine and 950 mg amisulpride
|
|
Quetiapine + haloperidol73
|
Two case reports
|
Riluzole 100 mg/day + risperidone up to
6 mg/day74
|
Glutamate modulating agent. One RCT demonstrated improvement in negative symptoms
|
Risperidone75-77
4-8 mg/day
|
Doubtful efficacy in true treatment-refractory schziophrenia but some supporting evidence. May also be tried in combination with glycine57 or lamotrigine58 or indeed with other atypicals78
|
|
Risperidone 50/100 mg 2/5279
|
One RCT showing good response for both doses in refractory schizophrenia. Plasma levels for 100 mg dose similar to 6-8 mg/day oral risperidone
|
Ritanserin + risperidone
(12 mg + 6 mg/day)80
|
5HT2A/2C antagonist. One RCT suggests small effect on negative symptoms
|
Sarcosine (2 g/day)81,82
(+antipsychotic)
|
Enhances glycine action. Supported by two RCTs
|
Sertindole83
(12-24 mg/day)
|
One large RCT (conducted in 1996-8 but published in 2011) suggested good effect and equivalence to risperidone. Around half of subjects responded. Another RCT84 showed no effect at all when added to clozapine. Little experience in practice
|
Topiramate (300 mg/day)
(+antipsychotic)85
|
Small effect shown in single RCT. Induces weight loss. Cognitive adverse effects likely
|
|
Transcranial magnetic stimulation86,87
|
Probably not effective
|
|
Valproate88
|
Doubtful effect but may be useful where there is a clear affective component
|
|
Ziprasidone 80-160 mg/day89-91
|
Two good RCTs. One91 suggests superior efficacy to chlorpromazine in refractory schziophrenia, the other89 suggests equivalence to clozapine in subjects with treatment intolerance/resistance. Disappointing results in practice, however. Supratherapeutic doses offer no advantage92
|
|
CBT, cognitive behavioural therapy; CGI, clinical global impression; COX, cyclo-oxgenase; ECT, electroconvulsive therapy; FGA, first-generation antipsychotic; NMDA, N-methyl-D-aspartate; PANSS, positive and negative syndrome scale; RCT, randomised controlled trial.
|
References
- Akhondzadeh S et al. Beneficial antipsychotic effects of allopurinol as add-on therapy for schizophrenia: a double blind, randomized and placebo controlled trial. Prog Neuropsychopharmacol Biol Psychiatry 2005; 29:253–259.
- Brunstein MG et al. A clinical trial of adjuvant allopurinol therapy for moderately refractory schizophrenia. J Clin Psychiatry 2005; 66:213–219.
- Buie LW et al. Allopurinol as adjuvant therapy in poorly responsive or treatment refractory schizophrenia. Ann Pharmacother 2006; 40:2200–2204.
- Dickerson FB et al. A double-blind trial of adjunctive allopurinol for schizophrenia. Schizophr Res 2009; 109:66–69.
- Kontaxakis VP et al. Switching to amisulpride monotherapy for treatment-resistant schizophrenia. Eur Psychiatry 2006; 21:214–217.
- Kane JM et al. Aripiprazole for treatment-resistant schizophrenia: results of a multicenter, randomized, double-blind, comparison study versus perphenazine. J Clin Psychiatry 2007; 68:213–223.
- Hsu WY et al. Aripiprazole in treatment-refractory schizophrenia. J Psychiatr Pract 2009; 15:221–226.
- Crossman AM et al. Tolerability of high-dose aripiprazole in treatment-refractory schizophrenic patients. J Clin Psychiatry 2006; 67:1158–1159.
- Lerner V et al. The retinoid X receptor agonist bexarotene relieves positive symptoms of schizophrenia: a 6-week, randomized, double-blind, placebo-controlled multicenter trial. J Clin Psychiatry 2013; 74:1224–1232.
- Valmaggia LR et al. Cognitive-behavioural therapy for refractory psychotic symptoms of schizophrenia resistant to atypical antipsychotic medication. Randomised controlled trial. Br J Psychiatry 2005; 186:324–330.
- Akhondzadeh S et al. Celecoxib as adjunctive therapy in schizophrenia: a double-blind, randomized and placebo-controlled trial. Schizophr Res 2007; 90:179–185.
- Lee BJ et al. A 12-week, double-blind, placebo-controlled trial of donepezil as an adjunct to haloperidol for treating cognitive impairments in patients with chronic schizophrenia. J Psychopharmacol 2007; 21:421–427.
- Keefe RSE et al. Efficacy and safety of donepezil in patients with schizophrenia or schizoaffective disorder: significant placebo/practice effects in a 12-week, randomized, double-blind, placebo-controlled trial. Neuropsychopharmacology 2007; 33:1217–1228.
- Akhondzadeh S et al. A 12-week, double-blind, placebo-controlled trial of donepezil adjunctive treatment to risperidone in chronic and stable schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:1810–1815.
- Tsai GE et al. D-alanine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry 2006; 59:230–234.
- Heresco-Levy U et al. d-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol Psychiatry 2005; 57:577–585.
- Ermilov M et al. A pilot double-blind comparison of d-serine and high-dose olanzapine in treatment-resistant patients with schizophrenia. Schizophr Res 2013; 150:604–605.
- Chanpattana W et al. Combined ECT and neuroleptic therapy in treatment-refractory schizophrenia: prediction of outcome. Psychiatry Res 2001; 105:107–115.
- Tang WK et al. Efficacy of electroconvulsive therapy in treatment-resistant schizophrenia: a prospective open trial. Prog Neuropsychopharmacol Biol Psychiatry 2003; 27:373–379.
- Chanpattana W et al. Acute and maintenance ECT with flupenthixol in refractory schizophrenia: sustained improvements in psychopathology, quality of life, and social outcomes. Schizophr Res 2003; 63:189–193.
- Chanpattana W et al. ECT for treatment-resistant schizophrenia: a response from the far East to the UK. NICE report. J ECT 2006; 22:4–12.
- Chanpattana W et al. Electroconvulsive therapy in treatment-resistant schizophrenia: prediction of response and the nature of symptomatic improvement. J ECT 2010; 26:289–298.
- Ravanic DB et al. Long-term efficacy of electroconvulsive therapy combined with different antipsychotic drugs in previously resistant schizophrenia. Psychiatr Danub 2009; 21:179–186.
- Meskanen K et al. A randomized clinical trial of histamine 2 receptor antagonism in treatment-resistant schizophrenia. J Clin Psychopharmacol 2013; 33:472–478.
- Zhou D et al. The effects of classic antipsychotic haloperidol plus the extract of ginkgo biloba on superoxide dismutase in patients with chronic refractory schizophrenia. Chin Med J 1999; 112:1093–1096.
- Zhang XY et al. A double-blind, placebo-controlled trial of extract of Ginkgo biloba added to haloperidol in treatment-resistant patients with schizophrenia. J Clin Psychiatry 2001; 62:878–883.
- Lieberman JA et al. A randomized, placebo-controlled study of memantine as adjunctive treatment in patients with schizophrenia. Neuropsychopharmacology 2009; 34:1322–1329.
- de Lucena D et al. Improvement of negative and positive symptoms in treatment-refractory schizophrenia: a double-blind, randomized, placebo-controlled trial with memantine as add-on therapy to clozapine. J Clin Psychiatry 2009; 70:1416–1423.
- Rezaei F et al. Memantine add-on to risperidone for treatment of negative symptoms in patients with stable schizophrenia: randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol 2013; 33:336–342.
- Shiloh R et al. Mianserin or placebo as adjuncts to typical antipsychotics in resistant schizophrenia. Int Clin Psychopharmacol 2002; 17:59–64.
- Levkovitz Y et al. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry 2010; 71:138–149.
- Miyaoka T et al. Minocycline as adjunctive therapy for schizophrenia: an open-label study. Clin Neuropharmacol 2008; 31:287–292.
- Kelly DL et al. Adjunct minocycline to clozapine treated patients with persistent schizophrenia symptoms. Schizophr Res 2011; 133:257–258.
- Chaudhry IB et al. Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J Psychopharmacol 2012; 26:1185–1193.
- Joffe G et al. Add-on mirtazapine enhances antipsychotic effect of first generation antipsychotics in schizophrenia: A double-blind, randomized, placebo-controlled trial. Schizophr Res 2009; 108:245–251.
- Berk M et al. Mirtazapine add-on therapy in the treatment of schizophrenia with atypical antipsychotics: a double-blind, randomised, placebo-controlled clinical trial. Hum Psychopharmacol 2009; 24:233–238.
- Delle CR et al. Add-on mirtazapine enhances effects on cognition in schizophrenic patients under stabilized treatment with clozapine. Exp Clin Psychopharmacol 2007; 15:563–568.
- Berk M et al. N-acetyl cysteine as a glutathione precursor for schizophrenia—a double-blind, randomized, placebo-controlled trial. Biol Psychiatry 2008; 64:361–368.
- Bulut M et al. Beneficial effects of N-acetylcysteine in treatment resistant schizophrenia. World J Biol Psychiatry 2009; 10:626–628.
- Breier A et al. Comparative efficacy of olanzapine and haloperidol for patients with treatment-resistant schizophrenia. Biol Psychiatry 1999; 45:403–411.
- Conley RR et al. Olanzapine compared with chlorpromazine in treatment-resistant schizophrenia. Am J Psychiatry 1998; 155:914–920.
- Sanders RD et al. An open trial of olanzapine in patients with treatment-refractory psychoses. J Clin Psychopharmacol 1999; 19:62–66.
- Taylor D et al. Olanzapine in practice: a prospective naturalistic study. Psychiatr Bull 1999; 23:178–180.
- Bitter I et al. Olanzapine versus clozapine in treatment-resistant or treatment-intolerant schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2004; 28:173–180.
- Tollefson GD et al. Double-blind comparison of olanzapine versus clozapine in schizophrenic patients clinically eligible for treatment with clozapine. Biol Psychiatry 2001; 49:52–63.
- Sheitman BB et al. High-dose olanzapine for treatment-refractory schizophrenia. Am J Psychiatry 1997; 154:1626.
- Fanous A et al. Schizophrenia and schizoaffective disorder treated with high doses of olanzapine. J Clin Psychopharmacol 1999; 19:275–276.
- Dursun SM et al. Olanzapine for patients with treatment-resistant schizophrenia: a naturalistic case-series outcome study. Can J Psychiatry 1999; 44:701–704.
- Conley RR et al. The efficacy of high-dose olanzapine versus clozapine in treatment-resistant schizophrenia: a double-blind crossover study. J Clin Psychopharmacol 2003; 23:668–671.
- Kumra S et al. Clozapine and "high-dose" olanzapine in refractory early-onset schizophrenia: a 12-week randomized and double-blind comparison. Biol Psychiatry 2008; 63:524–529.
- Kumra S et al. Clozapine versus "high-dose" olanzapine in refractory early-onset schizophrenia: an open-label extension study. J Child Adolesc Psychopharmacol 2008; 18:307–316.
- Meltzer HY et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry 2008; 69:274–285.
- Bronson BD et al. Adverse effects of high-dose olanzapine in treatment-refractory schizophrenia. J Clin Psychopharmacol 2000; 20:382–384.
- Kelly DL et al. Adverse effects and laboratory parameters of high-dose olanzapine vs. clozapine in treatment-resistant schizophrenia. Ann Clin Psychiatry 2003; 15:181–186.
- Zink M et al. Combination of amisulpride and olanzapine in treatment-resistant schizophrenic psychoses. Eur Psychiatry 2004; 19:56–58.
- Duggal HS. Aripirazole-olanzapine combination for treatment of schizophrenia. Can J Psychiatry 2004; 49:151.
- Heresco-Levy U et al. High-dose glycine added to olanzapine and risperidone for the treatment of schizophrenia. Biol Psychiatry 2004; 55:165–171.
- Kremer I et al. Placebo-controlled trial of lamotrigine added to conventional and atypical antipsychotics in schizophrenia. Biol Psychiatry 2004; 56:441–446.
- Dursun SM et al. Augmenting antipsychotic treatment with lamotrigine or topiramate in patients with treatment-resistant schizophrenia: a naturalistic case-series outcome study. J Psychopharmacol 2001; 15:297–301.
- Suzuki T et al. Effectiveness of antipsychotic polypharmacy for patients with treatment refractory schizophrenia: an open-label trial of olanzapine plus risperidone for those who failed to respond to a sequential treatment with olanzapine, quetiapine and risperidone. Hum Psychopharmacol 2008; 23:455–463.
- Kotler M et al. Sulpiride augmentation of olanzapine in the management of treatment-resistant chronic schizophrenia: evidence for improvement of mood symptomatology. Int Clin Psychopharmacol 2004; 19:23–26.
- Mellor JE et al. Omega-3 fatty acid supplementation in schizophrenic patients. Hum Psychopharmacol 1996; 11:39–46.
- Puri BK et al. Sustained remission of positive and negative symptoms of schizophrenia following treatment with eicosapentaenoic acid. Arch Gen Psychiatry 1998; 55:188–189.
- Zhang ZJ et al. Beneficial effects of ondansetron as an adjunct to haloperidol for chronic, treatment-resistant schizophrenia: a double-blind, randomized, placebo-controlled study. Schizophr Res 2006; 88:102–110.
- Akhondzadeh S et al. Added ondansetron for stable schizophrenia: A double blind, placebo controlled trial. Schizophr Res 2009; 107:206–212.
- Salimi S et al. A placebo controlled study of the propentofylline added to risperidone in chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:726–732.
- Reznik I et al. Long-term efficacy and safety of quetiapine in treatment-refractory schizophrenia: A case report. Int J Psychiatry Clin Pract 2000; 4:77–80.
- De Nayer A et al. Efficacy and tolerability of quetiapine in patients with schizophrenia switched from other antipsychotics. Int J Psychiatry Clin Pract 2003; 7:59–66.
- Larmo I et al. Efficacy and tolerability of quetiapine in patients with schizophrenia who switched from haloperidol, olanzapine or risperidone. Hum Psychopharmacol 2005; 20:573–581.
- Boggs DL et al. Quetiapine at high doses for the treatment of refractory schizophrenia. Schizophr Res 2008; 101:347–348.
- Lindenmayer JP et al. A randomized, double-blind, parallel-group, fixed-dose, clinical trial of quetiapine at 600 versus 1200 mg/d for patients with treatment-resistant schizophrenia or schizoaffective disorder. J Clin Psychopharmacol 2011; 31:160–168.
- Quintero J et al. The effectiveness of the combination therapy of amisulpride and quetiapine for managing treatment-resistant schizophrenia: a naturalistic study. J Clin Psychopharmacol 2011; 31:240–242.
- Aziz MA et al. Remission of positive and negative symptoms in refractory schizophrenia with a combination of haloperidol and quetiapine: Two case studies. J Psychiatr Pract 2006; 12:332–336.
- Farokhnia M et al. A double-blind, placebo controlled, randomized trial of riluzole as an adjunct to risperidone for treatment of negative symptoms in patients with chronic schizophrenia. Psychopharmacology (Berl) 2014; 231:533–542.
- Breier AF et al. Clozapine and risperidone in chronic schizophrenia: effects on symptoms, parkinsonian side effects, and neuroendocrine response. Am J Psychiatry 1999; 156:294–298.
- Bondolfi G et al. Risperidone versus clozapine in treatment-resistant chronic schizophrenia: a randomized double-blind study. The Risperidone Study Group. Am J Psychiatry 1998; 155:499–504.
- Conley RR et al. Risperidone, quetiapine, and fluphenazine in the treatment of patients with therapy-refractory schizophrenia. Clin Neuropharmacol 2005; 28:163–168.
- Lerner V et al. Combination of "atypical" antipsychotic medication in the management of treatment-resistant schizophrenia and schizoaffective disorder. Prog Neuropsychopharmacol Biol Psychiatry 2004; 28:89–98.
- Meltzer HY et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100 mg in treatment resistant schizophrenia. Schizophr Res 2014; 154:14–22.
- Akhondzadeh S et al. Effect of ritanserin, a 5HT2A/2C antagonist, on negative symptoms of schizophrenia: a double-blind randomized placebo-controlled study. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:1879–1883.
- Lane HY et al. Sarcosine or d-serine add-on treatment for acute exacerbation of schizophrenia: a randomized, double-blind, placebo-controlled study. Arch Gen Psychiatry 2005; 62:1196–1204.
- Tsai G et al. Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry 2004; 55:452–456.
- Kane JM et al. A double-blind, randomized study comparing the efficacy and safety of sertindole and risperidone in patients with treatmentresistant schizophrenia. J Clin Psychiatry 2011; 72:194–204.
- Nielsen J et al. Augmenting clozapine with sertindole: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol 2012; 32:173–178.
- Tiihonen J et al. Topiramate add-on in treatment-resistant schizophrenia: a randomized, double-blind, placebo-controlled, crossover trial. J Clin Psychiatry 2005; 66:1012–1015.
- Franck N et al. Left temporoparietal transcranial magnetic stimulation in treatment-resistant schizophrenia with verbal hallucinations. Psychiatry Res 2003; 120:107–109.
- Fitzgerald PB et al. A double-blind sham-controlled trial of repetitive transcranial magnetic stimulation in the treatment of refractory auditory hallucinations. J Clin Psychopharmacol 2005; 25:358–362.
- Basan A et al. Valproate as an adjunct to antipsychotics for schizophrenia: a systematic review of randomized trials. Schizophr Res 2004; 70:33–37.
- Sacchetti E et al. Ziprasidone vs clozapine in schizophrenia patients refractory to multiple antipsychotic treatments: the MOZART study. Schizophr Res 2009; 110:80–89.
- Loebel AD et al. Ziprasidone in treatment-resistant schizophrenia: a 52-week, open-label continuation study. J Clin Psychiatry 2007; 68:1333–1338.
- Kane JM et al. Efficacy and tolerability of ziprasidone in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol 2006; 21:21–28.
- Goff DC et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms: the ZEBRAS study. J Clin Psychopharmacol 2013; 33:485–490.
Re-starting clozapine after a break in treatment
Re-titration of clozapine is somewhat constrained by the manufacturer's recommendation that re-titration should be the same as initial titration if more than 48 hours' clozapine is missed. This recommendation does not seem to be based on any research but it certainly recognises the dangers of giving clozapine to those not tolerant to its effects. However, there is evidence that faster titrations are safe in both those naïve to clozapine and those re-starting it.93 Table 2.38 gives some broad recommendations on re-starting clozapine after gaps of various lengths. The advice takes into account the need to regain antipsychotic activity with clozapine while ensuring safety during titration. The key feature is that of flexibility: the dose given to the patient depends upon their ability to tolerate prior doses.
Table 2.38 Re-starting clozapine
|
Time since last clozapine dose
|
Action to re-start
|
|
Up to 48 hours
|
Restart at previous dose—no re-titration required
|
|
48-72 hours
|
Begin rapid re-titration as soon as possible
Restart with half of the previously prescribed total daily dose on day one (in divided doses 12 hours apart). Then give 75% of previous daily dose on day two and, if tolerated, the whole of the previous daily dose in the normal dosing schedule on day three
|
|
72 hours—one week
|
Begin re-titration with 12.5 mg or 25 mg clozapine
Increase according to patient tolerability over at least 3 days
|
|
More than one week
|
Re-titrate as if new patient
Aim to reach previously prescribed dose within 3-4 weeks
|
Reference
1. Ifteni P et al. Effectiveness and safety of rapid clozapine titration in schizophrenia. Acta Psychiatr Scand 2014;130:25–29.
Initiation of clozapine for community-based patients
Contraindications to community initiation
- History of seizures, significant cardiac disease, unstable diabetes, paralytic ileus, blood dyscrasia, neuroleptic malignant syndrome or other disorder that increases the risk of serious side-effects (initiation with close monitoring in hospital may still be possible).
- Unreliable or chaotic life-style that may affect adherence to the medication or the monitoring regimen.
- Significant abuse of alcohol or other drugs likely to increase the risk of side-effects (e.g. cocaine).
Suitability for community initiation
All the answers should be yes.
- Is the patient likely to be adherent with oral medication and to monitoring requirements?
- Has the patient understood the need for regular physical monitoring and blood tests?
- Has the patient understood the possible side-effects and what to do about them (particularly the rare but serious ones)?
- Is the patient readily contactable (e.g. in the event of a result that needs follow-up)?
- Is it possible for the patient to be seen every day during the early titration phase?
- Is the patient able to collect medication every week or can medication be delivered to their home?
- Is the patient likely to be able to seek help out-of-hours if they experience potentially serious side-effects (eg: indicators of myocarditis or infection such as fever, malaise, chest pain)?
initial work-up
To screen for risk factors and provide a baseline, carry out:
- physical examination, FBC (see below), LFTs, U&E, lipids, glucose/HbA1c. Consider troponin, CRP, beta-naturietic peptide, ESR (as baseline for further tests)
- ECG—particularly to screen for evidence of past myocardial infarction or ventricular abnormality
- echocardiogram if clinically indicated.
Mandatory blood monitoring and registration
- Register with the relevant monitoring service.
- Perform baseline blood tests (WCC and differential count) before starting clozapine.
- Further blood testing continues weekly for the first 18 weeks and then every 2 weeks for the remainder of the year. After that, the blood monitoring is usually done monthly.
- Inform the patient's GP.
Dosing
Starting clozapine in the community requires a slow and flexible titration schedule. Prior antipsychotics should be slowly discontinued during the titration phase (depots can usually be stopped at the start of titration). Clozapine can cause marked postural hypotension. The initial monitoring is partly aimed at detecting and managing this.
There are two basic methods for starting clozapine in the community. One is to give the first dose in the morning in clinic and then monitor the patient for at least three hours. If the dose is well tolerated, the patient is then allowed home with a dose to take before going to bed. This dosing schedule is described in Table 2.39. This is a very cautious schedule: most patients will tolerate faster titration. The second method involves giving the patient the first dose to take immediately before bed, so avoiding the need for close physical monitoring immediately after administration. Subsequent doses and monitoring is as for the first method. All initiations should take place early in the week (e.g. on a Monday) so that adequate staffing and monitoring are assured.
Adverse effects
Sedation, hypersalivation and hypotension are common at the start of treatment. These effects can usually be managed (see section on 'Common adverse effects' in this chapter) but require particular attention in community titration.
The formal carer (usually the Community Psychiatric Nurse) should inform the prescriber if:
- temperature rises above 38°C (this is very common and is not a good reason, on its own, for stopping clozapine)
- pulse is > 100 bpm (also common and not, on its own a reason for stopping, but may sometimes be linked to myocarditis)
- postural drop of > 30 mmHg
- patient is clearly over-sedated
- any other adverse effect is intolerable.
A doctor should see the patient at least once a week for the first month to assess mental and physical state. Recommended additional monitoring is summarised in Table 2.40.
Consider monitoring plasma troponin, beta-natriuretic peptide and C-reactive protein weekly in the first 6 weeks of treatment, particularly where there is any suspicion of myocarditis (see section on 'Myocarditis' in this chapter).
Switching from other antipsychotics
- The switching regime will be largely dependent on the patient's mental state.
- Consider potential additive side-effects of antipsychotics (e.g. hypotension, sedation, effect on QTc interval).
- Consider drug interactions (e.g. some SSRIs may increase clozapine levels).
- All depots, sertindole, pimozide and ziprasidone should be stopped before clozapine is started.
Table 2.39 Suggested titration regime for initiation of clozapine in the community. Note that much faster titrations can be undertaken in many patients where tolerability allows
| Day |
Day of the week |
Morning dose (mg) |
Evening dose (mg) |
Monitoring |
Percentage dose of previous antipsychotic |
| 1 |
Monday |
6.25 |
6.25 |
A |
100* |
| 2 |
Tuesday |
6.25 |
6.25 |
A |
|
| 3 |
Wednesday |
6.25 |
6.25 |
A |
|
| 4 |
Thursday |
6.25 |
12.5 |
A, B, FBC |
|
| 5 |
Friday |
12.5 |
12.5 |
A
Check results from day 4.
Remind patient of outof-hours arrangements for week-end |
|
| 6 |
Saturday |
12.5 |
12.5 |
No routine monitoring unless clinically indicated |
|
| 7 |
Sunday |
12.5 |
12.5 |
No routine monitoring unless clinically indicated |
|
| 8 |
Monday |
12.5 |
25 |
A |
75* |
| 9 |
Tuesday |
12.5 |
25 |
A |
|
| 10 |
Wednesday |
25 |
25 |
A |
|
| 11 |
Thursday |
25 |
37.5 |
A, B, FBC |
|
| 12 |
Friday |
25 |
37.5 |
A
Check results from day 1.
Remind patient of outof-hours arrangements for week-end |
|
| 13 |
Saturday |
25 |
37.5 |
No routine monitoring unless clinically indicated |
|
| 14 |
Sunday |
25 |
37.5 |
No routine monitoring unless clinically indicated |
|
| 15 |
Monday |
37.5 |
37.5 |
A |
50* |
| 16 |
Tuesday |
37.5 |
37.5 |
Not seen unless problems |
|
| 17 |
Wednesday |
37.5 |
50 |
A |
|
| 18 |
Thursday |
37.5 |
50 |
Not seen unless problems |
|
| 19 |
Friday |
50 |
50 |
A, B, FBC |
|
| 20 |
Saturday |
50 |
50 |
No routine monitoring unless clinically indicated |
|
| 21 |
Sunday |
50 |
50 |
No routine monitoring unless clinically indicated |
|
| 22 |
Monday |
50 |
75 |
A |
25* |
| 23 |
Tuesday |
50 |
75 |
Not seen unless problems |
|
| 24 |
Wednesday |
75 |
75 |
A |
|
| 25 |
Thursday |
75 |
75 |
Not seen unless problems |
|
| 26 |
Friday |
75 |
100 |
A, B, FBC |
|
| 27 |
Saturday |
75 |
100 |
No routine monitoring unless clinically indicated |
|
| 28 |
Sunday |
75 |
100 |
No routine monitoring unless clinically indicated |
|
|
Further increments should be 25–50 mg/day (generally 25 mg/day) until target dose is reached (use plasma levels). Beware sudden increase in plasma levels due to saturation of first pass metabolism (watch for increase in sedation/ other side-effects).
|
|
A, pulse, postural blood pressure, temperature, enquire about side-effects.
B, mental state, weight, review and actively manage side-effects (e.g. behavioural advice, slow clozapine titration or reduce dose of other antipsychotic, start adjunctive treatments—see sections on clozapine adverse effects in this chapter). Consider troponin, C-reactive protein, beta-naturietic peptide.
* May need to be adjusted depending on side-effects and mental state.
FBC, full blood count.
|
Table 2.40 Recommended additional monitoring
| Baseline |
1 month |
3 months |
4-6 months |
12 months |
| Weight/BMI/waist |
Weight/BMI/waist |
Weight/BMI/waist |
Weight/BMI/waist |
Weight/BMI/waist |
| Plasma glucose and lipids |
Plasma glucose and lipids |
|
Plasma glucose and lipids |
Plasma glucose and lipids |
| LFTs |
|
|
LFTs |
|
|
BMI, body mass index; LFTs, liver function tests.
|
- Other antipsychotics and clozapine may be cross-tapered with varying degrees of caution. ECG monitoring is prudent when clozapine is co-prescribed with other drugs known to affect QT interval.
Serious cardiac adverse effects
Patients should be closely observed for signs or symptoms of myocarditis, particularly during the first 2 months, and advised to inform staff if they experience these, and to seek out-of-hours review if necessary. These include persistent tachycardia (although commonly benign), palpitations, shortness of breath, fever, arrhythmia, symptoms mimicking myocardial infarction, chest pain and other unexplained symptoms of heart failure. (see section on 'Serious haematological and cardiovascular adverse effects' in this chapter.)
Further reading
Beck K et al. The practical management of refractory schizophrenia - the Maudsley Treatment Review and Assessment Team service approach. Acta Psychiatr Scand 2014; 130:427–438.
Lovett L. Initiation of clozapine treatment at home. Prog Neurol Psychiatry 2004; 8:19–21.
O'Brien A. Starting clozapine in the community: a UK perspective. CNS Drugs 2004;18:845–52.
CLOZAPINE—ADVERSE EFFECTS
Common adverse effects
Clozapine has a wide range of adverse effects many of which are serious or potentially life-threatening. Table 2.41 describes some more common adverse effects; Table 2.42 deals with rare and serious events.
Table 2.41 Common adverse effects of clozapine
|
Adverse effect
|
Time course
|
Action
|
|
Sedation
|
First few months
May persist, but usually wears off to some extent
|
Give smaller dose in the morning
Reduce dose if possible
|
|
Hypersalivation
|
First few months
May persist, but sometimes wears off
Often very troublesome at night
|
Give hyoscine 300 μg (Kwells) sucked and swallowed up to three times a day. Many other options (see section on 'Hypersalivation' in this chapter). Note: anticholinergics worsen constipation and cognition
|
|
Constipation
|
First 4 months are the highest risk1
Usually persists
|
Advise patients of the risks before starting, screen regularly, ensure adequate fibre, fluid and exercise. Bulk forming laxatives are usually first line, but have a low threshold for adding osmotic and/or stimulant laxatives early. Stop other medicines that may be contributing and reduce clozapine dose if possible. Effective treatment or prevention of constipation is essential as death may result1-5
|
|
Hypotension
|
First 4 weeks
|
Advise patient to take time when standing up. Reduce dose or slow down rate of increase. If severe, consider moclobemide and Bovril,6 or fludrocortisone. Over longer term, weight gain may lead to hypertension
|
|
Hypertension
|
First 4 weeks, sometimes longer
|
Monitor closely and increase dose as slowly as is necessary. Hypotensive therapy (e.g. atenolol 25 mg/day) is sometimes necessary7
|
|
Tachycardia
|
First 4 weeks, but sometimes persists
|
Very common in early stages of treatment but usually benign. Tachycardia, if persistent at rest and associated with fever, hypotension or chest pain, may indicate myocarditis8,9 (see section on 'Serious adverse effects of clozapine' in this chapter). Referral to a cardiologist is advised. Clozapine should be stopped if tachycardia occurs in the context of chest pain or heart failure. Benign sinus tachycardia can be treated with bisoprolol or atenolol.10 Ivabradine may be used if hypotension or contraindications limit the use of beta-blockers.11 Note that prolonged tachycardia can itself precipitate cardiomyopathy12
|
|
Weight gain
|
Usually during the first year of treatment
|
Dietary counselling is essential. Advice may be more effective if given before weight gain occurs. Weight gain is common and often profound (> 10 lb). Many treatments available (see section on 'Weight gain' in this chapter)
|
|
Fever
|
First 3 weeks
|
Clozapine induces inflammatory response (increased CRP and interleukin-6).13,14 Give paracetamol but check FBC for neutropenia. Reduce rate of dose titration.15 This fever is not usually related to blood dyscrasias16,17 but beware myocarditis and NMS (see section on 'Serious adverse effects of clozapine' in this chapter)
|
|
Seizures
|
May occur at any time18
|
Related to dose, plasma level and rapid dose escalation.19 Consider prophylactic, lamotrigine, gabapentin or valproate* if on high dose (≥ 500 mg/day) or with high plasma level (≥ 500 μg/L). Note that some suggest risk of seizures below 1300 μg/L (about 1 in 20 people) is not enough to support primary prophylaxis.20 After a seizure: withhold clozapine for one day; restart at half previous dose; give anticonvulsant.tEEG abnormalities are common in those on clozapine21
|
|
Nausea
|
First 6 weeks
|
May give anti-emetic. Avoid prochlorperazine and metoclopramide if previous EPS. Avoid domperidone if underlying cardiac risk or QTc prolongation. Ondansetron is a good choice
|
|
Nocturnal enuresis
|
May occur at any time
|
Try reducing the dose or manipulating dose schedule to avoid periods of deep sedation. Avoid fluids before bedtime. May resolve spontaneously,22 but may persist for months or years.23 May affect one in five people on clozapine.24 In severe cases, desmopressin nasal spray (10-20 μg nocte) is usually effective25 but is not without risk: hyponatraemia may result.26 Anticholinergic agents may be effective27 but support for this approach is weak and constipation and sedation may worsen
|
|
Neutropenia/ agranulocytosis
|
First 18 weeks (but may occur at any time)
|
Stop clozapine; admit to hospital if agranulocytosis confirmed
|
|
Gastro-oesophageal reflux disease (GORD)28
|
Any time
|
Proton pump inhibitors often prescribed. Reasons for GORD association unclear—clozapine is an H2 antagonist29
|
|
* Usual dose is 1000–2000 mg/day. Plasma levels may be useful as a rough guide to dosing—aim for 50–100 mg/L. Use of modified-release preparation (Epilim Chrono) may aid compliance: can be given once-daily and may be better tolerated.
† Use valproate if schizoaffective; lamotrigine if poor response to clozapine or continued negative symptoms; topiramate if weight loss required (but beware cognitive adverse effects); gabapentin if other anticonvulsants are poorly tolerated.19
CRP, C-reactive protein; EEG, electroencephalogram; EPS, extrapyramidal side-effects; FBC, full blood count; NMS, neuroleptic malignant syndrome.
|
References
- Palmer SE et al. Life-threatening clozapine-induced gastrointestinal hypomotility: an analysis of 102 cases. J Clin Psychiatry 2008; 69:759–768.
- Townsend G et al. Case report: rapidly fatal bowel ischaemia on clozapine treatment. BMC Psych 2006; 6:43.
- Rege S et al. Life-threatening constipation associated with clozapine. Australas Psychiatry 2008; 16:216–219.
- Leung JS et al. Rapidly fatal clozapine-induced intestinal obstruction without prior warning signs. Aust N Z J Psychiatry 2008; 42:1073–1074.
- Flanagan RJ et al. Gastrointestinal hypomotility: an under-recognised life-threatening adverse effect of clozapine. Forensic Sci Int 2011; 206:e31–e36.
- Taylor D et al. Clozapine-induced hypotension treated with moclobemide and Bovril. Br J Psychiatry 1995; 167:409–410.
- Henderson DC et al. Clozapine and hypertension: a chart review of 82 patients. J Clin Psychiatry 2004; 65:686–689.
- Committee on Safety of Medicines. Clozapine and cardiac safety: updated advice for prescribers. Curr Probl Pharmacovigil 2002; 28:8.
- Hagg S et al. Myocarditis related to clozapine treatment. J Clin Psychopharmacol 2001; 21:382–388.
- Stryjer R et al. Beta-adrenergic antagonists for the treatment of clozapine-induced sinus tachycardia: a retrospective study. Clin Neuropharmacol 2009; 32:290–292.
- Lally J et al. Ivabradine, a novel treatment for clozapine-induced sinus tachycardia: a case series. Ther Adv Psychopharmacol 2014; 4:117–122.
- Shinbane JS et al. Tachycardia-Induced Cardiomyopathy: A Review of Animal Models and Clinical Studies. J Am Coll Cardiol 1997; 29:709–715.
- Kohen I et al. Increases in C-reactive protein may predict recurrence of clozapine-induced fever. Ann Pharmacother 2009; 43:143–146.
- Kluge M et al. Effects of clozapine and olanzapine on cytokine systems are closely linked to weight gain and drug-induced fever. Psychoneuroendocrinology 2009; 34:118–128.
- Chung J.P.U. et al. The incidence and characteristics of clozapineinduced fever in a local psychiatric unit in Hong Kong. Can J Psychiatry 2008; 53:857–862.
- Tham JC et al. Clozapine-induced fevers and 1-year clozapine discontinuation rate. J Clin Psychiatry 2002; 63:880–884.
- Tremeau F et al. Spiking fevers with clozapine treatment. Clin Neuropharmacol 1997; 20:168–170.
- Pacia SV et al. Clozapine-related seizures: experience with 5,629 patients. Neurology 1994; 44:2247–2249.
- Varma S et al. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol 2011; 1:47–66.
- Caetano D. Use of anticonvulsants as prophylaxis for seizures in patients on clozapine. Australas Psychiatry 2014; 22:78–83.
- Centorrino F et al. EEG abnormalities during treatment with typical and atypical antipsychotics. Am J Psychiatry 2002; 159:109–115.
- Warner JP et al. Clozapine and urinary incontinence. Int Clin Psychopharmacol 1994; 9:207–209.
- Jeong SH et al. A 2-year prospective follow-up study of lower urinary tract symptoms in patients treated with clozapine. J Clin Psychopharmacol 2008; 28:618–624.
- Harrison-Woolrych M et al. Nocturnal enuresis in patients taking clozapine, risperidone, olanzapine and quetiapine: comparative cohort study. Br J Psychiatry 2011; 199:140–144.
- Steingard S. Use of desmopressin to treat clozapine-induced nocturnal enuresis. J Clin Psychiatry 1994; 55:315–316.
- Sarma S et al. Severe hyponatraemia associated with desmopressin nasal spray to treat clozapine-induced nocturnal enuresis. Aust N Z J Psychiatry 2005; 39:949.
- Praharaj SK et al. Amitriptyline for clozapine-induced nocturnal enuresis and sialorrhoea. Br J Clin Pharmacol 2007; 63:128–129.
- Taylor D et al. Use of antacid medication in patients receiving clozapine: a comparison with other second-generation antipsychotics. J Clin Psychopharmacol 2010; 30:460–461.
- Humbert-Claude M et al. Involvement of histamine receptors in the atypical antipsychotic profile of clozapine: a reassessment in vitro and in vivo. Psychopharmacology (Berl) 2011; 220:225–241.
Further reading
Iqbal MM et al. Clozapine: a clinical review of adverse effects and management. Ann Clin Psychiatry 2003; 15: 33–48.
Lieberman JA. Maximizing clozapine therapy: managing side-effects. J Clin Psychiatry 1998; 59(Suppl. 3): 38–43.
Clozapine: uncommon or unusual adverse effects
Pharmacoepidemiological monitoring of clozapine is more extensive than with any other drug used in psychiatry. Awareness of adverse effects related to clozapine treatment is therefore enhanced. Table 2.42 gives brief details of unusual or uncommon adverse effects of clozapine reported since its relaunch in 1990.
Table 2.42 Uncommon or unusual adverse effects of clozapine
|
Adverse effect
|
Comment
|
|
Agranulocytosis/ neutropenia (delayed)1-3
|
Occasional reports of apparent clozapine-related blood dyscrasia even after 1 year of treatment. It is possible that clozapine is not the causative agent in some cases4,5
|
|
Colitis6-8
|
A few reports in the literature, but clear causative link to clozapine not determined. Any severe or chronic diarrhoea should prompt specialist referral as there is a substantial risk of death. Anticholinergic use probably increases risk of colitis and necrosis9
|
|
Delirium10,11
|
Reported to be fairly common, but rarely seen in practice if dose is titrated slowly and plasma level determinations are used
|
|
Eosinophilia12,13
|
Reasonably common but significance unclear. Some suggestion that eosinophilia predicts neutropenia but this is disputed. May be associated with colitis and related symptoms.14 Occasional reports linking eosinophilia with myocarditis15
|
|
Heat stroke16
|
Occasional case reported. May be mistaken for neuroleptic malignant syndrome
|
|
Hepatic failure/enzyme abnormalities17-20
|
Benign changes in liver function tests are common (up to 50% of patients) but worth monitoring because of the very small risk of fulminant hepatic failure.21 Rash may be associated with clozapine-related hepatitis22
|
|
Interstitial nephritis23,24
|
A handful of reports implicating clozapine. May occur after only a few doses
|
|
Ocular pigmentation25
|
Single case report
|
|
Pancreatitis26,27
|
Rare reports of asymptomatic and symptomatic pancreatitis sometimes associated with eosinophilia. Some authors recommend monitoring serum amylase in all patients treated with clozapine. No cases of successful re-challenge after pancreatitis28
|
|
Parotid gland swelling29
|
A few case reports. Unclear mechanism, possibly immunological. May be recurrent. Treatment of hypersalivation with terazosin in combination with benzatropine may be helpful
|
|
Pericardial effusion30,31
|
Several reports in the literature. Symptoms include fatigue, dyspnoea and tachycardia. Use echocardiogram to confirm/rule out effusion
|
|
Pneumonia32,33
|
Very rarely results from saliva aspiration. Pneumonia is a common cause of death in people on clozapine.33 Infections in general may be more common in those on clozapine34 and use of antibiotics is also increased.35 Note that respiratory infections may give rise to elevated clozapine levels.36,37 (Possibly an artefact: smoking usually ceases during an infection)
|
|
Reflux oesophagitis38
|
Those treated with clozapine are more than three times as likely to be treated with antacids than those on other antipsychotics. Reasons unclear
|
|
Stuttering39,40
|
Case reports. May be a result of extrapyramidal side-effects or epileptiform activity. Check plasma levels, consider dose reduction and/or anticonvulsant drug
|
|
Thrombocytopenia41
|
Few data but apparently fairly common. Probably transient and clinically unimportant
|
|
Vasculitis42
|
One report in the literature in which patient developed confluent erythematous rash on lower limbs
|
References
- Thompson A et al. Late onset neutropenia with clozapine. Can J Psychiatry 2004; 49:647–648.
- Bhanji NH et al. Late-onset agranulocytosis in a patient with schizophrenia after 17 months of clozapine treatment. J Clin Psychopharmacol 2003; 23:522–523.
- Sedky K et al. Clozapine-induced agranulocytosis after 11 years of treatment (Letter). Am J Psychiatry 2005; 162:814.
- Panesar N et al. Late onset neutropenia with clozapine. Aust N Z J Psychiatry 2011; 45:684.
- Tourian L et al. Late-onset agranulocytosis in a patient treated with clozapine and lamotrigine. J Clin Psychopharmacol 2011; 31:665–667.
- Hawe R et al. Response to clozapine-induced microscopic colitis: a case report and review of the literature. J Clin Psychopharmacol 2008; 28:454–455.
- Shah V et al. Clozapine-induced ischaemic colitis. BMJ Case Rep 2013; 2013.
- Linsley KR et al. Clozapine-associated colitis: case report and review of the literature. J Clin Psychopharmacol 2012; 32:564–566.
- Peyriere H et al. Antipsychotics-induced ischaemic colitis and gastrointestinal necrosis: a review of the French pharmacovigilance database. Pharmacoepidemiol Drug Saf 2009; 18:948–955.
- Centorrino F et al. Delirium during clozapine treatment: incidence and associated risk factors. Pharmacopsychiatry 2003; 36:156–160.
- Shankar BR. Clozapine-induced delirium. J Neuropsychiatry Clin Neurosci 2008; 20:239–240.
- Hummer M et al. Does eosinophilia predict clozapine induced neutropenia? Psychopharmacology 1996; 124:201–204.
- Ames D et al. Predictive value of eosinophilia for neutropenia during clozapine treatment. J Clin Psychiatry 1996; 57:579–581.
- Karmacharya R et al. Clozapine-induced eosinophilic colitis (Letter). Am J Psychiatry 2005; 162:1386–1387.
- Chatterton R. Eosinophilia after commencement of clozapine treatment. Aust N Z J Psychiatry 1997; 31:874–876.
- Kerwin RW et al. Heat stroke in schizophrenia during clozapine treatment: rapid recognition and management. J Psychopharmacol 2004; 18:121–123.
- Erdogan A et al. Management of marked liver enzyme increase during clozapine treatment: a case report and review of the literature. Int J Psychiatry Med 2004; 34:83–89.
- Macfarlane B et al. Fatal acute fulminant liver failure due to clozapine: a case report and review of clozapine-induced hepatotoxicity. Gastroenterology 1997; 112:1707–1709.
- Chang A et al. Clozapine-induced fatal fulminant hepatic failure: a case report. Can J Gastroenterol 2009; 23:376–378.
- Chaplin AC et al. Re: Recent case report of clozapine-induced acute hepatic failure. Can J Gastroenterol 2010; 24:739–740.
- Tucker P. Liver toxicity with clozapine. Aust N Z J Psychiatry 2013; 47:975–976.
- Fong SY et al. Clozapine-induced toxic hepatitis with skin rash. J Psychopharmacol 2005; 19:107.
- Hunter R et al. Clozapine-induced interstitial nephritis - a rare but important complication: a case report. J Med Case Reports 2009; 3:8574.
- Elias TJ et al. Clozapine-induced acute interstitial nephritis. Lancet 1999; 354:1180–1181.
- Borovik AM et al. Ocular pigmentation associated with clozapine. Med J Aust 2009; 190:210–211.
- Bergemann N et al. Asymptomatic pancreatitis associated with clozapine. Pharmacopsychiatry 1999; 32:78–80.
- Raja M et al. A case of clozapine-associated pancreatitis. Open Neuropsychopharmacol J 2011; 4:5–7.
- Huang YJ et al. Recurrent pancreatitis without eosinophilia on clozapine rechallenge. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33:1561–1562.
- Immadisetty V et al. A successful treatment strategy for clozapine-induced parotid swelling: a clinical case and systematic review. Ther Adv Psychopharmacol 2012; 2:235–239.
- Raju P et al. Pericardial effusion in patients with schizophrenia: are they on clozapine? Emerg Med J 2008; 25:383–384.
- Dauner DG et al. Clozapine-induced pericardial effusion. J Clin Psychopharmacol 2008; 28:455–456.
- Hinkes R et al. Aspiration pneumonia possibly secondary to clozapine-induced sialorrhea. J Clin Psychopharmacol 1996; 16:462–463.
- Taylor DM et al. Reasons for discontinuing clozapine: matched, case-control comparison with risperidone long-acting injection. Br J Psychiatry 2009; 194:165–167.
- Landry P et al. Increased use of antibiotics in clozapine-treated patients. Int Clin Psychopharmacol 2003; 18:297–298.
- Nielsen J et al. Increased use of antibiotics in patients treated with clozapine. Eur Neuropsychopharmacol 2009; 19:483–486.
- Raaska K et al. Bacterial pneumonia can increase serum concentration of clozapine. Eur J Clin Pharmacol 2002; 58:321–322.
- de Leon J et al. Serious respiratory infections can increase clozapine levels and contribute to side effects: a case report. Prog Neuropsychopharmacol Biol Psychiatry 2003; 27:1059–1063.
- Taylor D et al. Use of antacid medication in patients receiving clozapine: a comparison with other second-generation antipsychotics. J Clin Psychopharmacol 2010; 30:460–461.
- Kumar T et al. Dose dependent stuttering with clozapine: a case report. Asian J Psychiatr 2013; 6:178–179.
- Grover S et al. Clozapine-induced stuttering: a case report and analysis of similar case reports in the literature. Gen Hosp Psychiatry 2012; 34:703.
- Jagadheesan K et al. Clozapine-induced thrombocytopenia: a pilot study. Hong Kong J Psychiatry 2003; 13:12–15.
- Penaskovic KM et al. Clozapine-induced allergic vasculitis (Letter). Am J Psychiatry 2005; 162:1543–1542.
Clozapine: serious haematological and cardiovascular adverse effects
Agranulocytosis, thromboembolism, cardiomyopathy and myocarditis
Clozapine is a somewhat toxic drug, but it may reduce overall mortality in schizophrenia, largely because of a reduction in the rate of suicide.1,2 Clozapine can cause serious, life-threatening adverse effects, of which agranulocytosis is the best known. In the UK, the risk of death from agranulocytosis is probably less than 1 in 10,000 patients exposed (Novartis report 4 deaths from 47,000 exposed).3 Risk is well managed by the approved clozapine-monitoring systems.
Thromboembolism
A possible association between clozapine and thromboembolism has been suggested.4 Initially, Walker et al.1 uncovered a risk of fatal pulmonary embolism of 1 in 4500—about 20 times the risk in the population as a whole. Following a case report of non-fatal pulmonary embolism possibly related to clozapine,5 data from the Swedish authorities were published.6 Twelve cases of venous thromboembolism were described, of which five were fatal. The risk of thromboembolism was estimated to be 1 in 2000 to 1 in 6000 patients treated. Thromboembolism may be related to clozapine's observed effects on antiphospholipid antibodies7 and platelet aggregation.8 It seems most likely to occur in the first 3 months of treatment but can occur at any time. Other antipsychotics are also strongly linked to thromboembolism9–15 although clozapine appears to have the most reports.16
With all drugs, the causes of thromboembolism are probably multifactorial.10 Encouraging exercise and ensuring good hydration are essential precautionary measures.17
Myocarditis and cardiomyopathy
It has also been suggested that clozapine is associated with myocarditis and cardiomyopathy. Australian data initially identified 23 cases (15 myocarditis, eight cardiomyopathy), of which six were fatal.18 Risk of death from either cause was estimated from these data to be 1 in 1300. Similar findings were reported in New Zealand.19 Myocarditis seems to occur within 6–8 weeks of starting clozapine (median 3 weeks20); cardiomyopathy may occur later in treatment (median 9 months20) but both may occur at any time. It is notable that other data sources give rather different risk estimates: in Canada the risk of fatal myocarditis was estimated to be 1 in 12,500; in the USA, 1 in 67,000.21 Conversely, another Australian study identified nine cases of possible (non-fatal) myocarditis in 94 patients treated.22 A later Australian study estimated the risk of myocarditis to be around 1% of those treated (in whom 1 in 10 died).23
Despite this uncertainty over incidence, patients should be closely monitored for signs of myocarditis especially in the first few months of treatment.24 Symptoms include hypotension, tachycardia, fever, flu-like symptoms, fatigue, dyspnoea (with increased respiratory rate) and chest pain.25 Signs include ECG changes (ST depression), enlarged heart on radiography/echo and eosinophilia. Many of these symptoms occur in patients on clozapine not developing myocarditis26 and conversely, their absence does not rule out myocarditis.27 Nonetheless, signs of heart failure should provoke immediate cessation of clozapine. Re-challenge has been successfully completed22 (the use of beta-blockers and angiotensin-converting enzyme [ACE] inhibitors may help28,29) but recurrence is possible.30–32 Use of echocardiography, CRP and troponin are essential in cases of re-challenge.33–35
Autopsy findings suggest that fatal myocarditis can occur in the absence of clear cardiac symptoms, although tachycardia and fever are usually present.36 A group from Melbourne, Australia has put forward a monitoring programme which is said to detect 100% of symptomatic cases of myocarditis37 using measurement of troponin I or Tand C-reactive protein (Table 2.43).
Factors that may increase the risk of developing myocarditis include rapid dose increases, concurrent use of sodium valproate, and older age (31% increased risk for each additional decade).38 Other psychotropics, including lithium, risperidone, haloperidol, chlorpromazine and fluphenazine have also been associated with myocarditis.39 It is probably preferable to avoid concomitant use of other medicines that may contribute to the risk, but this may be practically difficult. Any pre-existing cardiac disorder, previous cardiac event or family history of cardiac disease should provoke extra caution.
Cardiomyopathy should be suspected in any patient showing signs of heart failure, which should provoke immediate cessation of clozapine and referral. Presentation of cardiomyopathy varies somewhat40,41 so any reported symptoms of palpitations, chest pain, syncope, sweating, decreased exercise capacity or breathing difficulties should be closely investigated.
Note also that, despite an overall reduction in mortality, younger patients may have an increased risk of sudden death,42 perhaps because of clozapine-induced ECG changes.43
Table 2.43 Suggested monitoring for myocarditis36,37,46
|
Time/condition
|
Signs/symptoms to monitor
|
|
Baseline
|
Pulse, blood pressure, temperature, respiratory rate
Full blood count (FBC)
C-reactive protein (CRP)
Troponin
Echocardiography (if available)
|
|
Daily, if possible
|
Pulse, blood pressure, temperature, respiratory rate
Ask about: chest pain, fever, cough, shortness of breath, exercise capacity
|
|
On days 7, 14, 21, and 28
|
CRP
Troponin
FBC
ECG if possible
|
|
If CRP > 100 mg/L or troponin > twice upper limit of normal
|
Stop clozapine; repeat echo
|
|
If fever + tachycardia + raised CRP or troponin (but not as above)
|
Daily CRP and troponin measures
|
|
ECG, electrocardiogram.
|
The overall picture remains very unclear but caution is required. There may, of course, be similar problems with other antipsychotics.44,39,45
Summary
- Overall mortality may be lower for those on clozapine than in schizophrenia as a whole.
- Risk of fatal agranulocytosis is less than 1 in 10000 patients treated in the UK.
- Risk of fatal pulmonary embolism is estimated to be around 1 in 4500 patients treated.
- Risk of fatal myocarditis or cardiomyopathy may be as high as 1 in 1000 patients.
- Careful monitoring is essential during clozapine treatment, particularly during the first 3 months (see Table 2.39).
References
- Walker AM et al. Mortality in current and former users of clozapine. Epidemiology 1997; 8:671–677.
- Munro J et al. Active monitoring of 12760 clozapine recipients in the UK and Ireland. Br J Psychiatry 1999; 175:576–580.
- Thuillier S. Clozapine and agranulocytosis. 2006. Personal Communication
- Paciullo CA. Evaluating the association between clozapine and venous thromboembolism. Am J Health Syst Pharm 2008; 65:1825–1829.
- Lacika S et al. Pulmonary embolus possibly associated with clozapine treatment (Letter). Can J Psychiatry 1999; 44:396–397.
- Hagg S et al. Association of venous thromboembolism and clozapine. Lancet 2000; 355:1155–1156.
- Davis S et al. Antiphospholipid antibodies associated with clozapine treatment. Am J Hematol 1994; 46:166–167.
- Axelsson S et al. In vitro effects of antipsychotics on human platelet adhesion and aggregation and plasma coagulation. Clin Exp Pharmacol Physiol 2007; 34:775–780.
- Liperoti R et al. Venous thromboembolism among elderly patients treated with atypical and conventional antipsychotic agents. Arch Intern Med 2005; 165:2677–2682.
- Lacut K. Association between antipsychotic drugs, antidepressant drugs, and venous thromboembolism. Clin Adv Hematol Oncol 2008; 6:887–890.
- Borras L et al. Pulmonary thromboembolism associated with olanzapine and risperidone. J Emerg Med 2008; 35:159–161.
- Maly R et al. Four cases of venous thromboembolism associated with olanzapine. Psychiatry Clin Neurosci 2009; 63:116–118.
- Hagg S et al. Associations between venous thromboembolism and antipsychotics. A study of the WHO database of adverse drug reactions. Drug Saf 2008; 31:685–694.
- Lacut K et al. Association between antipsychotic drugs, antidepressant drugs and venous thromboembolism: results from the EDITH case-control study. Fundam Clin Pharmacol 2007; 21:643–650.
- Zink M et al. A case of pulmonary thromboembolism and rhabdomyolysis during therapy with mirtazapine and risperidone. J Clin Psychiatry 2006; 67:835.
- Allenet B et al. Antipsychotic drugs and risk of pulmonary embolism. Pharmacoepidemiol Drug Saf 2012; 21:42–48.
- Maly R et al. Assessment of risk of venous thromboembolism and its possible prevention in psychiatric patients. Psychiatry Clin Neurosci 2008; 62:3–8.
- Killian JG et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet 1999; 354:1841–1845.
- Hill GR et al. Clozapine and myocarditis: a case series from the New Zealand Intensive Medicines Monitoring Programme. N Z Med J 2008; 121:68–75.
- La Grenade L et al. Myocarditis and cardiomyopathy associated with clozapine use in the United States (Letter). N Engl J Med 2001; 345:224–225.
- Warner B et al. Clozapine and sudden death. Lancet 2000; 355:842.
- Reinders J et al. Clozapine-related myocarditis and cardiomyopathy in an Australian metropolitan psychiatric service. Aust N Z J Psychiatry 2004; 38:915–922.
- Haas SJ et al. Clozapine-associated myocarditis: a review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993-2003. Drug Saf 2007; 30:47–57.
- Marder SR et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry 2004; 161:1334–1349.
- Annamraju S et al. Early recognition of clozapine-induced myocarditis. J Clin Psychopharmacol 2007; 27:479–483.
- Wehmeier PM et al. Chart review for potential features of myocarditis, pericarditis, and cardiomyopathy in children and adolescents treated with clozapine. J Child Adolesc Psychopharmacol 2004; 14:267–271.
- McNeil JJ et al. Clozapine-induced myocarditis: characterisation using case-control design. Eur Heart J 2013; 34 ( Suppl 1):688–.
- Rostagno C et al. Beta-blocker and angiotensin-converting enzyme inhibitor may limit certain cardiac adverse effects of clozapine. Gen Hosp Psychiatry 2008; 30:280–283.
- Floreani J et al. Successful re-challenge with clozapine following development of clozapine-induced cardiomyopathy. Aust N Z J Psychiatry 2008; 42:747–748.
- Roh S et al. Cardiomyopathy associated with clozapine. Exp Clin Psychopharmacol 2006; 14:94–98.
- Masopust J et al. Repeated occurrence of clozapine-induced myocarditis in a patient with schizoaffective disorder and comorbid Parkinson's disease. Neuro Endocrinol Lett 2009; 30:19–21.
- Ronaldson KJ et al. Observations from 8 cases of clozapine rechallenge after development of myocarditis. J Clin Psychiatry 2012; 73:252–254.
- Hassan I et al. Monitoring in clozapine rechallenge after myocarditis. Australas Psychiatry 2011; 19:370–371.
- Bray A et al. Successful clozapine rechallenge after acute myocarditis. Aust N Z J Psychiatry 2011; 45:90.
- Rosenfeld AJ et al. Successful clozapine retrial after suspected myocarditis. Am J Psychiatry 2010; 167:350–351.
- Ronaldson KJ et al. Clinical course and analysis of ten fatal cases of clozapine-induced myocarditis and comparison with 66 surviving cases. Schizophr Res 2011; 128:161–165.
- Ronaldson KJ et al. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust N Z J Psychiatry 2011; 45:458–465.
- Ronaldson KJ et al. Rapid clozapine dose titration and concomitant sodium valproate increase the risk of myocarditis with clozapine: a case-control study. Schizophr Res 2012; 141:173–178.
- Coulter DM et al. Antipsychotic drugs and heart muscle disorder in international pharmacovigilance: data mining study. BMJ 2001; 322:1207–1209.
- Pastor CA et al. Masked clozapine-induced cardiomyopathy. J Am Board Fam Med 2008; 21:70–74.
- Sagar R et al. Clozapine-induced cardiomyopathy presenting as panic attacks. J Psychiatr Pract 2008; 14:182–185.
- Modai I et al. Sudden death in patients receiving clozapine treatment: a preliminary investigation. J Clin Psychopharmacol 2000; 20:325–327.
- Kang UG et al. Electrocardiographic abnormalities in patients treated with clozapine. J Clin Psychiatry 2000; 61:441–446.
- Thomassen R et al. Antipsychotic drugs and venous thromboembolism (Letter). Lancet 2000; 356:252.
- Hagg S et al. Antipsychotic-induced venous thromboembolism: a review of the evidence. CNS Drugs 2002; 16:765–776.
- Ronaldson KJ et al. Diagnostic characteristics of clozapine-induced myocarditis identified by an analysis of 38 cases and 47 controls. J Clin Psychiatry 2010; 71:976–981.
Further reading
Caforio AL et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34:2636–2648d.
Razminia M et al. Clozapine induced myopericarditis: early recognition improves clinical outcome. Am J Ther 2006;13:274–6.
Wehmeier PM et al. Myocarditis, pericarditis and cardiomyopathy in patients treated with clozapine. J Clin Pharm Ther 2005;30:91–6.
Clozapine-induced hypersalivation
Clozapine is well known to be causally associated with apparent hypersalivation (drooling, particularly at night). This seems to be chiefly problematic in the early stages of treatment and is probably dose-related. Clinical observation suggests that hypersalivation reduces in severity over time (usually several months) but may persist. Clozapine-induced hypersalivation is socially embarrassing and potentially life-threatening,1 so treatment is a matter of some urgency.
The pharmacological basis of clozapine-related hypersalivation remains unclear.2 Suggested mechanisms include muscarinic M4 agonism, α2-adrenergic antagonism and inhibition of the swallowing reflex.3,4 The last of these is supported by trials which suggest that saliva production is not increased in clozapine-treated patients,5,6 although at least one study has observed marked increases in salivary flow in the first 3 weeks of treatment.7
Whatever the mechanism, drugs which reduce saliva production are likely to diminish the severity of this adverse effect. Non-drug treatments may be used if appropriate—these include chewing gum, elevating pillows and placing a towel on the pillow to prevent soaking of clothes.2 Table 2.44 describes drug treatments so far examined.
Table 2.44 Clozapine-related hypersalivation
|
Treatment
|
Comments
|
Amisulpride
100-400 mg/day8,9
|
Supported by a positive RCT compared with placebo, one other in which it was compared with moclobemide and numerous case studies.10-13 May allow dose reduction of clozapine
|
Amitriptyline
25-100 mg/day14,15
|
Limited literature support. Adverse effects may be troublesome. Worsens constipation
|
Atropine eye drops (1%)
given sublingually16,17 or as solution (1 mg/10 mL)
used as a mouthwash
|
Limited literature support. Rarely used
|
Benzhexol (trihexyphenidyl)
5-15 mg/day18
|
Small, open study suggests useful activity. Used in some centres but may impair cognitive function. Lower doses (2 mg) may be effective19
|
Benztropine 2 mg/day
+ terazosin 2 mg/day20
|
Combination shown to be better than either drug alone. Terazosin is an ^-antagonist so may cause hypotension
|
Botulinum toxin21-23
(Botox) Bilateral parotid gland injections
(150 IU into each gland)
|
Effective in treating sialorrhoea associated with neurological disorders. Five cases of successful treatment of clozapine-related hypersalivation in the literature
|
Bupropion
100-150 mg/day24
|
Single case report. May lower seizure threshold
|
Clonidine
0.1-0.2 mg patch weekly or 0.1 mg orally at night25,26
|
α2-partial agonist. Limited literature support. May exacerbate psychosis, depression and cause hypotension
|
Glycopyrrolate
0.5-4 mg twice daily27-29
|
One RCT showed glycopyrrolate to be more effective than biperiden without worsening cognitive function
|
Guanfacine
1 mg daily30
|
α2-agonist. Single case report. May cause hypotension
|
Hyoscine
0.3 mg tablet sucked or chewed up to 3 times daily or 1.5 mg/72 hour patch31,32
|
Peripheral and central anticholinergic. Very widely used but no published data available on oral treatment. May cause cognitive impairment, drowsiness and worsens constipation.
|
Ipratropium Nasal spray
(0.03% or 0.06%)—given sublingually33,34 up to 2 sprays three times a day of the 0.06% or intranasally30 1 spray into each nostril daily of the 0.03%
|
Limited literature support. The only placebo-controlled RCT conducted was negative35
|
Lofexidine
0.2 mg twice daily36
|
α2-agonist. Very few data. May exacerbate psychosis, depression and cause hypotension
|
Moclobemide
150-300 mg/day37
|
Effective in 9 out of 14 patients treated in one open study. Appears to be as effective as amisupride (see above)
|
Oxybutynin
5 mg up to twice daily38
|
Single case report
|
Pirenzepine
50-1 50 mg/day39-41
|
Selective M1, M4 antagonist. Extensive clinical experience suggests efficacy in some but one RCT suggested no effect. Still widely used. Does not have a UK licence for any indication. May cause constipation
|
Propantheline
7.5 mg at night42
|
Peripheral anticholinergic. No central effects. Two Chinese RCTs (one positive). May worsen constipation
|
|
Quetiapine43
|
May reduce hypersalivation by allowing lower doses of clozapine to be used
|
Sulpiride
150-300 mg/day44,45
|
Supported by one, small positive RCT and a Cochrane Review of clozapine augmentation with sulpiride (at higher sulpiride doses). May allow dose reduction of clozapine
|
|
RCT, randomised controlled trial.
|
References
- Hinkes R et al. Aspiration pneumonia possibly secondary to clozapine-induced sialorrhea. J Clin Psychopharmacol 1996; 16:462–463.
- Praharaj SK et al. Clozapine-induced sialorrhea: pathophysiology and management strategies. Psychopharmacology 2006; 185:265–273.
- Davydov L et al. Clozapine-induced hypersalivation. Ann Pharmacother 2000; 34:662–665.
- Rogers DP et al. Therapeutic options in the treatment of clozapine-induced sialorrhea. Pharmacotherapy 2000; 20:1092–1095.
- Rabinowitz T et al. The effect of clozapine on saliva flow rate: a pilot study. Biol Psychiatry 1996; 40:1132–1134.
- Ben Aryeh H et al. Salivary flow-rate and composition in schizophrenic patients on clozapine: subjective reports and laboratory data. Biol Psychiatry 1996; 39:946–949.
- Praharaj SK et al. Salivary flow rate in patients with schizophrenia on clozapine. Clin Neuropharmacol 2010; 33:176–178.
- Kreinin A et al. Amisulpride treatment of clozapine-induced hypersalivation in schizophrenia patients: a randomized, double-blind, placebocontrolled cross-over study. Int Clin Psychopharmacol 2006; 21:99–103.
- Kreinin A et al. Amisulpride versus moclobemide in treatment of clozapine-induced hypersalivation. World J Biol Psychiatry 2010; 12:620–626.
- Praharaj SK et al. Amisulpride treatment for clozapine-induced sialorrhea. J Clin Psychopharmacol 2009; 29:189–190.
- Aggarwal A et al. Amisulpride for clozapine induced sialorrhea. Psychopharmacol Bull 2009; 42:69–71.
- Croissant B et al. Reduction of side effects by combining clozapine with amisulpride: case report and short review of clozapine-induced hypersalivation-a case report. Pharmacopsychiatry 2005; 38:38–39.
- Praharaj SK et al. Amisulpride improved debilitating clozapine-induced sialorrhea. Am J Ther 2011; 18:e84–e85.
- Copp P et al. Amitriptyline in clozapine-induced sialorrhoea. Br J Psychiatry 1991; 159:166.
- Praharaj SK et al. Amitriptyline for clozapine-induced nocturnal enuresis and sialorrhoea. Br J Clin Pharmacol 2007; 63:128–129.
- Antonello C et al. Clozapine and sialorrhea: a new intervention for this bothersome and potentially dangerous side effect. J Psychiatry Neurosci 1999; 24:250.
- Mustafa FA et al. Sublingual atropine for the treatment of severe and hyoscine-resistant clozapine-induced sialorrhea. Afr J Psychiatry (Johannesbg ) 2013; 16:242.
- Spivak B et al. Trihexyphenidyl treatment of clozapine-induced hypersalivation. Int Clin Psychopharmacol 1997; 12:213–215.
- Praharaj SK et al. Complete resolution of clozapine-induced sialorrhea with low dose trihexyphenidyl. Psychopharmacol Bull 2010; 43:73–75.
- Reinstein M et al. Comparative efficacy and tolerability of benzatropine and terazosin in the treatment of hypersalivation secondary to clozapine. Clin Drug Invest 1999; 17:97–102.
- Kahl KG et al. Botulinum toxin as an effective treatment of clozapine-induced hypersalivation. Psychopharmacology 2004; 173:229–230.
- Steinlechner S et al. Botulinum toxin B as an effective and safe treatment for neuroleptic-induced sialorrhea. Psychopharmacology (Berl) 2010; 207:593–597.
- Kahl KG et al. [Pharmacological strategies for clozapine-induced hypersalivation: treatment with botulinum toxin B in one patient and review of the literature]. Nervenarzt 2005; 76:205–208.
- Stern RG et al. Clozapine-induced sialorrhea alleviated by bupropion—a case report. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33:1578–1580.
- Grabowski J. Clonidine treatment of clozapine-induced hypersalivation. J Clin Psychopharmacol 1992; 12:69–70.
- Praharaj SK et al. Is clonidine useful for treatment of clozapine-induced sialorrhea? J Psychopharmacol 2005; 19:426–428.
- Duggal HS. Glycopyrrolate for clozapine-induced sialorrhea. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31:1546–1547.
- Robb AS et al. Glycopyrrolate for treatment of clozapine-induced sialorrhea in three adolescents. J Child Adolesc Psychopharmacol 2008; 18:99–107.
- Liang CS et al. Comparison of the efficacy and impact on cognition of glycopyrrolate and biperiden for clozapine-induced sialorrhea in schizophrenic patients: a randomized, double-blind, crossover study. Schizophr Res 2010; 119:138–144.
- Webber MA et al. Guanfacine treatment of clozapine-induced sialorrhea. J Clin Psychopharmacol 2004; 24:675–676.
- McKane JP et al. Hyoscine patches in clozapine-induced hypersalivation. Psychiatr Bull 2001; 25:277.
- Gaftanyuk O et al. Scolpolamine patch for clozapine-induced sialorrhea. Psychiatr Serv 2004; 55:318.
- Calderon J et al. Potential use of ipatropium bromide for the treatment of clozapine-induced hypersalivation: a preliminary report. Int Clin Psychopharmacol 2000; 15:49–52.
- Freudenreich O et al. Clozapine-induced sialorrhea treated with sublingual ipratropium spray: a case series. J Clin Psychopharmacol 2004; 24:98–100.
- Sockalingam S et al. Treatment of clozapine-induced hypersalivation with ipratropium bromide: a randomized, double-blind, placebo-controlled crossover study. J Clin Psychiatry 2009; 70:1114–1119.
- Corrigan FM et al. Clozapine-induced hypersalivation and the alpha 2 adrenoceptor. Br J Psychiatry 1995; 167:412.
- Kreinin A et al. Moclobemide treatment of clozapine-induced hypersalivation: pilot open study. Clin Neuropharmacol 2009; 32:151–153.
- Leung JG et al. Immediate-release oxybutynin for the treatment of clozapine-induced sialorrhea. Ann Pharmacother 2011; 45:e45.
- Fritze J et al. Pirenzepine for clozapine-induced hypersalivation. Lancet 1995; 346:1034.
- Bai YM et al. Therapeutic effect of pirenzepine for clozapine-induced hypersalivation: a randomized, double-blind, placebo-controlled, crossover study. J Clin Psychopharmacol 2001; 21:608–611.
- Schneider B et al. Reduction of clozapine-induced hypersalivation by pirenzepine is safe. Pharmacopsychiatry 2004; 37:43–45.
- Syed Sheriff RJ et al. Pharmacological interventions for clozapine-induced hypersalivation. Schizophr Bull 2008; 34:611–612.
- Reinstein MJ et al. Use of quetiapine to manage patients who experienced adverse effects with clozapine. Clin Drug Invest 2003; 23:63–67.
- Kreinin A et al. Sulpiride addition for the treatment of clozapine-induced hypersalivation: preliminary study. Isr J Psychiatry Relat Sci 2005; 42:61–63.
- Wang J et al. Sulpiride augmentation for schizophrenia. Cochrane Database Syst Rev 2010; CD008125.
Further reading
Bird AM et al. Current treatment strategies for clozapine-induced sialorrhea. Ann Pharmacother 2011; 45:667–675.
Clozapine-induced gastrointestinal hypomotility (cigh)
Constipation is a common adverse effect of clozapine treatment with a prevalence of up to 60%.1 The mechanism of action is not completely understood but is thought to be a combination of the drug's anticholinergic2,3 and antihistaminergic properties4 which are further complicated by antagonism at 5-HT3 receptors.2,3,5 In addition, clozapine-induced sedation can result in a sedentary lifestyle,4 which is itself a risk factor for constipation. Essentially clozapine causes constipation by slowing transit time through the gut.
Clozapine-induced constipation is much more common than blood dyscrasias, and mortality rates are also higher;4 when constipation is severe, the fatality rate (calculated from severe cases reported in the literature) is around 20–30%.4,6,7 Enhanced monitoring for CIGH is urgently needed to reduce constipation-related fatalities.
A gastrointestinal history and abdominal examination is recommended prior to starting treatment and if the patient is constipated, clozapine should not be initiated until this has resolved.7 CIGH is most severe during the first 4 months of treatment7 and so weekly assessments are recommended during this time frame.8 Adopting the Rome III criteria9 at routine FBCs might be a successful strategy to combat preventable deaths due to CIGH.
Opinions differ on the relationship between clozapine dose and constipation, and clozapine plasma level and constipation,7,10,11 however, the deaths that have occurred as a result of CIGH were in people taking higher daily doses (mean 535 mg/day)7.
The risk factors for developing clozapine-induced constipation are summarised in Box 2.2.
Prevention and simple management of CIGH
A slower clozapine titration may reduce the risk of developing constipation with dose increments not exceeding 25 mg/day or 100 mg/week.1 Increasing dietary fibre intake to at least 20–25 g per day can increase stool weight and decrease gut transit time.14,15
|
Box 2.2 Risk factors for developing clozapine-induced constipation7,8,12–14
|
- Increasing age
- Female sex
- Anticholinergic medication
- Hypersalivation
- Higher clozapine dose/plasma level
- Hypercalcaemia
- Gastrointestinal disease
- Obesity
- Diaphoresis
- Low fibre diet
- Poor bowel habit
- Dehydration
- Diabetes
- Hypothyroidism
- Parkinson's disease
- Multiple sclerosis
|
If fibre intake is increased it is important that adequate fluid intake (1.5–2 L/day) is also maintained to avoid intestinal obstruction.7,14,16 Daily food and fluid charts would be ideal to monitor fibre and fluid intake especially during the titration phase of clozapine. Regular exercise (150 minutes/week)17 in addition to a high fibre diet and increased fluid intake also assist in the prevention of CIGH.18,19
Weekly stool charts,14,20 should be used for all patients starting clozapine. If there is a change from usual baseline bowel habit or fewer than three bowel movements (BM) per week9 an abdominal examination is indicated.7 Where this excludes intestinal obstruction, both a stimulant and stool-softening laxative should be started (for example senna and docusate7,21). Bulk forming laxatives are not effective in slow-transit constipation2,22 and therefore should be avoided. There is some evidence that lactulose and polyethylene glycol (for example Movicol) are effective2,23 and could be considered as second line options or alternatives to the stimulant and softener combination. Choice of laxative should also be guided by the patients' previous response to certain agents in association with the required speed of action.24 It would not be appropriate for example to start lactulose treatment (takes up to 72 hours of regular use to work25) for someone in need of urgent treatment.
Management of suspected acute CIGH
Signs and symptoms that warrant immediate medical attention are abdominal pain, distension, vomiting, overflow diarrhoea, absent bowel sounds, acute abdomen, feculent vomitus and symptoms of sepsis,7 1,6,26–33 There have been case reports of fatalities occurring only hours after first symptoms present,34 which emphasises the urgency for prompt assessment and management. There should therefore be a low threshold for referral to a gastroenterologist when conservative management fails or constipation is severe and acute.7,35
Physicians may not be familiar with clozapine-induced constipation and may welcome information on the associated morbidity and mortality; a copy of this section of the guidelines may facilitate timely treatment. The following may be helpful to communicate to staff in emergency departments.
- Stop clozapine,7 and all other anti-muscarinic medicines.
- Assess for bowel obstruction.
- If obstruction is not present, consider use of an enema or digital disimpaction.7 In some cases, the off-label use of neostigmine or physostigmine has been employed to accelerate gastrointestinal transit time and has shown good results for acute colonic pseudo-obstruction.7,36,37
- If obstruction is present, consider urgent surgical referral.
Clozapine re-challenge following severe constipation
Some patients have been successfully re-challenged following severe cases of CIGH, however, this does not come without risk. Prophylactic measures should therefore be considered for those with a history of CIGH or who are deemed high risk of developing CIGH. Where conventional laxatives have not been tried in regular and adequate doses, this should be done. However, when this approach has previously failed, a number of more experimental options are available. Prescribers must familiarise themselves with the literature (at the very least by reading the SPC) before using any of these treatments.
The prostaglandin E1 analogue lubiprostone is licensed in the UK for the treatment of chronic idiopathic constipation and associated symptoms in adults, when response to diet and other non-pharmacological measures (e.g. educational measures, physical activity) are inappropriate.38 The recommended dose for the licensed indication is 24 μg twice daily for a maximum of 2 weeks duration.38 Lubiprostone has been reported to be effective in obviating the need for other laxatives in a clozapine re-challenge following a severe case of CIGH,39 and is used in some centres for this indication.39
Orlistat, a drug used to aid weight loss, is also known to have a laxative effect particularly when a high-fat diet is consumed. It has been successfully used for three patients with severe constipation associated with opioid use (hypomotility-induced constipation).40 A small, randomised placebo controlled study of orlistat for clozapine-induced constipation found a statistically significant favourable difference at study endpoint (week 16) for the prevalence of constipation, diarrhoea, and normal stools for orlistat compared with placebo;41 note that 47 of the 54 participants required conventional laxatives. Note also that orlistat is known to reduce the absorption of some drugs from the GI tract. It is therefore important to monitor plasma clozapine levels if starting treatment with orlistat.
Bethanechol, a cholinergic agonist, has been described as being effective in reducing the amount of laxatives and enemas required to maintain regular bowel movements for a patient diagnosed with CIGH.42 Bethanechol in this scenario was used at a dose of 10 mg three times daily. Bethanechol should only ever be initiated after other options have failed and in consultation with a gastroenterologist.42
References
- Hayes G et al. Clozapine-induced constipation. Am J Psychiatry 1995; 152:298.
- Hibbard KR et al. Fatalities associated with clozapine-related constipation and bowel obstruction: a literature review and two case reports. Psychosomatics 2009; 50:416–419.
- Rege S et al. Life-threatening constipation associated with clozapine. Australas Psychiatry 2008; 16:216–219.
- De Hert M et al. Second-generation antipsychotics and constipation: a review of the literature. Eur Psychiatry 2011; 26:34–44.
- Meltzer HY et al. Effects of antipsychotic drugs on serotonin receptors. Pharmacol Rev 1991; 43:587–604.
- Cohen D et al. Beyond white blood cell monitoring: screening in the initial phase of clozapine therapy. J Clin Psychiatry 2012; 73:1307–1312.
- Palmer SE et al. Life-threatening clozapine-induced gastrointestinal hypomotility: an analysis of 102 cases. J Clin Psychiatry 2008; 69:759–768.
- Nielsen J et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry 2013; 74:603–613; quiz 613.
- Rome Foundation. Rome III Disorders and Criteria. http://www.theromefoundation.org/criteria/, 2006.
- Chengappa KN et al. Anticholinergic differences among patients receiving standard clinical doses of olanzapine or clozapine. J Clin Psychopharmacol 2000; 20:311–316.
- Vella-Brincat J et al. Clozapine-induced gastrointestinal hypomotility. Australas Psychiatry 2011; 19:450–451
- Nielsen J et al. Risk factors for ileus in patients with schizophrenia. Schizophr Bull 2012; 38:592–598.
- Longmore M et al. Oxford Handbook of Clinical Medicine Oxford, UK: OUP Oxford, 2010.
- ZTAS. Zaponex Fact Sheet - Constipation. https://www.ztas.com/Manuals/ZFS_Constipation2.pdf, 2013.
- Muller-Lissner SA. Effect of wheat bran on weight of stool and gastrointestinal transit time: a meta analysis. Br Med J (Clin Res Ed) 1988; 296:615–617.
- National Prescribing Centre. The management of constipation. MedRec Bulletin 2011; 21:1–8.
- NHS Choices. Physical activity guidelines for adults. http://www.nhs.uk/Livewell/fitness/Pages/physical-activity-guidelines-for-adults.aspx, 2013.
- Fitzsimons J et al. A review of clozapine safety. Expert Opin Drug Saf 2005; 4:731–744.
- Young CR et al. Management of the adverse effects of clozapine. Schizophr Bull 1998; 24:381–390.
- Lewis SJ et al. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997; 32:920–924.
- Swegle JM et al. Management of common opioid-induced adverse effects. Am Fam Physician 2006; 74:1347–1354.
- Voderholzer WA et al. Clinical response to dietary fiber treatment of chronic constipation. Am J Gastroenterol 1997; 92:95–98.
- Brandt LJ et al. Systematic review on the management of chronic constipation in North America. Am J Gastroenterol 2005; 100 Suppl 1:S5–s21.
- Bleakley S et al. Clozapine Handbook. Warwickshire, UK: Lloyd-Reinhold Communications LLP, 2013.
- Intrapharm Laboratories Limited. Summary of Product Characteristics. Lactulose 10 g/15 ml oral solution sachets. https://www.medicines. org.uk/, 2013.
- Leong QM et al. Necrotising colitis related to clozapine? A rare but life threatening side effect. World J Emerg Surg 2007; 2:21.
- Shammi CM et al. Clozapine-induced necrotizing colitis. J Clin Psychopharmacol 1997; 17:230–232.
- Rondla S et al. A case of clozapine-induced paralytic ileus. Emerg Med J 2007; 24:e12.
- Levin TT et al. Death from clozapine-induced constipation: case report and literature review. Psychosomatics 2002; 43:71–73.
- Townsend G et al. Case report: rapidly fatal bowel ischaemia on clozapine treatment. BMC Psychiatry 2006; 6:43.
- Karmacharya R et al. Clozapine-induced eosinophilic colitis. Am J Psychiatry 2005; 162:1386–1387.
- Erickson B et al. Clozapine-associated postoperative ileus: case report and review of the literature. Arch Gen Psychiatry 1995; 52:508–509.
- Schwartz BJ et al. A case report of clozapine-induced gastric outlet obstruction. Am J Psychiatry 1993; 150:1563.
- Drew L et al. Clozapine and constipation: a serious issue. Aust N Z J Psychiatry 1997; 31:149.
- Ikai S et al. Reintroduction of clozapine after perforation of the large intestine—a case report and review of the literature. Ann Pharmacother 2013; 47:e31.
- Loftus CG et al. Assessment of predictors of response to neostigmine for acute colonic pseudo-obstruction. Am J Gastroenterol 2002; 97:3118–3122.
- Saunders MD et al. Systematic review: acute colonic pseudo-obstruction. Aliment Pharmacol Ther 2005; 22:917–925.
- Sucampo Pharma Europe Ltd. AMITIZA 24 microgram soft capsules. https://www.medicines.org.uk/, 2013.
- Meyer,JM et al. Lubiprostone for treatment-resistant constipation associated with clozapine use. Acta Psychiatr Scand 2014; 130:71–72.
- Guarino AH. Treatment of intractable constipation with orlistat: a report of three cases. Pain Med 2005; 6:327–328.
- Chukhin E et al. In a randomized placebo-controlled add-on study orlistat significantly reduced clozapine-induced constipation. Int Clin Psychopharmacol 2013; 28:67–70.
- Poetter CE et al. Treatment of clozapine-induced constipation with bethanechol. J Clin Psychopharmacol 2013; 33:713–714.
Clozapine, neutropenia and lithium
Risk of clozapine-induced neutropenia
Around 2.7% of patients treated with clozapine develop neutropenia. Of these, half do so within the first 18 weeks of treatment and three-quarters by the end of the first year.1 Risk factors1 include being Afro-Caribbean (77% increase in risk) and young (17% decrease in risk per decade increase in age), and having a low baseline white cell count (WCC) (31% increase in risk for each 1 × 109/L drop). Risk is not dose-related. Approximately 0.8% will develop agranulocytosis. The mechanism of clozapine-induced neutropenia/ agranulocytosis is unclear and it is possible that immune-mediated and direct cytotoxic effects may both be important. The mechanism may differ between individuals and also between mild and severe forms of marrow suppression.2 One-third of patients who stop clozapine because they have developed neutropenia or agranulocytosis will develop a blood dyscrasia on re-challenge. In almost all cases, the second reaction will occur more rapidly, be more severe and last longer than the first.3
Benign ethnic neutropenia (BEN)
After being released from the bone marrow, neutrophils can either circulate freely in the bloodstream or be deposited next to vessel walls (margination).4 All of these neutrophils are available to fight infection. The proportion of marginated neutrophils is greater in people of Afro-Caribbean or African origin than in Caucasians, leading to lower apparent white cell counts (WCC) in the former. This is benign ethnic neutropenia. Many countries allow registration of BEN status with the clozapine supplier whereby different (lower) limits are set for neutrophil counts in these patients.
Many patients develop neutropenia on clozapine but not all are clozapine-related or even pathological. Benign ethnic neutropenia very probably accounts for a proportion of observed or apparent clozapine-associated neutropenias (hence higher rates among AfroCaribbeans). Distinguishing between true clozapine toxicity and neutropenia unrelated to clozapine is not possible with certainty but some factors are important. True clozapine-induced neutropenia generally occurs early in treatment. White cell counts are normal to begin with but then fall precipitously (over 1–2 weeks or less) and recover slowly once clozapine is withdrawn. In benign ethnic neutropenia, WCCs are generally low and may frequently fall below the lower limit of normal. This pattern may be observed before, during and after the use of clozapine. Of course, true clozapine-induced neutropenia can occur in the context of benign ethnic neutropenia. Partly because of this, any iatrogenic manipulation of WCCs in benign ethnic neutropenia carries significant risk.
Effect of lithium on the WCC
Lithium increases the neutrophil count and total WCC both acutely5 and chronically.6,7 The magnitude of this effect is poorly quantified, but a mean neutrophil count of 11 × 109/L has been reported in lithium-treated patients5 and a mean rise in neutrophil count of 2 × 109/L was seen in clozapine-treated patients after the addition of lithium.8 This effect does not seem to be clearly dose-related5,6 although a minimum lithium serum level of 0.4 mmol/L may be required.9 The mechanism is not completely understood: both stimulation of granulocyte-macrophage colony-stimulating factor (GM-CSF)10 and demargination8 have been suggested. Lithium has been successfully used to raise the WCC during cancer chemotherapy.11–13 White cells are fully formed and function normally—there is no 'left shift'.
Case reports
Lithium has been used to increase the WCC in patients who have developed neutropenia whilst taking clozapine, allowing clozapine treatment to continue. Several case reports in adults9,14–18 and in children19,20 have been published. Almost all patients had serum lithium levels of > 0.6 mmol/L. Lithium has also been reported to speed the recovery of the WCC when prescribed after the development of clozapine-induced agranulocytosis.9 In a case series (n = 25) of patients who had stopped clozapine because of a blood dyscrasia and were re-challenged in the presence of lithium, only one developed a subsequent dyscrasia; a far lower proportion than would be expected21 (see above).
Other potential benefits of lithium–clozapine combinations
Combinations of clozapine and lithium may improve symptoms in schizoaffective patients8 and refractory bipolar illness.22,23 There are no data pertaining to schizophrenia.
Agranulocytosis
At least 0.8% of clozapine-treated patients develop agranulocytosis, which is potentially fatal. Over 80% of cases develop within the first 18 weeks of treatment.1 Risk factors include increasing age and Asian race.1 Some patients may be genetically predisposed.24 Although the timescale and individual risk factors for the development of agranulocytosis are different from those associated with neutropenia, it is impossible to be certain in any given patient that neutropenia is not a precursor to agranulocytosis. Lithium does not seem to protect against true clozapine-induced agranulocytosis: one case of fatal agranulocytosis has occurred with this combination25 and a second case of agranulocytosis has been reported where the bone marrow was resistant to treatment with granulocyte-colony stimulating factor (G-CSF).26 Note also that up to 20% of patients who receive clozapine–lithium combinations develop neurological symptoms typical of lithium toxicity despite lithium levels being maintained well within the therapeutic range.8,27
Management options
The use of iatrogenic agents to elevate WCC in patients with clear prior clozapineinduced neutropenia is not recommended. Lithium or other medicines should only be used to elevate WCC where it is strongly felt that prior neutropenic episodes were unrelated to clozapine. Patients who have had a previous clear clozapine-induced agranulocytosis should not be re-challenged with clozapine.
The patient's individual clinical circumstances should be considered. In particular, patients in whom the first dyscrasia:
- was inconsistent with previous WCCs (i.e. not part of a pattern of repeated low WCCs)
- occurred within the first 18 weeks of treatment
- was severe (neutrophils < 0.5 × 109/L), and
- was prolonged
should be considered to be very high risk if re-challenged with clozapine. Generally re-exposure to clozapine should not be attempted.
Management of patients with either of the following conditions:
- low initial WCC (< 4 × 109/L) or neutrophils (< 2.5 × 109/L), or
- leucopenia (WCC < 3 × 109/L) or neutropenia (neutrophils < 1.5 × 109/L) thought to be linked to benign ethnic neutropenia. Such patients may be of African or Middle Eastern descent, have no history of susceptibility to infection and have morphologically normal white blood cells.
should be treated as outlined in Figure 2.6.

Figure 2.6 The use of lithium with clozapine.
Granulocyte-colony stimulating factor
The use of granulocyte-colony stimulating factor ( G-CSF) to facilitate uninterrupted clozapine therapy in patients with previous neutropenia is a strategy that is attracting increasing interest, but is somewhat controversial. There are both successful28–30 and unsuccessful30,31 case reports of patients receiving regular long-term G-CSF to enable clozapine therapy. As well as the commonly reported side-effects of bone pain32 and neutrophil dysplasia,33 the administration of G-CSF in the face of a low or declining neutrophil count may mask an impending neutropenia or agranulocytosis, leading to severe consequences. The long term safety of G-CSF has not been determined—bone density and spleen size should probably be monitored.
'When required' G-CSF, to be administered if neutrophils drop below a defined threshold, may allow re-challenge with clozapine of patients in whom lithium is insufficient to prevent 'dipping' of WCC below the normal range. Again, this strategy risks masking a severe neutropenia or agranulocytosis. It is also likely to be practically difficult to manage outside a specialist unit, as frequent blood testing (twice to three times a week) is required, as well as immediate access to medical review and the G-CSF itself.
Consultation with a haematologist is essential before considering the use of G-CSF.
References
- Munro J et al. Active monitoring of 12760 clozapine recipients in the UK and Ireland. Br J Psychiatry 1999; 175:576–580.
- Whiskey E et al. Restarting clozapine after neutropenia: evaluating the possibilities and practicalities. CNS Drugs 2007; 21:25–35.
- Dunk LR et al. Rechallenge with clozapine following leucopenia or neutropenia during previous therapy. Br J Psychiatry 2006; 188:255–263.
- Abramson N et al. Leukocytosis: basics of clinical assessment. Am Fam Physician 2000; 62:2053–2060.
- Lapierre G et al. Lithium carbonate and leukocytosis. Am J Hosp Pharm 1980; 37:1525–1528.
- Carmen J et al. The effects of lithium therapy on leukocytes: a 1-year follow-up study. J Natl Med Assoc 1993; 85:301–303.
- Palominao A et al. Leukocytosis after lithium and clozapine combination therapy. Ann Clin Psychiatry 2010; 22:205–206.
- Small JG et al. Tolerability and efficacy of clozapine combined with lithium in schizophrenia and schizoaffective disorder. J Clin Psychopharmacol 2003; 23:223–228.
- Blier P et al. Lithium and clozapine-induced neutropenia/agranulocytosis. Int Clin Psychopharmacol 1998; 13:137–140.
- Ozdemir MA et al. Lithium-induced hematologic changes in patients with bipolar affective disorder. Biol Psychiatry 1994; 35:210–213.
- Johnke RM et al. Accelerated marrow recovery following total-body irradiation after treatment with vincristine, lithium or combined vincristine-lithium. Int J Cell Cloning 1991; 9:78–88.
- Greco FA et al. Effect of lithium carbonate on the neutropenia caused by chemotherapy: a preliminary clinical trial. Oncology 1977; 34:153–155.
- Ridgway D et al. Enhanced lymphocyte response to PHA among leukopenia patients taking oral lithium carbonate. Cancer Invest 1986; 4:513–517.
- Adityanjee A. Modification of clozapine-induced leukopenia and neutropenia with lithium carbonate. Am J Psychiatry 1995; 152:648–649.
- Silverstone PH. Prevention of clozapine-induced neutropenia by pretreatment with lithium. J Clin Psychopharmacol 1998; 18:86–88.
- Boshes RA et al. Initiation of clozapine therapy in a patient with preexisting leukopenia: a discussion of the rationale of current treatment options. Ann Clin Psychiatry 2001; 13:233–237.
- Papetti F et al. Treatment of clozapine-induced granulocytopenia with lithium (two observations). Encephale 2004; 30:578–582.
- Kutscher EC et al. Clozapine-induced leukopenia successfully treated with lithium. Am J Health Syst Pharm 2007; 64:2027–2031.
- Sporn A et al. Clozapine-induced neutropenia in children: management with lithium carbonate. J Child Adolesc Psychopharmacol 2003; 13:401–404.
- Mattai A et al. Adjunctive use of lithium carbonate for the management of neutropenia in clozapine-treated children. Hum Psychopharmacol 2009; 24:584–589.
- Kanaan RA et al. Lithium and clozapine rechallenge: a restrospective case analysis. J Clin Psychiatry 2006; 67:756–760.
- Suppes T et al. Clozapine treatment of nonpsychotic rapid cycling bipolar disorder: a report of three cases. Biol Psychiatry 1994; 36:338–340.
- Puri BK et al. Low-dose maintenance clozapine treatment in the prophylaxis of bipolar affective disorder. Br J Clin Pract 1995; 49:333–334.
- Dettling M et al. Further evidence of human leukocyte antigen-encoded susceptibility to clozapine-induced agranulocytosis independent of ancestry. Pharmacogenetics 2001; 11:135–141.
- Gerson SL et al. Polypharmacy in fatal clozapine-asscoaited agranulocytosis. Lancet 1991; 338:262–263.
- Valevski A et al. Clozapine-lithium combined treatment and agranulocytosis. Int Clin Psychopharmacol 1993; 8:63–65.
- Blake LM et al. Reversible neurologic symptoms with clozapine and lithium. J Clin Psychopharmacol 1992; 12:297–299.
- Spencer BW et al. Granulocyte colony stimulating factor (G-CSF) can allow treatment with clozapine in a patient with severe benign ethnic neutropaenia (BEN): a case report. J Psychopharmacol 2012; 26:1280–1282.
- Hagg S et al. Long-term combination treatment with clozapine and filgrastim in patients with clozapine-induced agranulocytosis. Int Clin Psychopharmacol 2003; 18:173–174.
- Joffe G et al. Add-on filgrastim during clozapine rechallenge in patients with a history of clozapine-related granulocytopenia/agranulocytosis. Am J Psychiatry 2009; 166:236.
- Mathewson KA et al. Clozapine and granulocyte colony-stimulating factor: potential for long-term combination treatment for clozapineinduced neutropenia. J Clin Psychopharmacol 2007; 27:714–715.
- Puhalla S et al. Hematopoietic growth factors: personalization of risks and benefits. Mol Oncol 2012; 6:237–241.
- Bain BJ et al. Neutrophil dysplasia induced by granulocyte colony-stimulating factor. Am J Hematol 2010; 85:354.
Further reading
Paton C et al. Managing clozapine-induced neutropenia with lithium. Psychiatr Bull 2005;29:186–8.
Clozapine and chemotherapy
The use of clozapine with agents that cause neutropenia is formally contraindicated. Most chemotherapy treatments cause significant bone marrow suppression. When the white blood cell count drops below 3.0 × 109/L clozapine is usually discontinued; this is an important safety precaution outlined in the formal licence/labelling. In many regimens it can be predicted that chemotherapy will reduce the white blood cell count below this level, irrespective of the use of clozapine.
If possible clozapine should be discontinued before chemotherapy. However, this will place most patients at high risk of relapse or deterioration which may affect their capacity to consent to chemotherapy. This poses a therapeutic dilemma in patients prescribed clozapine and requiring chemotherapy. Many patients, perhaps even a majority, continue clozapine during chemotherapy.
There are a number of case reports supporting the continued use of clozapine during chemotherapy.1–13 Before initiating chemotherapy in a patient who takes clozapine, it is essential to put in place a treatment plan that is agreed with all relevant staff involved in the patient's care, and of course, the patient themself; this will include the oncologist/ physician, psychiatrist, pharmacist and the clozapine monitoring service. Plans should be made in advance for the action that should be taken when the white blood count drops below the normally accepted minimum. This plan should cover the frequency of haematological monitoring, increased vigilance regarding the clinical consequences of neutropenia/agranulocytosis, if and when clozapine should be stopped, and the place of 'antidote' medication such as lithium and G-CSF.
In the UK, the clozapine monitoring service will normally ask for the psychiatrist to sign an 'unlicensed use' form and will request additional blood monitoring. Complications appear to be rare, but there is one case report of neutropenia persisting for 6 months after doxorubicin, radiotherapy and clozapine.7 G-CSF has been used to treat agranulocytosis associated with chemotherapy and clozapine in combination.8 Risks of life-threatening blood dyscrasia are probably lowest in those who have received clozapine for longer than a year in whom clozapine-induced neutropenia would be highly unusual.
Summary
- If possible clozapine should be discontinued before starting chemotherapy. For most patients withdrawal is not possible.
- The risk of relapse or deterioration must be considered before discontinuing clozapine.
- If the patient's mental state deteriorates they may retract their consent for chemotherapy.
- When clozapine is continued during chemotherapy a collaborative approach between the oncologist, psychiatrist, pharmacy, patient and clozapine monitoring service is strongly recommended.
References
- Wesson ML et al. Continuing clozapine despite neutropenia. Br J Psychiatry 1996; 168:217–220.
- Bareggi C et al. Clozapine and full-dose concomitant chemoradiation therapy in a schizophrenic patient with nasopharyngeal cancer. Tumori 2002; 88:59–60.
- Avnon M et al. Clozapine, cancer, and schizophrenia. Am J Psychiatry 1993; 150:1562–1563.
- Hundertmark J et al. Reintroduction of clozapine after diagnosis of lymphoma. Br J Psychiatry 2001; 178:576.
- McKenna RC et al. Clozapine and chemotherapy. Hosp Community Psychiatry 1994; 45:831.
- Haut FA. Clozapine and chemotherapy. J Drug Dev Clin Pract 1995; 7:237–239.
- Rosenstock J. Clozapine therapy during cancer treatment. Am J Psychiatry 2004; 161:175.
- Lee SY et al. Combined antitumor chemotherapy in a refractory schizophrenic receiving clozapine (Korean). J Korean Neuropsychiatr Assoc 2000; 39:234–239.
- Rosenberg I et al. Restarting clozapine treatment during ablation chemotherapy and stem cell transplant for Hodgkin's lymphoma. Am J Psychiatry 2007; 164:1438–1439.
- Goulet K et al. Case report: clozapine given in the context of chemotherapy for lung cancer. Psychooncology 2008; 17:512–516.
- Frieri T et al. Maintaining clozapine treatment during chemotherapy for non-Hodgkin's lymphoma. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:1611–1612.
- Sankaranarayanan A et al. Clozapine, cancer chemotherapy and neutropenia - dilemmas in management. Psychiatr Danub 2013; 25:419–422.
- De Berardis D et al. Safety and efficacy of combined clozapine-azathioprine treatment in a case of resistant schizophrenia associated with Behcet's disease: a 2-year follow-up. Gen Hosp Psychiatry 2013; 35:213–11.






