CHAPTER 4
Laboratory Testing and Imaging Studies in Psychiatry
Laboratory and diagnostic testing traditionally have not held a central role in the diagnosis and treatment of patients with psychiatric disorders, although other specialties of modern medicine have come to rely heavily on laboratory and imaging modalities to provide the necessary information to diagnose and treat patients with disorders such as cancer, heart disease, and pulmonary problems. Psychiatric diagnoses continue to be made primarily on clinical grounds, with laboratory and diagnostic testing being relegated to informing clinicians about medical causes of psychiatric symptoms that might be excluded from the differential diagnosis or used to monitor psychotropic drug levels during treatment. However, clinical laboratory and diagnostic imaging is on the threshold of a new era.
New methods such as pharmacogenetic and pharmacogenomic testing are becoming widespread and more widely available for clinical use. Research into structural and functional neuroimaging abnormalities in psychiatric disorders is providing valuable information about the possible pathophysiology underlying these disease states. Research into the use of combined laboratory and imaging modalities may lead to the eventual goal of early identification, treatment, and ultimately prevention of psychiatric illnesses. In this chapter, we present currently available information regarding clinical diagnostic testing and imaging of psychiatric patients and discuss what the future holds for these modalities as the armamentarium of diagnostic modalities grows ever larger for the clinical psychiatrist.
Laboratory assessment is essential to the workup of the psychiatric patient because any number of neurological and medical illnesses can give rise to psychiatric symptomatology. A careful neuropsychiatric history and physical examination and judicious clinical laboratory testing are still the first and very important steps in the workup. They can focus or even obviate neuroimaging or electro-physiological testing, which can be expensive, invasive, and physically and emotionally uncomfortable to the patient.
Moreover, psychiatric symptoms that on the surface may appear to be similar may, in fact, have dissimilar etiologies. For example, hallucinations can occur in the context of schizophrenia, as well as some major and mild neurocognitive disorders, substance-related disorders, and delirium. Table 4-1 lists some of the many medical and neurological illnesses that may present with prominent neuropsychiatric symptoms. Clinical laboratory assessment and diagnostic testing can help determine which of these many causes is responsible for a patient's hallucinations. Importantly, a number of these etiologies may have potentially curative remediations, and hence accurate diagnosis is critical.
A complete psychiatric assessment, including a medical and psychiatric history, physical examination, and mental status examination, must be conducted before the initiation of any clinical and diagnostic testing. Such initial assessments will guide the clinician in making choices for relevant, cost-effective laboratory testing. Laboratory costs account for a substantial portion of total health care costs, and unnecessary tests should be avoided if they are unlikely to alter the patient's treatment and outcome (Sheline and Kehr 1990).
No consensus guidelines currently exist for the initial laboratory screening of psychiatric patients without known medical illnesses. Clinicians are generally guided by the history, physical examination, and mental status examination and by their own clinical judgment to decide which tests are appropriate.
Studies of patient populations with general medical illnesses have shown that the history and review of systems obtained from the patient are superior to the physical examination in the diagnosis and management of patients and that screening laboratory testing can be the least helpful modality (Amin and Wang 2009; Anfinson and Kathol 1992). Furthermore, other studies indicate that there is little relationship between physical complaints and the presence of physical disease (Honig et al. 1991).
Which screening laboratory tests, then, are most helpful for the psychiatric patient? Most studies investigating the utility of screening laboratory testing in the psychiatric patient have been conducted in a retrospective manner, drawing from varied patient populations (Amin and Wang 2009; Mookhoek and Sterrenburg-vdNieuwegiessen 1998; Sheline and Kehr 1990). Results from these studies suggest that patients with psychiatric complaints alone, without other medical problems or complaints, will benefit from a few screening tests such as serum glucose concentration, blood urea nitrogen (BUN) concentration, creatinine clearance, and urinalysis (Anfinson and Kathol 1992). More extensive screening panels appear to be unnecessary (Lukens et al. 2006). Screening of female psychiatric patients ages 50 years and older, especially those with mood symptoms, may be justified due to a high prevalence of hypothyroidism in the patients. Thyroid screening of men and younger women, among whom the prevalence of thyroid dysfunction is estimated to be 0.1%, should be limited to patients with two or more clinical signs of hypothyroidism (Anfinson and Stoudemire 2000).
|
Table 4-1. Selected medical conditions with psychiatric manifestations |
NeurologicalCerebrovascular disease [major or mild vascular neurocognitive disorder] Multiple sclerosis Multiple systems atrophy Parkinson's disease [major or mild neurocognitive disorder due to Parkinson's disease] Progressive supranuclear palsy Alzheimer's disease [major or mild neurocognitive disorder due to Alzheimer's disease] Frontotemporal dementias [major or mild frontotemporal neurocognitive disorder] Dementia associated with Lewy bodies [major or mild neurocognitive disorder with Lewy bodies] Seizure disorder Huntington's disease [major or mild neurocognitive disorder due to Huntington's disease] Traumatic brain injury [major or mild neurocognitive disorder due to traumatic brain injury] Anoxic brain injury Migraine headache Sleep disorders [narcolepsy breathing-related sleep disorders] Normal pressure hydrocephalus NeoplasticCentral nervous system tumors, primary and metastatic Pancreatic carcinoma Paraneoplastic syndromes Endocrine tumors Pheochromocytoma InfectiousHIV Neurosyphilis Creutzfeldt-Jakob's disease Systemic viral and bacterial infections Viral and bacterial meningitis and encephalitis Tuberculosis Infectious mononucleosis Pediatric acute-onset neuropsychiatric syndrome (PANS) NutritionalVitamin deficiencies B12: pernicious anemia Folate: megaloblastic anemia Nicotinic acid deficiency: pellagra Thiamine deficiency: Wernicke-Korsakoff's syndrome Trace mineral deficiency (zinc, magnesium) AutoimmuneSystemic lupus erythematosus Sarcoidosis Sjögren's syndrome Behcet's syndrome N-methyl-D-aspartate (NMDA) receptor encephalitis Potassium channel antibody-mediated encephalitis Endocrine/metabolicWilson's disease Fluid and electrolyte disturbances (syndrome of inappropriate antidiuretic hormone secretion [SIADH], central pontine myelinolysis) Porphyrias Uremias Hypercapnia Hepatic encephalopathy Hypercalcemia/hypocalcemia Hyperglycemia/hypoglycemia Thyroid and parathyroid disease Diabetes mellitus Pheochromocytoma Pregnancy Gonadotropic hormonal disturbances Panhypopituitarism Drugs and toxinsEnvironmental toxins: organophosphates, heavy metals, carbon monoxide Substance-related intoxication/withdrawal or delirium or substance-induced neurocognitive disorder Adverse effects of prescription and over-the-counter medications |
Note. DSM-5 (American Psychiatric Association 2013) diagnostic labels for selected cognitive disorders associated with specific etiologies appear in brackets.
Source. Adapted from Ringholz 2001; Sadock and Sadock 2007; Wallach 2000.
More extensive laboratory screening may be required for several categories of patients: elderly individuals, institutionalized persons, persons of low socioeconomic status, individuals with a high degree of self-neglect, persons with alcohol or drug dependence, and those with cognitive impairment or fluctuating mental status (Anfinson and Kathol 1992). These patients may be less able to give a coherent or complete clinical history, or may have a higher burden of complex medical illnesses, and thus require more "detective" work in the form of laboratory workup. In these situations, screening laboratory tests will vary according to the patient's clinical presentation, the clinical situation (outpatient clinic, emergency department, inpatient setting), and concomitant medical illnesses. Laboratory screening becomes anything but routine for the patients in these categories, and it must be tailored to the patient's specific presenting complaints and physical findings.
In several studies, investigators have retrospectively reviewed the utility of the screening chest radiograph in the evaluation of psychiatric patients and concluded that there is little evidence that a routine chest radiograph will yield beneficial information for a patient without respiratory or neurological symptoms (Berkemeier et al. 2008; Mookhoek and Sterrenburg-vdNieuwegiessen 1998). These data, in addition to the absence of current screening guidelines for chest radiographs in the general population, indicate that the routine screening chest radiograph is not indicated for a person being evaluated for the presence of a psychiatric disorder. However, chest radiographs are clearly indicated for specific clinical situations. For example, a chest radiograph should be ordered on an emergency basis for an elderly patient with sudden onset of fever, shortness of breath, chest pain, or delirium.
Several studies have shown that the routine performance of screening electrocardiograms (ECGs) on young, medically healthy psychiatric patients who do not have cardiovascular symptoms is unnecessary (Hollister 1995). However, studies differ regarding the importance of electrocardiography in the elderly, with some studies finding an increased prevalence of electrocardiographic abnormalities in people older than 50 years. Furthermore, the conclusions of these studies differ with regard to the clinical importance or outcome that these abnormalities might have for a patient's health (Hollister 1995; Mookhoek and Sterrenburg-vdNieuwegiessen 1998). However, all agree that an ECG is indicated, regardless of a patient's age, when the history, review of systems, or findings from the physical examination suggest cardiovascular disease, or when a patient is initiating treatment with a psychotropic drug, such as a tricyclic antidepressant (TCA) or an antipsychotic that is known to alter cardiac function or increase cardiac conduction times.
The electroencephalogram (EEG) can be very useful when a patient has altered mental status, such as delirium or encephalopathy. It can be useful for distinguishing between possible diagnoses. For example, it can diagnose complex partial status epilepticus. It can also be useful for diagnosing metabolic encephalopathy, which is generally due to a systemic illness that is having an effect on the nervous system, such as a urinary tract infection, endocrine disorder, toxin, or metabolic derangement. The EEG is also useful for distinguishing some specific etiologies of encephalopathy. For example, it might show the di- and triphasic waves characteristic of renal failure, hepatic failure, or anoxia. In the patient who is frankly comatose, the EEG can be very valuable for identifying the level of nervous system impairment. For example, it can show an alpha coma pattern or a theta coma pattern characteristic of brain stem lesions producing coma or may show a delta coma pattern characteristic of bihemispheric disease. In the patient who appears to be obtunded, the EEG can be useful for demonstrating whether a patient is catatonic, and hence has a normal awake-looking EEG, versus encephalopathic, where there might be diffuse slowing or triphasic waves (metabolic encephalopathy).
Although the acute computed tomography (CT) scan has generally superseded the EEG for diagnosing strokes, strokes may not be demonstrable in the first 24 hours after they occur. In that case, an EEG may be useful for diagnosing a focal deficit before it is visible on a CT scan. Thus, an EEG might be useful, for example, to distinguish a functional right hemiparesis and aphasia due to a stroke that is not yet visible on a head CT scan. When these symptoms are due to a large middle cerebral artery stroke, focal slowing will be evident on the EEG. The EEG will be normal, in contrast, if the symptoms are due to functional hemiparesis and aphasia.
The evidence to date suggests that routine screening with structural neuroimaging in patients younger than age 65 years is unlikely to disclose findings that are not also evident from a full clinical history and neurological examination, and that, in the majority of cases, such screening would not alter clinical management (Albon et al. 2008). However, in patients in select categories, imaging may be useful. A screening head CT scan is very easy to perform, takes only a few minutes, produces little discomfort, and has a fairly high resolution and sensitivity. It can thus be easily performed in any psychiatric patient admitted with clinical features that do not appear to be classic for the disorder diagnosed. For example, if a patient has late-onset depression or mood disorder, then a head CT scan can be useful for screening for vascular disease, demyelinating disease, subdural hematoma, subarachnoid hemorrhage, and so on.
Magnetic resonance imaging (MRI) of the brain has the advantage over the head CT scan of being more sensitive. It is much more likely to detect vascular disease and demyelinating disease. It is also useful for detecting mild neurodegenerative changes that might point to degenerative neurocognitive disorders. However, brain MRI does take longer than head CT (45 minutes vs. 3-5 minutes), may be at least twice as expensive, and may not be performed in patients with MRI contraindications. In most places, MRI is also not available at night and hence is not useful for rapid screening.
The consensus of studies evaluating the role and value of laboratory testing is that patients who have psychiatric signs and symptoms but who do not exhibit other physical complaints or symptoms will benefit from a small screening battery that includes serum glucose concentration, BUN concentration, creatinine clearance, and urinalysis. Female patients over age 50 will also benefit from a screening thyroid-stimulating hormone (TSH) test regardless of the presence or absence of mood symptoms. Broader screening panels are generally unnecessary and costly. However, for psychiatric patients who have concomitant physical complaints or findings on physical examination, a more extensive laboratory workup may become necessary. Likewise, a more extensive laboratory workup is warranted for patients who are of higher risk, such as elderly or institutionalized patients or those with low socioeconomic status, selfneglect, alcohol or drug dependence, or cognitive impairment. Imaging may also be helpful when atypical features are present, such as an older age at onset of psychiatric illness, or when cognitive impairment is present.
In this section, we discuss the specific clinical situations that may arise with the psychiatric patient that would warrant more extensive laboratory and diagnostic workup. These situations include, but are not limited to, new-onset psychosis, new-onset mood symptoms, anxiety symptoms, altered mental status, cognitive decline, and substance-related disorders.
A careful evaluation is important for a patient with a first episode of psychosis to rule out the many possible medical and neurological causes of psychosis. Routine screening tests often include serum chemistries including sodium, potassium, chloride, carbon dioxide, BUN, and creatinine; liver function tests such as total protein, total and direct bilirubin, serum aspartate transaminase/serum glutamic-oxaloacetic transaminase (AST/SGOT), and alanine aminotransferase/serum glutamate pyruvate transaminase (AAT/SGPT); complete blood count (CBC) with platelets and differential; TSH; a rapid plasma reagin for syphilis; HIV serology; serum alcohol level; urinalysis; and urine toxicology screen for drugs of abuse. Other tests to consider during the initial workup include structural neuroimaging (head CT or brain MRI) and electroencephalography. If appropriate, the clinician should also consider ordering a urine pregnancy test and baseline ECG, especially if he or she is planning to initiate or change antipsychotic medication. If these initial tests do not immediately yield an etiology, the clinician may also consider a lumbar puncture to analyze cerebrospinal fluid (CSF) for the presence of red and white blood cells, protein, and glucose; opening pressure; and bacterial culture, cryptococcal antigen, and viral serologies. Antinuclear antibodies, rheumatoid factor, erythrocyte sedimentation rate, urine porphyrins, blood cultures, and assays for heavy metals (manganese and mercury) and bromides are other tests to consider. There are many causes of psychosis that need to be considered, including central nervous system (CNS) or systemic infections, temporal lobe epilepsy, substance intoxication and withdrawal, metabolic or endocrine disorders, CNS tumors, and heavy metal poisoning. Table 4-2 summarizes some of the recommended tests in the diagnostic approach to a patient with new-onset psychosis.
|
Table 4-2. Recommended diagnostic workup for a patient with new-onset psychosis |
||
Routine screeningComplete blood count with differential and platelets Serum chemistries, including liver and renal function tests Thyroid-stimulating hormone Rapid plasma reagin HIV serology Erythrocyte sedimentation rate Serum alcohol level Urine toxicology screen Head computed tomography or brain magnetic resonance imaging scan Electroencephalogram Urine pregnancy test Baseline electrocardiogram Therapeutic drug levels Consider per clinical suspicionAntinuclear antibody Rheumatoid factor Blood cultures Serum B12 and folate levels Metal assays: serum and urine copper, serum ceruloplasmin, lead, mercury, manganese Cerebrospinal fluid analysis: red blood cell count; white blood cell count; protein; glucose; opening pressure; bacterial cultures; cryptococcal antigen; viral serologies Urine porphyrins |
||
A thorough laboratory screening is also recommended for the evaluation of adult patients with new-onset mood symptoms such as depression or mania. Tests might include TSH, serum chemistries, CBC, urinalysis, and urine toxicology screen for drugs of abuse. If appropriate, the clinician should also consider ordering a urine pregnancy test and ECG, especially if he or she is considering prescribing a mood-stabilizing medication. Measuring levels of therapeutic drugs can be helpful to confirm the presence of a drug if non-compliance is suspected or if therapeutic effect is not obtained, to determine whether toxicity may be contributing to the patient's clinical presentation, or to determine whether drug interactions have altered the desired therapeutic levels (Wallach 1992). Serum trough levels of mood stabilizers such as lithium, valproate, or carbamazepine and TCAs can be obtained to monitor therapeutic response in accordance with therapeutic levels. (For additional information, see sections "Medication Monitoring and Maintenance" and "Pharmacogenetics" later in the chapter.)
Neuroimaging and electroencephalography are often helpful as well in understanding the etiology of a patient's mood symptoms. Multiple neurological and medical disorders have mood manifestations that may often be the presenting complaint. For example, neurocognitive disorders associated with stroke, seizure disorders, Parkinson's disease, Huntington's disease, frontotemporal disease, and thyroid and other endocrine abnormalities may all present with depression, mania/hypomania, or psychosis as the primary complaint, with only subtle physical and cognitive manifestations that may be missed by cursory clinical examination. Further workup with laboratory tests, structural and sometimes functional imaging, and electroencephalography can uncover medical or neurological etiologies, thus providing the patient with effective treatment or prophylaxis against further episodes. The diagnostic approach to a patient with new-onset depressive or manic symptoms is summarized in Table 4-3.
The initial workup for anxiety symptoms should include serum chemistries, serum glucose, and TSH and other endocrine measures (Table 4-4). Many different medical diseases can also manifest with anxiety, including angina and myocardial infarction, mitral valve prolapse, substance intoxication and withdrawal, and metabolic and endocrine disorders such as thyroid abnormalities, pheochromocytoma, and hypoglycemia. Neurological disorders, such as many forms of neurocognitive disorders, can also present with anxiety. A cardiac workup is important because cardiac symptoms may masquerade as panic attacks and are often misdiagnosed as such, especially in female patients. Therefore, electrocardiography, Holter monitoring, stress testing, and/or echocardiography may be necessary. Respiratory function should also be evaluated with a chest radiograph or pulmonary function tests to rule out chronic obstructive pulmonary disease as a contributory factor. Other tests to consider if one has clinical suspicion include electroencephalography, urine porphyrins, and urine vanillylmandelic acid.
Patients with a fluctuating mental status of acute onset most likely will have one or more underlying medical or neurological causes for their impaired consciousness. This often constitutes a medical emergency, and comprehensive laboratory and diagnostic testing are indicated on an emergency basis, as summarized in Table 4-5. In addition to a complete physical examination and as much history as can be obtained from the patient and ancillary sources, the clinician should order serum chemistries, CBC, erythrocyte sedimentation rate, HIV serology, urinalysis and urine toxicology, ECG, and a chest radiograph. A CT scan, blood cultures, lumbar puncture with CSF analysis, and electroencephalography can be helpful as well, if clinically indicated. Impairment in mental status can be caused by many medical and neurological disorders, including seizures, CNS and systemic infection, kidney or liver failure, cardiac arrhythmias, stroke, myocardial infarction, and substance intoxication and withdrawal.
As noted previously (see section "Screening Electroencephalograms"), the EEG can be very helpful in the workup of patients with encephalopathy. It can diagnose seizures. It can also suggest that an encephalopathy is due to a nonneurological etiology. For example, the EEG can show a metabolic etiology (metabolic encephalopathy), which often suggests that systemic issues are at the root of the encephalopathy. Such etiologies include electrolyte disturbances, infections, and toxins.
|
Table 4-3. Recommended diagnostic workup for a patient with new-onset depressive or manic symptoms |
Routine screeningComplete blood count with differential and platelets Serum chemistries, including liver and renal function tests Thyroid-stimulating hormone Rapid plasma reagin HIV serology Urinalysis Urine toxicology screen Serum alcohol level (if suspected) Urine pregnancy test Electrocardiogram Therapeutic drug levels (if patient is already on psychiatric medications) Consider per clinical suspicionStructural neuroimaging (brain magnetic resonance imaging) Electroencephalogram |
|
Table 4-4. Recommended diagnostic workup for a patient with new-onset anxiety symptoms |
Routine screeningSerum chemistries, including liver and renal function tests Serum glucose Thyroid-stimulating hormone Referral for cardiac evaluation: electrocardiogram, Holter monitoring, stress test, and/or echocardiogram Consider per clinical suspicionReferral for respiratory evaluation: chest radiograph; pulmonary function tests Electroencephalogram Urine porphyrins and vanillylmandelic acid levels Urine metanephrines Blood gas |
The head CT scan can also be helpful in the workup of the patient with altered mental status. It can detect subdural hematomas or subarachnoid hemorrhage, and a CT scan with contrast can suggest infections such as meningitis or an abscess. Strokes do not typically present as altered mental status. However, a right middle cerebral artery stroke or a thalamic stroke can occasionally present with altered mental status, and the head CT scan can be very useful for detecting these etiologies.
|
Table 4-5. Recommended diagnostic workup for a patient with altered mental status |
Routine screeningSerum chemistries, including liver and renal function tests Complete blood count Erythrocyte sedimentation rate HIV serology Antinuclear antibody Rheumatoid factor B12 Folate Rapid plasma reagin Urinalysis Urine toxicology Serum alcohol level Therapeutic drug levels Electrocardiogram Chest radiograph Head computed tomography scan Electroencephalogram Consider per clinical suspicionCerebrospinal fluid analysis: red blood cell count; white blood cell count; protein; glucose; opening pressure; bacterial cultures; cryptococcal antigen; viral serologies Urine porphyrins Serum ammonia level Brain magnetic resonance imaging Arterial blood gases Blood cultures |
Laboratory testing is a major component of the comprehensive evaluation of cognitive decline. The current American Academy of Neurology (2007) practice recommendations for evaluation of neurocognitive disorders (or "reversible causes of dementia") include testing for vitamin B12 deficiency and hypothyroidism. These laboratory tests are recommended in addition to structural imaging (noncontrast head CT scan or MRI studies) and evaluation of depression to rule out so-called pseudodementia, or neurocognitive disorder-like symptoms that stem from depression. Syphilis serology screening is necessary only in patients with dementia who are at risk for neurosyphilis. Neuropsychological testing is also recommended; it can be very useful for differentiating between neurocognitive disorder and pseudodementia, for distinguishing among the many types of neurocognitive disorders, and for determining whether a patient is responding to treatment.
Other imaging modalities—such as linear and volumetric imaging, singlephoton emission computed tomography (SPECT), and positron emission tomography (PET)—are not recommended routinely at this time because there are insufficient data on the validity of these tests to diagnose illnesses that lead to neurocognitive disorder. However, under certain circumstances, PET and SPECT imaging can be useful. Fluorodeoxyglucose PET is approved to distinguish between neurocognitive disorders including Alzheimer's disease and frontotemporal disease. Florbetapir PET scans can differentiate between amyloid-based neurocognitive disorders, such as Alzheimer's disease and neurocognitive disorder with Lewy bodies, and non-amyloid-based neurocognitive disorders, such as frontotemporal disease. DaTscan ([123I] ioflupaine) is also useful for differentiating between parkinsonism and other disorders with similar symptoms, such as essential tremor.
Likewise, no serum or CSF biomarkers or genetic tests are currently recommended for routine use in the diagnosis of neurocognitive disorder, although the clinical utility of several tests is being investigated. One exception is the immunoassay for CSF 14-3-3 protein, which is useful for the confirmation of Creutzfeldt-Jakob disease in a patient with rapidly progressive neurocognitive disorder and pathognomonic neurological symptoms (i.e., myoclonic jerks). False-positive results can occur with some other neurological conditions, such as viral encephalitis, stroke, and paraneoplastic neurological disorders. Table 4-6 lists the laboratory and diagnostic tests that would be included in the workup of a patient with cognitive impairment.
There are no current clinical recommendations for the laboratory assessment of patients who have mild neurocognitive disorder (also termed "mild cognitive impairment"). Patients with mild neurocognitive disorder are at very high risk for developing a major neurocognitive disorder or Alzheimer's disease (Petersen et al. 2005). However, the utility of a diagnostic workup, aside from cognitive screening, is as yet unknown. Patients who have symptoms of a mild neurocognitive disorder will likely benefit from a thyroid screen. Other laboratory tests typically ordered for the evaluation of neurocognitive disorder may be of use should signs and symptoms be elicited from the history, review of systems, or physical examination. For example, it may be useful to measure folate and vitamin B12 levels in a patient with mild cognitive impairment who has a long history of an alcohol-related disorder or who is discovered to have peripheral neuropathy on the neurological examination. Because one-third of patients with mild cognitive impairment progress to Alzheimer's disease over 3 years (Petersen et al. 2005), many clinicians feel it prudent to order the same tests they would to rule out reversible causes of neurocognitive disorder. However, as of yet, there are no studies that have proven the clinical utility of this strategy.
|
Table 4-6. Recommended diagnostic workup for a patient with cognitive decline |
||
Routine screeningComplete blood count with differential and platelets Serum chemistries including liver and renal function tests Erythrocyte sedimentation rate Antinuclear antibody Rheumatoid factor B12 and folate levels Thyroid-stimulating hormone Structural neuroimaging studies (head computed tomography or brain magnetic resonance imaging scan) Consider per clinical suspicionRapid plasma reagin HIV serology C-reactive protein Cerebrospinal fluid (CSF) analysis: red blood cell count; white blood cell count; protein; glucose; opening pressure; bacterial cultures; cryptococcal antigen; viral serologies; CSF 14-3-3 protein immunoassay (if Creutzfeldt-Jakob disease is suspected); CSF tau and Abeta 42 levels for frontotemporal dementia vs. Alzheimer's disease Urine porphyrins Functional neuroimaging studies (single photon emission computed tomography or positron emission tomography) Electroencephalogram Apolipoprotein E genotyping Neuropsychological testing Fasting lipids, triglycerides, and blood sugar when a vascular etiology is suspected |
||
In a study of 345 consecutive patients who presented to the emergency department of an urban teaching hospital with primary psychiatric complaints, 141 (41%) had positive urine toxicology screens for substances, and 90 (26%) had positive ethanol screens (Olshaker et al. 1997). Clearly, laboratory testing is essential to the evaluation, monitoring, and subsequent treatment of patients who abuse alcohol, prescribed addictive medications, or illicit drugs.
Laboratory detection of drugs of abuse, as well as test results indicative of end-organ damage related to the abuse, can provide valuable hard evidence that the treating clinician can use to inform and monitor his or her patient's progress. These data are also frequently useful in confronting the denial of substance-related disorders by the patient or his or her family. Laboratory testing can be conducted with blood and urine specimens or with saliva and hair samples. Urine specimens are typically preferred, because the detectable length of time that a particular drug of abuse and its metabolites are present is longer in urine than in blood. However, some substances, such as alcohol or barbiturates, are best detected in blood specimens.
The length of time that a drug of abuse is detectable in the urine varies based on the amount and duration of substance consumed, kidney and liver function, and the specific drug itself. Laboratory methodologies vary.-'If the screening tests yield a positive result, follow-up with more specific tests, including quantitative analyses, can be ordered for confirmation. Table 4-7 lists common drugs of abuse, their toxic levels, and the length of detection time in urine.
Measuring levels of therapeutic drugs to evaluate for toxicity and effective levels can be extremely helpful in the workup and treatment of the psychiatric patient. Therapeutic drug monitoring should be used to confirm the presence and level of the drug if noncompliance is suspected, if the desired therapeutic effect is not obtained, or if signs or symptoms of toxicity occur; to determine whether toxicity may be contributing to the patient's clinical presentation; or to determine whether drug interactions have altered desired levels of therapeutic drugs (Wallach 1992). Serum trough levels of mood stabilizers (e.g., lithium, valproate, carbamazepine) and TCAs can be obtained to monitor therapeutic response in accordance with therapeutic levels for acute exacerbation and maintenance treatment of bipolar disorder.
Blood tests are important for screening for end-organ damage before the initiation of treatment with mood stabilizers such as lithium, valproate, and carbamazepine. Follow-up testing during maintenance treatment is recommended at regular intervals, although the utility of these routine screens in detecting asymptomatic end-organ damage—such as an increase in liver function with valproate or renal impairment with lithium—is unclear. No clear consensus exists as to the appropriate interval for routine monitoring during the use of mood stabilizers. Most experts recommend screening every 3-6 months; however, some experts recommend that clinical monitoring of signs of toxicity may be more effective than periodic screening. That may especially be the case for drugs like valproate for which the routine monitoring of liver function tests may have little predictive value in terms of hepatotoxicity (Marangell et al. 2002). Although there is a lack of consensus regarding the recommended screening tests, we provide in Table 4-8 a set of guidelines, which most authors appear to support. The table shows the psychotropic medications for which therapeutic drug monitoring may be useful, as well as therapeutic and toxic drug levels and ancillary tests that are recommended to monitor for the prevention of end-organ damage.
|
Table 4-7. Substances of abuse |
||
| Agent | Toxic level | Urine detection time |
|
Alcohol |
300 mg/dL at any time or >100 g ingested |
7-12 hours |
|
Amphetamines |
Varies with medication |
48 hours |
|
Barbiturates |
>6 μg/mL |
24 hours (short-acting) 3 weeks (long-acting) |
|
Benzodiazepines |
Varies with medication Lorazepam: >25-100 mg Diazepam: >250 mg |
3 days |
|
Cannabis |
50-200 μg/kg |
4-6 weeks |
|
Cocaine |
>1.2 g |
6-8 hours 2-4 days (metabolites) |
|
Opiates |
Varies with medication Heroin: > 100-250 mg Codeine: >500-1,000 mg Morphine: >50-100 pig/kg |
2-3 days |
|
Phencyclidine |
> 10-20 mg |
1-2 weeks |
Source. Adapted from Wallach 2000.
Drug levels of TCAs may also be obtained, although it is unclear whether blood levels of antidepressants correlate with therapeutic response. Four TCAs—imipramine, desipramine, amitriptyline, and nortriptyline—have been well studied, and generalizations can be made about the relationship of drug levels to therapeutic response. For imipramine, optimal response rates occur as blood levels reach 200-250 ng/mL; levels greater than 250 ng/mL often produce more side effects but no change in antidepressant response (American Psychiatric Association Task Force on the Use of Laboratory Tests in Psychiatry 1985). Nortriptyline, in contrast, appears to have a specific therapeutic window between 50 and 150 ng/mL, and poor clinical response occurs both above and below that window. Desipramine also appears to have a linear relationship between drug concentration and clinical outcome, with plasma concentrations greater than 125 ng/mL being significantly more effective. Amitriptyline has been fairly well studied; however, some studies have found a linear relationship similar to that of imipramine, others have found a curvilinear relationship, and others have found no relationship between blood levels and clinical outcomes (American Psychiatric Association Task Force on the Use of Laboratory Tests in Psychiatry 1985). For the other TCAs that have been less well studied, drug levels can still be useful to confirm the presence of the drug or to confirm extremely high serum levels (Hyman and Arana 1991).
The monitoring of blood levels for antipsychotics is not routine in clinical practice. Different methods for monitoring antipsychotic drugs have been developed, but a reliable therapeutic range has not been established because there does not appear to be a consistent relationship between blood levels of anti-psychotics and clinical response (Curry 1985). However, obtaining blood levels of antipsychotics may be useful in several clinical situations.
|
Table 4-8. Medication monitoring |
||||
| Medication type | Medication | Therapeutic range | Toxic level | Recommended screening |
|
Mood stabilizer |
Lithium |
0.8-1.2 mEq/L |
>1.5 mEq/L |
Initiation: sodium, potassium, calcium, phosphate, BUN, creatinine, TSH, T4, CBC, urinalysis, beta-HCG if appropriate; ECG in patient older than 50 years or with preexisting cardiac disease Maintenance: TSH, BUN/creatinine recommended every 6 months; ECGs as needed inpatient older than 40 years or with preexisting cardiac disease |
|
Valproate |
50-150 μg/mL |
>150 μg/mL |
Initiation: CBC with platelets, LFTs; beta-HCG if appropriate Maintenance: LFTs, CBC recommended every 6 months |
|
|
Carbamazepine |
8-12 μg/mL |
>12 μg/mL |
Initiation: CBC with platelets, LFTs, BUN/creatinine Maintenance: CBC with platelets, LFTs, BUN/creatinine |
|
|
Tricyclic antidepressants (TCAs) |
Imipramine + desipramine |
125-250 ng/mL |
>500 ng/mL or >1 g ingested |
Desipramine is a metabolite of imipramine Initiation: ECG in patient older than 40 years or with preexisting cardiac disease for all TCAs |
|
Doxepin + metabolite desmethyldoxepin |
100-275 ng/mL |
>500 ng/mL |
Initiation: ECG in patient older than 40 years or with preexisting cardiac disease for all TCAs |
|
|
Amitriptyline + nortriptyline |
75-225 ng/mL |
> 500 ng/mL |
Initiation: ECG in patient older than 40 years or with preexisting cardiac disease for all TCAs |
|
|
Nortriptyline only |
50-150 ng/mL |
>50 ng/mL |
Initiation: ECG in patient older than 40 years or with preexisting cardiac disease for all TCAs |
|
|
Antipsychotics |
Olanzapine, quetiapine, risperidone, ziprasidone |
Fasting serum glucose Triglycerides |
||
Note. BUN=blood urea nitrogen; CBC=complete blood count; ECG=electrocardiogram; HCG=human chorionic gonadotropin; LFT=liver function test; T4=thyroxine; TSH=thyroid-stimulating hormone.
Source. Adapted from Wallach 2000; Hyman SE, Arana GW, Rosenbaum JF: Handbook of Psychiatric Drug Therapy, 3rd Edition. Boston, MA, Little, Brown & Co., 1995. Used with permission.
Blood-level monitoring may be useful to confirm the presence of the antipsychotic when adherence is a concern. It may be used to ascertain the presence of drug interactions in a patient who has relapsed or experienced an exacerbation of symptoms after a period of stabilization and who has been taking drugs that may interact with antipsychotics, such as carbamazepine or fluoxetine. This monitoring may also be helpful to obtain drug levels in patients who develop excessive side effects from moderate dosages of antipsychotics (Bernardo et al. 1993).
Diagnostic and laboratory monitoring are important components of care for patients receiving antipsychotic medications. In patients older than 50 years or those with preexisting cardiac disease, a screening ECG should be ordered before institution of antipsychotic medications such as thioridazine or ziprasidone that may cause prolongation of the QTc interval (a marker for potentially life-threatening cardiac arrhythmias such as torsades de pointes). Follow-up ECGs should be ordered for any patient receiving treatment with antipsychotic medications in whom symptoms indicative of cardiac compromise appear. It is also recommended that screening laboratory studies be performed at regular intervals (every 6 months) to test for glucose and metabolic dysregulation (hyperlipidemias, diabetes, hypothyroidism), which are often associated with atypical antipsychotic medications.
Progress in drug metabolism research has resulted in tests that may have significant clinical utility for psychopharmacology. Human drug metabolism is highly variable, making it difficult to predict therapeutic dosage levels and ranges, and can lead to unanticipated adverse outcomes, toxicity, and therapeutic failure. Clearly, adverse drug reactions are a serious problem.
Most psychiatric drugs are metabolized by microsomal enzymes in the cytochrome P450 (CYP) enzyme system. The CYP enzymes are a superfamily of more than 20 related enzymes, although only six metabolize more than 90% of all medications (Streetman 2000). These six enzymes that are important to human drug metabolism are CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A. Enzymes are identified by numbers and letters that identify the family and subfamily grouping. For example, CYP2D6 is in family 2 and subfamily 2D and is structurally related to CYP2C19 in the same family, but it is not similar to CYP3A, which is in a different family (Streetman 2000).
The majority of CYP enzyme metabolism occurs in the liver, although metabolism can occur elsewhere in the body, such as in the small intestine (CYP3A4), the brain (CYP2D6), and the lung (CYP1A1). The CYP enzyme system, in addition to metabolizing drugs, also metabolizes exogenous substances, such as environmental toxins and dietary nutrients, and endogenous substances, such as steroids and prostaglandins. Through drug metabolism, a medication is made more hydrophilic, or water soluble, in order to be excreted by the kidneys. Table 4-9 lists many of the psychiatric drugs that are metabolized by selected CYP enzymes (substrates) as well as those that may decrease enzyme activity (inhibitors). CYP drug metabolism is highly variable due to several factors, including genetic polymorphisms, effects of concomitant medications (inhibition or induction of enzymes), physiological or disease status, and environmental or exogenous factors such as toxins and diet (Ingelman-Sundberg et al. 1999).
Pharmacogenetics is the study of genetic variation as it relates to drug response and metabolism. Research in pharmacogenetics to date has focused largely on genes that encode receptors targeted by drugs such as the serotonin and dopamine receptor subtypes or those that encode CYP enzymes. Research on the latter has been significantly more helpful than the former to an understanding of the genetic basis of variability in medication response.
The pharmacokinetic effects of the CYP enzyme system, specifically CYP2D6 and CYP2C19 polymorphisms, on psychiatric medications have been studied extensively. The allele sequence that produces normally functioning enzyme is coded by the wild-type gene (given the suffix *1). Thereafter, differing genetic sequence polymorphisms are numbered sequentially (i.e.,*2, *3). Thus, multiple copies of a functional CYP enzyme gene can occur, resulting in enzyme overactivity. Conversely, polymorphisms may be inactivating, resulting in decreased CYP enzyme activity or even a complete loss of activity.
Four general phenotypes have been used to describe the outcomes of these CYP genetic polymorphisms (Table 4-10): ultrarapid metabolizers, extensive metabolizers, intermediate metabolizers, and poor metabolizers. Extensive metabolizers have the normal two copies of fully active CYP enzyme alleles for a particular microsomal enzyme. Poor metabolizers do not have the active enzyme gene allele, resulting in increased concentrations of medications due to reduced metabolism, and may have more adverse effects at usual, recommended dosages. In contrast, ultrarapid metabolizers will have multiple copies of the functional enzyme allele, resulting in an increased rate of drug metabolism, and may not reach therapeutic concentrations at the recommended dosage.
There is significant ethnic variability in allele frequencies, with 4%-10% of whites completely lacking the CYP2D6 enzyme, compared with only l%-3% of African Americans and Chinese. Similarly, discrepancy in allele frequencies occur for the CYP2C19 enzyme, with up to 20% of Asians lacking the active enzyme gene allele, compared with only 2%-5% of whites (de Leon et al. 2006).
Genotyping tests for CYP enzyme DNA sequence variants are now available. They utilize DNA microarray assays to detect single nucleotide polymorphisms (SNPs) or DNA sequence variations in the genes encoding CYP enzymes. These tests are not yet in routine clinical practice because of prohibitive costs and lack of insurance reimbursement. As studies become available that provide evidence of health care cost savings, CYP genotyping has the potential to revolutionize psychiatric approaches to medication management. These tests in their current form are most helpful when phenotype closely mirrors genotype. Genotyping needs to be performed only once in a patient's life. If performed prior to initiation of medications, it could prevent adverse drug reactions.
In the future, perhaps specific dosing adjustment recommendations can be compiled for a patient based on his or her drug metabolism genotype profile. The sensitivity for predicting poor metabolizers in white populations is 99% for the CYP2D6 enzyme genotyping test and 98%-100% for the CYP2C19 test (Sachse et al. 1997; Sagar et al. 1998). Data on the effects of genotyping on treatment outcomes and health care costs are not yet available, but the potential benefits of genotyping are numerous. For example, genotyping may eventually obviate the need for costly and lengthy drug trials, potentially allowing the physician to choose the best medication for the patient at treatment outset. Furthermore, for medications with narrow therapeutic windows, genotyping may reduce the frequency of toxicity and other adverse events.
|
Table 4-9. Psychiatric drug metabolism by specific P450 enzymes |
||
| Enzyme | ||
| CYP2D6 | CYP2C19 | |
|
Substrates (drugs metabolized by specific enzyme) |
Antidepressants Amitriptyline Desipramine Duloxetine Imipramine Fluoxetine Fluvoxamine Nortriptyline Paroxetine Sertraline Trazodone Venlafaxine Antipsychotics Aripiprazole Clozapine Haloperidol Fluphenazine Perphenazine Olanzapine Risperidone Thioridazine Other drugs Donepezil Methadone |
Antidepressants Citalopram Escitalopram Amitriptyline Clomipramine Imipramine Other drugs Diazepam |
|
Inhibitors |
Antidepressants Amitriptyline Bupropion Desipramine Fluoxetine Paroxetine Sertraline Antipsychotics Thioridazine Clomipramine Clozapine |
Amitriptyline Citalopram Clomipramine Fluvoxamine Fluoxetine |
Source. Data adapted from Kirchheiner et al. 2001; Streetman 2000.
|
Table 4-10. Drug metabolizer phenotype classification |
||
| Type | Number of active enzyme gene alleles | Expected response to substrate drug |
|
Poor metabolizer |
None |
Reduced metabolism of drug may result in increased concentrations and more adverse effects |
|
Intermediate metabolizer |
One active and one inactive allele, or two gene alleles with reduced activity |
Lesser degree of adverse effects related to reduced metabolism |
|
Extensive metabolizer (normal) |
2 |
Expected response to standard medication dosage |
|
Ultrarapid metabolizer |
>2 |
Rapid clearance of medications, so may not reach therapeutic concentrations at recommended dosages |
Source. Adapted from Ingelman-Sundberg et al. 1999; Mrazek 2006.
Limited data exist regarding antidepressant effectiveness based on CYP genotype. Even fewer data are available for antipsychotic efficacy and CYP genotype. Preliminary antidepressant dosage recommendations are being developed based on CYP drug metabolism phenotype. Kirchheiner et al. (2001) presented preliminary practical dosage recommendations for several antidepressant medications according to metabolizer status. Recommended dosages for poor metabolizers were 20%-70%, dosages for intermediate metabolizers were 80%-90%, and dosages for ultrarapid metabolizers were 100%-130% of those recommended for extensive metabolizers of CYP2D6 or CYP2C19. Several antidepressants, including mirtazapine, nefazodone, sertraline, and trazodone, are metabolized by CYP3A4. However, poor and ultrarapid metabolizers of CYP3A4 have not been identified due to the relative lack of variability in the 3A4 gene; thus, dosing recommendations relating to CYP3A4 have not been developed.
In general, dosages of TCAs are reduced by 50% for poor metabolizers of CYP2D6 or CYP2C19 substrates, with less dramatic dosage reductions for selective serotonin reuptake inhibitors (de Leon 2006; Kirchheiner et al. 2001). A very small proportion of poor metabolizers are lacking both CYP2D6 and CYP2C19 functional alleles. These patients are likely to have adverse reactions to most available antidepressant medications. Thus, the use of antidepressant medications such as bupropion and mirtazapine, which are not dependent on these metabolic pathways, would be prudent in these patients (de Leon et al. 2006).
Similar guidelines for practical dosage recommendations for antipsychotic medications have yet to be defined, largely because data on their clinical efficacy based on CYP genotyping are extremely limited. A conservative estimate is to lower the dosage of typical antipsychotics and risperidone by one-half in poor metabolizers of CYP2D6 (de Leon 2006).
Interpretation of clinical drug response in the context of CYP genotyping is still fraught with complications because the effects of medical comorbidities, environment, and medication interactions must be addressed. Concomitant medications can be powerful inducers or inhibitors of CYP metabolism and must also be taken into account when predicting drug response. Despite these complications, these advances in pharmacogenetics bring the field one step closer to the "individualized" or "personalized" approach to medicine, with the potential to decrease possible adverse events, reduce costly trials of ineffective medication treatments, and hasten recovery times.
Much research has been conducted on the relationship of endocrine abnormalities to primary psychiatric disorders. This research has been engendered by the observation that both endogenous endocrine disorders, such as Cushing syndrome or hypo- and hyperthyroidism, and the administration of exogenous hormones, such as glucocorticoid steroids, can produce mood and psychotic episodes identical to those of endogenous primary mood and psychotic disorders. In general, neuroendocrine evaluation measures include 1) basal hormone levels, 2) circadian secretion patterns, and 3) secretion response to a hormonal challenge or provocation. The challenge or provocation tests have received the most attention. For this method, a hypothalamic releasing factor, such as thyrotropin-releasing hormone (TRH), corticotropin-releasing hormone (CRH), gonadotropin-releasing hormone (GnRH), or growth hormone-releasing hormone (GHRH), is administered to stimulate the release of corresponding downstream pituitary hormone (TRH → TSH, CRH → adrenocorticotropic hormone, GnRH → follicle-stimulating hormone and luteinizing hormone, and GHRH → growth hormone).
Several psychiatric disorders have been associated with abnormal secretion in response to these hormonal challenges. The best known of these challenge tests is the dexamethasone suppression test, in which secretion of serum cortisol is measured at several time points for 24 hours after a "challenge" of dexamethasone administration. An abnormal response is a failure to suppress serum cortisol levels below 5 μg/dL. Initially, this test was believed to be useful in the diagnosis of melancholic depression (Carroll 1984). However, it has limited sensitivity, because it is positive in only 40%-50% of depressed patients (Wallach 2000). Furthermore, there are multiple factors that can interfere with the test results, including drugs that can cause nonsuppression, such as barbiturates, carbamazepine, and chronic alcohol use, as well as those that can enhance suppression, such as high-dose benzodiazepines, corticosteroids, and dextroamphetamine. Medical conditions such as pregnancy, systemic infections, endocrine and liver disease, and other severe medical illnesses may result in a false-positive test (Wallach 2000).
Unfortunately, neither the dexamethasone suppression test nor any other neuroendocrine testing method has clinical applications at this time. Given that neuroendocrine systems are highly complex feedback loops, affected by numerous endogenous and environmental factors, perhaps these tests will attain clinical utility in the future as the workings and relationships of these psychoneuroendocrine systems become better understood.
The standard EEG is a noninvasive recording of electrical activity of the brain. Electrodes placed on the scalp record extracellular current flow of neurons. The EEG is used in the evaluation of the psychiatric patient to exclude the contribution of a general medical condition, such as epilepsy or delirium, to a patient's clinical presentation. In general, an abnormal EEG will consist of one or more of the following: 1) paroxysmal activity indicative of transient, episodic neuronal discharges, as seen in epilepsy; 2) nonparoxysmal slowing of activity, as seen in delirium; 3) asymmetric activity, as observed with mass lesions or infarction; or 4) sleep abnormalities consistent with sleep-wake disorders, including sleep apneas, narcolepsy, and parasomnias such as rapid eye movement sleep behavior disorder.
No clear guidelines exist for the use of electroencephalographic evaluation in routine screening of the psychiatric patient. An EEG would be prudent to obtain in a patient with new-onset psychosis, episodic behavioral disturbance, or altered mental status. In a patient with altered mental status, the EEG can be diagnostically useful because it can differentiate between a diffuse encephalopathy, nonmotoric status epilepticus, or focal lesion (Boutros and Struve 2004). A normal EEG does not exclude seizure disorder from the differential diagnosis, because 20% of patients with epilepsy will have normal EEGs, and 2% of patients without epilepsy will have spike and wave formations (Engel 1992). The diagnosis of epilepsy is a clinical one, based on observation of the patient or the report of someone who has observed the patient having a seizure. Although the EEG can support the diagnosis, it cannot exclude it. Several techniques can be implemented to increase the diagnostic yield of the EEG, including sleep deprivation, serial EEGs, 24-hour electroencephalographic monitoring, or adjustments in electrode placement, including nasopharyngeal, sphenoidal, and anterior temporal electrodes. Despite the fact that electroencephalography is widely available, noninvasive, inexpensive, and useful for diagnosing neurological disorders, it has fairly limited utility in the differentiation of psychiatric disorders.
Polysomnography entails the recording of multiple physiological variables during sleep to determine the presence of sleep disorders. It is a useful technique to implement in the psychiatric patient if a sleep disorder is suspected to be responsible for or is exacerbating psychiatric symptoms. Hypnagogic hallucinations, which occur at the interface between sleep and wakefulness, can often be mistaken for symptoms of a primary psychotic disorder. Furthermore, there is considerable overlap in symptoms of depression and sleep disorders, such as insomnia, daytime fatigue, or excessive daytime sleepiness. A typical polysomnogram will consist of an EEG, ECG, electro-oculogram, and electromyogram, and measurement of respiratory airflow and oxygenation, blood pressure, and body temperature. Again, no definitive guidelines exist as to the usefulness of polysomnography in the clinical workup of the psychiatric patient. Although psychiatric disorders often go hand in hand with disturbed sleep, sleep studies are not ordered for the routine evaluation of the psychiatric patient. Instead, a poly-somnogram is ordered when there is clinical suspicion of parasomnia or hypersomnia (narcolepsy), a breathing disorder such as sleep apnea, or limb movements during sleep.
Auditory, visual, somatosensory, or cognitive stimuli can be used to evoke electrical potentials that can be recorded. Repetitive stimuli result in small-magnitude electrical changes that are mathematically manipulated or "averaged," resulting in the evoked potential. Evoked potential testing provides clinically useful information about processing of sensory stimuli, which is helpful in discerning medical versus psychogenic causes of some symptoms. For example, visual evoked potentials can be useful to differentiate psychogenic blindness from true blindness, and auditory evoked potentials can be used to differentiate psychogenic deafness from catatonia in a mute, unresponsive patient.
Initial evoked potentials are followed by other evoked potential components such as midlatency evoked responses and even later event-related potentials. The latter have been the focus of much research, because they are elicited by a psychological event. For example, the P300 event-related potential, a positive peak that occurs 250-500 milliseconds poststimulus, has been found to be abnormal in amplitude and latency in multiple psychiatric disorders.
Quantitative electroencephalography uses 1-2 minutes of a resting EEG that is analyzed using fast Fourier transform to quantify the power at each frequency of the EEG averaged across the entire sample (Hughes and John 1999). For each of the four frequency bands (delta [1.5-3.5 Hz], theta [3.5-7.5 Hz], alpha [7.5-12.5 Hz], and beta [12.5-20 Hz]), results obtained include absolute power (total microV2), relative power (percentage of total power for each band), coherence (synchronization between bands), and symmetry between bands. Thus, quantification allows comparison of these variables between patient groups. Despite numerous studies of quantitative electroencephalography in neurocognitive disorders, cerebrovascular disease, schizophrenia, mood and anxiety disorders, learning disorders, and substance-related disorders, few data are available to support its use in the clinical evaluation of psychiatric patients. However, this analytical tool holds great promise for the future.
Brain imaging research in psychiatry has exploded in the past two decades, spurred on by increasingly sophisticated neuroimaging modalities. Although neuroimaging does not yet play a diagnostic role for any of the primary psychiatric disorders, it is still an integral part of the clinical workup for psychiatric patients to rule out underlying medical causes of psychiatric symptoms. In this section, we discuss current clinical and research neuroimaging modalities as they relate to psychiatric disorders.
Current neuroimaging methods provide both structural and functional data about the brain. Structural imaging techniques such as CT and MRI provide a fixed image of the brain's anatomy and spatial distribution. Newer functional neuroimaging techniques such as PET (PET-CT and PET-MRI), SPECT, magnetic resonance spectroscopy (MRS), and arterial spin labeling (ASL) provide information about brain metabolism, blood flow, the presynaptic uptake of transmitter precursors, neurotransmitter transporter activity, and postsynaptic receptor activity. Functional scans should always be interpreted in the context of the underlying structural images. With these techniques, one can find a grossly normal brain, structurally speaking, with abnormal function. Alternately, a person can have abnormal brain structures that can lead to reduced or increased metabolic function (e.g., a brain tumor).
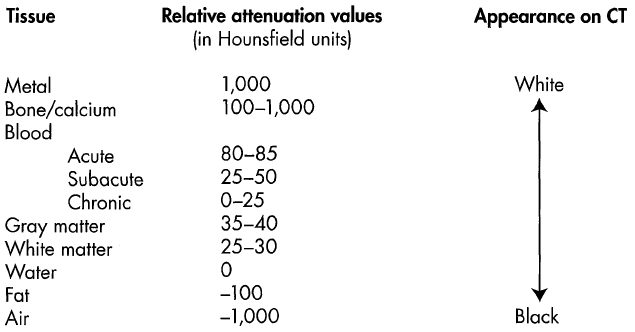
CT scanning enlists a focused beam of X rays that passes through the brain at many angles. The many images evoked are then joined together to provide a cross-sectional view of the brain. The X rays are attenuated as they pass through tissue, which absorbs their energy. The degree of energy absorbed varies, based on the radiodensity of the tissue. This differential X-ray attenuation is transformed into a two-dimensional gray-scale map of the brain by computers, with bone appearing most radiopaque, or white, and air the least radiopaque, or black. Brain tissue, CSF, and water have varying degrees of radiopacity (Figure 4-1).
CT has many advantages. It is widely available, is less expensive than MRI, has a quick scanning time, and is relatively more comfortable and convenient than other structural imaging modalities. Thus, CT is quick and efficient and is used to rule out life-threatening conditions such as skull fracture, hemorrhage, or brain tumor.
CT also has limitations. A brain CT scan involves some radiation exposure. Deep brain structures, including those of the posterior fossa such as the brain stem and cerebellum, are poorly visualized with CT because of the surrounding bony structures. Furthermore, discrimination between gray and white matter in the brain is limited due to their similar radiodensities.
MRI relies on nuclear magnetic resonance. Hydrogen nuclei in the body have paramagnetic properties, and their spins align when placed in a static magnetic field. The magnetic field is pulsed, causing the hydrogen protons to align. When the magnetic pulses are terminated, the protons relax toward their original positions and release energy at a detectable radiofrequency. The collective magnetic behavior of the realigning hydrogen atoms within the magnetic field constitutes T1, or longitudinal relaxation, and T2, or transverse relaxation. The bulk of the MRI signaling comes from hydrogen atoms in water. MRI can distinguish between hydrogen nuclei in free water and those in blood, fat, or muscle based on differential relaxation rates in different tissues. These resonant frequencies are nonionizing and not harmful. The spatial resolution of the images produced is determined by the strength of the static magnet. Most clinical MRI scanners use a superconducting magnet of 1.5 or 3.0 tesla strength.

Figure 4-1. Computed tomography (CT) tissue attenuation values and appearance.
Source. Adapted from J Levine lecture "Structural Neuroimaging in Psychiatry," given as part of the Neuroimaging in Psychiatry lecture series, Department of Psychiatry, Baylor College of Medicine, March 2006.
In clinical practice, T2-weighted images can be very useful for visualizing lesions because they show edema as an increase in signal intensity. T1-weighted images are useful for demonstrating structural anatomy. Gradient echo images can reveal past hemorrhages. Fluid-attenuated inversion recovery images are useful for removing fluids like CSF while retaining fluid changes as observed with the gliosis of past infarcts. One can thus observe, for example, the extent of past small-vessel ischemic changes. Table 4-11 lists the characteristic appearance of tissue signals on T1-weighted, T2-weighted, and proton density (PD)-weighted MRI images. Figure 4-2 demonstrates the classic appearance of the brain on CT and different conventional MRI sequences commonly used.
MRI has many advantages over CT. First and foremost, it has superior visualization of brain tissue, providing enhanced gray/white matter discrimination compared with that of CT and allowing quantitative or volumetric measurement of brain regions. Deep brain structures such as the cerebellum and brain stem are better visualized with MRI. Furthermore, axial, coronal, and sagittal images maybe acquired. MRI image acquisition is complex and, depending on parameters, can produce T1-, T2-, or PD-weighted images, spin-echo, and inversion-recovery images. Table 4-12 provides a summary comparison of CT and MRI imaging modalities. Figure 4-3 is a comparison of images available with CT and MRI.
|
Table 4-11. Comparison of tissue signal on T1-weighted, T2-weighted, and proton density (PD)-weighted magnetic resonance imaging (MRI) |
|||
| T1 | T2 | PD | |
|
Gray matter |
Intermediate (gray) |
Intermediate to high (light gray) |
Intermediate to high (light gray) |
|
White matter |
High (white) |
Intermediate to low (dark gray) |
Intermediate (gray) |
|
Cerebrospinal fluid or water |
Low (black) |
High (white) |
Intermediate to low (dark gray) |
|
Fat |
High (white) |
Low (black) |
Low (black) |
|
Air |
Low (black) |
Low (black) |
Low (black) |
|
Edema |
Intermediate (gray) |
High (white) |
High (white) |
|
Demyelination or gliosis |
Intermediate (gray) |
High (white) |
High (white) |
|
Ferritin deposits (e.g., in basal ganglia) |
Intermediate to low (dark gray) |
Low (black) |
Low (black) |
|
Calcium bound to protein |
High (white) |
Intermediate to low (dark gray) |
Intermediate to low (dark gray) |
|
Proteinaceous fluid |
High (white) |
Variable |
Variable |
Note. On fast spin echo (FSE) sequences (a faster variant of the spin echo sequence), fat appears bright in T2- and PD-weighted images.
Source. Adapted from Wilde EA, Hunter JV, Bigler ED: "A Primer of Neuroimaging Analysis in Neurorehabilitation Outcome Research." NeuroRehabilitation 31:227-242, 2012.
For the primary psychiatric disorders, the clinical use of structural neuroimaging such as CT and MRI is largely limited to the identification of medical causes of psychiatric symptomatology. Structural imaging is ordered to evaluate for evidence of tangible abnormalities such as stroke, brain tumor, trauma, or developmental abnormalities that might underlie psychiatric symptoms. The clinical utility of structural imaging modalities has been evaluated in several retrospective studies (Agzarian et al. 2006; Albon et al. 2008; Hollister and Shah 1996; Moles et al. 1998). There appears to be little justification for routine screening of psychiatric patients (Agzarian et al. 2006). In one retrospective study, 397 consecutive psychiatric patients without focal neurological signs were screened with CT scans over a 2-year period; 95% (377) of these scans were normal. Although 5 of the 20 abnormal scans showed cortical atrophy, all of the abnormal findings were considered to be unrelated to the patient's psychiatric condition and symptoms. The authors concluded that routine screening with CT scan is unlikely to be helpful for the evaluation of psychiatric patients without neurological signs on clinical examination (Agzarian et al. 2006). Moles et al. (1998) retrospectively attempted to identify which clinical features of psychiatric patients might be predictive of abnormal CT findings that would influence treatment recommendations. The authors found that an abnormal cognitive examination (the Folstein Mini-Mental State Examination was used in this study), an abnormal neurological examination, and age were the most sensitive predictors of abnormal CT findings that would influence treatment.

Figure 4-2. Comparison of computed tomography (CT) and various magnetic resonance imaging (MRI) modalities.
The images are derived at the same level within the same individual and demonstrate the characteristic appearance of white matter, gray matter, and cerebrospinal fluid (CSF) on CT and various conventional sequences in common use in clinical practice. FLAIR=fluid attenuated inversion recovery; GRE=gradient echo; PD=proton density.
Source. Images courtesy of Elisabeth A. Wilde, Ph.D., Departments of Physical Medicine and Rehabilitation, Neurology and Radiology, Baylor College of Medicine, Houston, Texas, and Erin D. Bigler, Ph.D., Departments of Psychology and Neuroscience, Brigham Young University, Provo, Utah.
The clinical utility of MRI in the evaluation of adult psychiatric patients has been addressed in a few studies (e.g., Er-hart et al. 2005; Hollister and Shah 1996). In a retrospective chart review of psychiatric patients referred for brain MRI evaluation (excluding those referred for evaluation of neurocognitive disorder) over a 6-year period, 15% (38 of 253) had MRI findings that modified treatment recommendations (Erhart et al. 2005). For 6 patients (2%), MRI identified a new medical condition requiring treatment. Thus, the authors concluded that MRI evaluation can be valuable in patients with suspected underlying medical problems causing psychiatric manifestations. In a study of CT and MRI scans ordered in a psychiatric hospital over a 2-year period, 17% (12 of 68) of scans were abnormal (Hollister and Shah 1996). The authors concluded that brain imaging scans are indicated for psychiatric patients with cognitive impairment (to evaluate for neurocognitive disorder), a first psychotic break, personality change in a patient older than 50 years, or new or unexplained focal neurological signs.
|
Table 4-12. Comparison of computed tomography (CT) and magnetic resonance imaging (MRI) |
||
| CT | MRI | |
|
Mechanism |
X-ray attenuation |
Proton magnetic resonance |
|
Imaging planes |
Axial (transverse) only |
Axial, coronal, sagittal |
|
Image acquisition time |
Short (5-10 minutes) |
Longer (45 minutes) |
|
Slice thickness |
2-5 mm |
1-3 mm |
|
Spatial resolution |
1-2 mm |
<1 mm |
|
Cost |
$300-$500 |
$800-$l,000 |
|
Advantages |
Widely available Rapid acquisition Useful in evaluating for acute, life-threatening conditions such as hemorrhage or trauma |
No radiation exposure Gray-white contrast excellent Excellent visualization of posterior fossa |
|
Disadvantages |
Radiation exposure Limited visualization of posterior fossa |
Unable to use if metal or pacemakers present Slow acquisition |
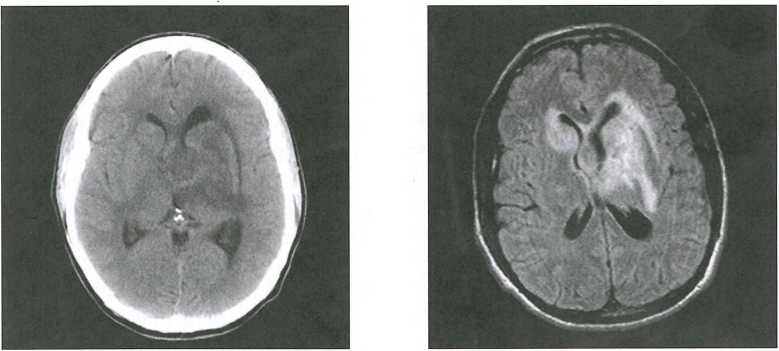
Figure 4-3. Side-by-side comparison of structural imaging modalities: computed tomography (CT) and magnetic resonance imaging (MRI).
The sensitivity of head CT versus MRI of the brain in the same patient is demonstrated here in a patient who presented with memory loss. Head CT scan at left shows a large area of decreased density consistent with edema. It is difficult to ascertain whether there is an underlying mass or what its shape might be. The image on the right is from a brain MRI (T2 image) and also demonstrates an area of increased intensity of about the same shape as the CT abnormality. The patient was found to be HIV positive, and a subsequent brain biopsy demonstrated that the mass was a B-cell lymphoma.
Source. Images courtesy of Paul E. Schulz, M.D., Department of Neurology, Baylor College of Medicine, Houston, Texas.
Albon et al. (2008) performed a systematic review of 24 studies with a diagnostic before-after type of design to evaluate the clinical benefit of CT, structural MRI, or combinations of these in treatment-naïve first-episode or unspecified psychotic patients. The authors concluded that structural neuroimaging identified very little that would influence patient management that was not suspected based on a medical history and/or physical examination. In the four MRI studies, approximately 5% of patients had findings that would influence clinical management, whereas in the CT studies, approximately 0.5% of patients had such findings.
Although evidence is limited, structural neuroimaging appears to be indicated for psychiatric patients prior to the initiation of electroconvulsive therapy or for the following clinical situations: new or unexplained focal neurological signs, cognitive changes or impairment, or new-onset psychosis. For psychiatric patients older than age 50 years, any change in mental status, mood, personality, or behavior may warrant an MRI (Rauch and Renshaw 1995). A CT scan is valuable when evaluating for suspected hemorrhage or skull fracture or when MRI is contraindicated (e.g., because of metal implants) (Table 4-13).
MRS is based on the same nuclear magnetic resonance principles as MRI, but rather than relying on the resonance of hydrogen protons, MRS detects other signals of interest, including protium (1H), phosphorus 31 (31P), lithium 7 (7Li), fluorine 19 (19F), and carbon 13 (13C). MRS provides information about neuronal damage by measuring several markers of cellular integrity and function, including N-acetyl aspartate, creatinine, choline, and myoinositol. Each of these compounds produces a characteristic spectral peak, thus allowing quantification and distribution of the compound within regions of the brain. MRS has been applied extensively to research of numerous psychiatric disorders and is even used to assess pharmacokinetics and pharmacodynamics of psychotropic medications. Its clinical use, however, is currently somewhat limited for primary psychiatric disorders. However, it is quite useful in the neurological arena for brain tumor typing and grading, differentiating brain tumor from infection or inflammation, stroke management, and seizure disorders.
Diffusion tensor imaging (DTI) is a tool to probe microstructural properties of tissue, including the brain. DTI measures the diffusion of water in brain tissues, allowing quantification of orientation and structure via various metrics such as fractional anisotropy, mean diffusivity, and others, as well as qualitative aspects of white matter tracts via tractography. In DTI, diffusion-weighted pulse sequences that are sensitive to the random motion of water are used to quantify how water diffuses along axes. A matrix of water diffusion speed, the diffusion tensor, is calculated for every voxel in an image. The speed of water diffusion is generally constant in all directions. However, in white matter, water diffusion is faster parallel to axons rather than perpendicular to axons, ostensibly because myelin sheaths and white matter tracts constrain and direct water diffusion (Taber et al. 2002). Alterations in diffusion are used to identify damage to the structural integrity of white matter tracts as seen in traumatic brain injury, stroke, or multiple sclerosis. This information can also be used to map white matter tracts that have been compromised by pathology or developmental anomaly. DTI and other advanced diffusion methods, including free water imaging, diffusion kurtosis imaging, and diffusion spectrum imaging, have been used with some enthusiasm in research studies of psychiatric disorders such as schizophrenia (Pasternak et al. 2012) to elucidate the presumed structural causes and consequences of psychiatric disorders. Figure 4-4 illustrates white matter tracts that can be visualized with DTI.
|
Table 4-13. Indications for computed tomography (CT) prior to or instead of magnetic resonance imaging (MRI) |
Noncontrast CTEvaluation of new-onset or acute neurological abnormality Acute stroke Subarachnoid hemorrhage Trauma Mass with edema, hydrocephalus, mass effect Evaluation of ventricular size Sinus disease CT with or without contrastBone pathology (with or without contrast) |
Source. Adapted from J. Levine lecture "Structural Neuroimaging in Psychiatry," given as part of the Neuroimaging in Psychiatry lecture series, Department of Psychiatry, Baylor College of Medicine, March 2006.
DTI is a fairly new imaging technique. It is the subject of intense psychiatric and neurological research, including neurocognitive disorders, schizophrenia, mood and anxiety disorders, substance-related disorders, and brain injury. It should prove promising in the future, but at this time its clinical utility is limited.
SPECT provides images of cerebral blood flow and brain activity. For this particular scan, a radioactive tracer attached to a drug, generally technetium-99m-HMPAO (hexamethylpropyleneamine oxime) or technetium-99m-ECD (ethyl cysteinate dimer), is administered intravenously. HM-PAO and ECD are lipophilic drugs and are able to diffuse across the blood-brain barrier and into neurons. Once inside the cell, they are converted into hydrophilic compounds and are unable to diffuse out of the cell. Physical decay of the radionuclide attached to HMPAO or ECD leads to high-energy photon emissions that are measured by a SPECT detector. A computer creates visual images from this information, using various algorithms and filtering techniques to correct background noise and motion. Tracer uptake and cerebral blood flow are high in the gray matter, where neuronal bodies and synapses reside, and are low in the white matter, which is composed of metabolically less active axons. Thus, the cortex and subcortical structures will appear bright, or "hot," on SPECT, whereas white matter will appear "cold," or dark. The ventricles will appear even darker. Though not widely used in routine clinical psychiatric diagnosis, SPECT has been cited as a modality that may add clinically meaningful information in complex or treatment-resistant cases (Amen et al. 2012).
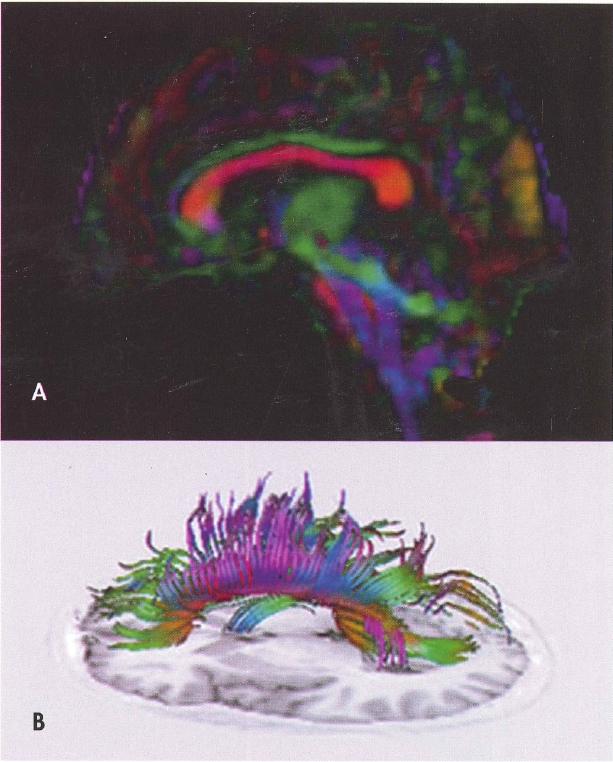
Figure 4-4. Diffusion tensor imaging (DTI).
A, Fractional anisotropy color map derived from DTI in the sagittal plane. Red indicates white matter fibers coursing in a right-left direction, blue indicates fibers running in a superior-inferior direction, and green reflects fibers oriented in an anterior-posterior direction. B, Fiber tracking using DTI of the total corpus callosum overlaid on a T1-weighted inversion recovery image from the same brain.
To view this figure in color, see Plate 1 in Color Gallery in middle of book.
Source. Images courtesy of Elisabeth A. Wilde, Ph.D., Department of Physical Medicine and Rehabilitation, Baylor College of Medicine, Houston, Texas.
PET assesses metabolism within the brain. In this technique, a radioactive tracer (e.g., oxygen 15 [15O], carbon 11 [11C], or fluorine 18 [18F]) administered intravenously enters biologically active molecules and decays, thereby emitting positrons that collide with electrons within the tissue (termed coincidence event). Each collision produces two high-energy photons, which travel in paths at a 180-degree angle from each other and are detected by a pair of detectors near the event. In the detector, the detected photons are converted into photons in the visible light range, which are then converted into an electrical signal. These electrical signals from opposing detectors enter a coincidence circuit where coincidence logic selects photon pairs, which are detected within a narrow time window. The PET image is then constructed. For example, PET utilizing the radiotracer [18F-2]fluorodeoxyglucose provides direct information about cerebral glucose metabolism by calculating average glucose metabolic rate per volume. PET can also be applied to study of regional blood flow, neuroreceptor imaging, and neurotransmitter kinetics.
Although the temporal resolution of PET coincidence events is very high (i.e., a few nanoseconds), generation of the PET image requires a large number of events. In addition, the data acquisition time may be limited by tracer kinetics, metabolism, and binding, which limit the temporal resolution vis-à-vis the physiological process being measured. For example, the measurement of brain glucose metabolism using [18F]fluorodeoxyglucose averages activity in the brain over a 20- to 30-minute period, and the measurement of cerebral blood flow with [15O] water averages activity over approximately 60 seconds (Volkow et al. 1997a). PET imaging has a lower spatial resolution (>2 mm) than MRI. However, the major limitation of the technique is that most radiotracers are short lived and therefore have to be processed in the proximity of the imaging facility. The use of radioactivity also frequently limits its application mostly to adults because of safety concerns in children and adolescents, despite a relatively low absorbed dose (Parvas et al. 2011).
SPECT is more widely available than other functional imaging modalities, less expensive, and technically easier than PET imaging. Because PET tracers have much shorter half-lives than those of SPECT tracers, they require an on-site cyclotron and radiopharmaceutical laboratory for compounding immediately prior to each study. In comparison, SPECT tracers are stable for 4-6 hours after preparation. Thus, although temporal and spatial resolution is generally superior with PET, it is used less often for clinical reasons due to practical considerations of tracer acquisition, insurance reimbursement, and cost. Both imaging modalities provide only limited visualization of anatomical structures; thus, they often require structural MRI to be superimposed on the functional scan. Table 4-14 provides a comparison of SPECT, PET, and functional MRI modalities.
Increasingly, structural and functional imaging techniques are used together in the evaluation of neuropsychiatric and neurological disorders. For the primary psychiatric disorders, functional imaging techniques hold promise for the future but currently have limited clinical utility. However, for broader neuropsychiatric problems such as evaluation of suspected cognitive impairment, epilepsy, and traumatic brain injury, functional neuroimaging is playing an increasingly useful role. Functional imaging techniques such as SPECT and PET are now being used in several clinical situations, including the evaluation of neurocognitive disorders, presurgical evaluation of medically refractory seizures, vascular disease to localize compromised vascular reserve, and brain injury. The exact clinical utility of SPECT and PET for some of these circumstances remains debatable. Figure 4-5 provides a comparison of structural versus functional neuroimaging modalities, and Figure 4-6 compares SPECT and PET images.
SPECT and PET can be helpful in the workup of a patient who is experiencing cognitive decline but shows normal or nonspecific brain structural changes on MRI. Functional imaging can be particularly helpful for providing clues regarding anatomical areas of involvement and thus clues as to the type of early or mild neurocognitive disorder that maybe present. In this case, PET or SPECT can reveal areas of reduced brain metabolic activity in areas that the MRI suggests are structurally normal.
PET and SPECT can be particularly useful to the clinician for differentiating between neurocognitive disorders such as Alzheimer's disease and frontotemporal neurocognitive disorder (also termed "frontotemporal dementia"). Alzheimer's disease is often associated with bilateral, symmetric, posterior temporal, and parietal lobe perfusion defects, whereas frontotemporal dementia is generally associated with reduced perfusion of the frontal and/or lateral temporal lobes bilaterally. In contrast, patients with vascular neurocognitive disorder (also termed "multi-infarct dementia") may show patchy perfusion defects corresponding to the site of strokes, whereas patients with depression, which may take the form of a pseudodementia, may show normal brain perfusion or only mildly reduced prefrontal perfusion.
|
Table 4-14. Comparison of SPECT, PET, and fMRI |
|||
| SPECT | PET | fMRI | |
|
Measures |
Cerebral perfusion |
Cerebral glucose metabolism |
Oxygen saturation of blood |
|
Typical radiotracer half-life |
99mTc T½ = 6 hrs |
18F T½ = 110 min 15O T½ = 2 min 13N T½ = 10 min 12C T½ = 20 min |
N/A |
|
Temporal resolution |
Fair |
Good |
Great |
|
Spatial resolution |
6-9 mm |
4-5 mm |
3 mm |
|
Scan time |
30 min |
10-30 min |
30-60 min |
|
Cost |
$1,500 |
$2,000-$4,000 |
$800-$l,000 |
|
Advantages |
Less expensive Technically easier method Relative stability of radiotracer |
More precise and direct quantification of brain function Shorter radiation exposure time Markers for some receptors or enzymes of interest may be available |
No ionizing radiation exposure Ability to scan subject multiple times Superior temporal and spatial resolution |
|
Disadvantages |
Limited structural anatomic visualization Radiation exposure |
Limited structural anatomic visualization Prohibitive cost Short half-life of radiotracer Radiation exposure Problematic for diabetes patients due to glucose load from tracer (fluorodeoxyglucose-PET) |
Limited clinical utility |
Note. fMRI=functional magnetic resonance imaging; N/A=not applicable; PET=positron emission tomography; SPECT=single photon emission computed tomography.
SPECT and PET can be used to identify seizure foci in the interictal period or to localize deep subcortical foci that may not be apparent on EEG. They are also useful techniques for the presurgical localization of seizure foci in patients with medically refractory seizures. SPECT is often used rather than PET in this clinical situation because the relative stability of radioactive tracers (usable up to 4-6 hours after preparation) is helpful for imaging of seizure activity. SPECT is used in the context of partial or focal seizures to identify a localized brain region that can be removed surgically to eradicate the seizures. Seizures can be localized during both ictal and interictal phases.
Figure 4-5. Side-by-side comparison of structural and functional neuroimaging: magnetic resonance imaging (MRI) and positron emission tomography (PET).
To view this figure in color, see Plate 2 in Color Gallery in middle of book.
Seizures are associated with intense increases in glucose metabolism and regional cerebral blood flow. Thus, the seizure foci appear bright or hypermetabolic on SPECT scan during the seizure (ictal scan) and are dark or hypometabolic between seizures (interictal phase= interictal scan). Ictal scans are the most sensitive but most difficult method of localizing seizures. Radioactive tracer is injected within the first minute of seizure onset and accumulates in the brain. The SPECT images are acquired after cessation of seizure activity and subsequent recovery of the patient, usually an hour later. An easier but less sensitive method of localizing seizures with SPECT or PET is scanning between seizures in order to look for hypometabolic areas; these areas are believed to be a result of neuronal damage that occurs as a result of the seizure. Ictal and interictal scans are now often used together to localize a focus that is hypermetabolic on ictal scan but hypometabolic on interictal scan (Henry and van Heertum 2003).
SPECT is an extremely sensitive test for stroke. It is able to visualize perfusion defects and define the size of the stroke. However, CT is still used in the acute setting because it is quick and easy to obtain. In addition, CT is superior to SPECT in differentiating between hemorrhagic and nonhemorrhagic stroke, which is essential to know prior to starting thrombolytic medications. SPECT holds great promise for the evaluation and treatment of stroke, and its role will likely expand in the future.
Studies have found SPECT to be more sensitive than CT or conventional MRI sequences in the diagnosis of traumatic brain injury. Structural neuroimaging modalities can detect serious head injuries but often do not detect mild traumatic brain injuries. Patients with mild traumatic brain injuries often complain of persistent neuropsychiatric symptoms despite having normal CT or MRI scans. Because of its increased sensitivity, SPECT may show regional cerebral blood flow hypoperfusion despite normal CT or MRI scans (Bonne et al. 2003). However, the prognosis of patients with an abnormal SPECT scan is unclear. It may be further complicated by difficulties in recognizing which specific SPECT abnormalities are attributable to brain injury as opposed to motion artifact, normal variation, and processing errors. Thus, the clinical utility of the SPECT scan in mild traumatic brain injury is not clear and requires further investigation. Figures 4-7 and 4-8 illustrate the uses of SPECT and PET in patients with brain injuries.
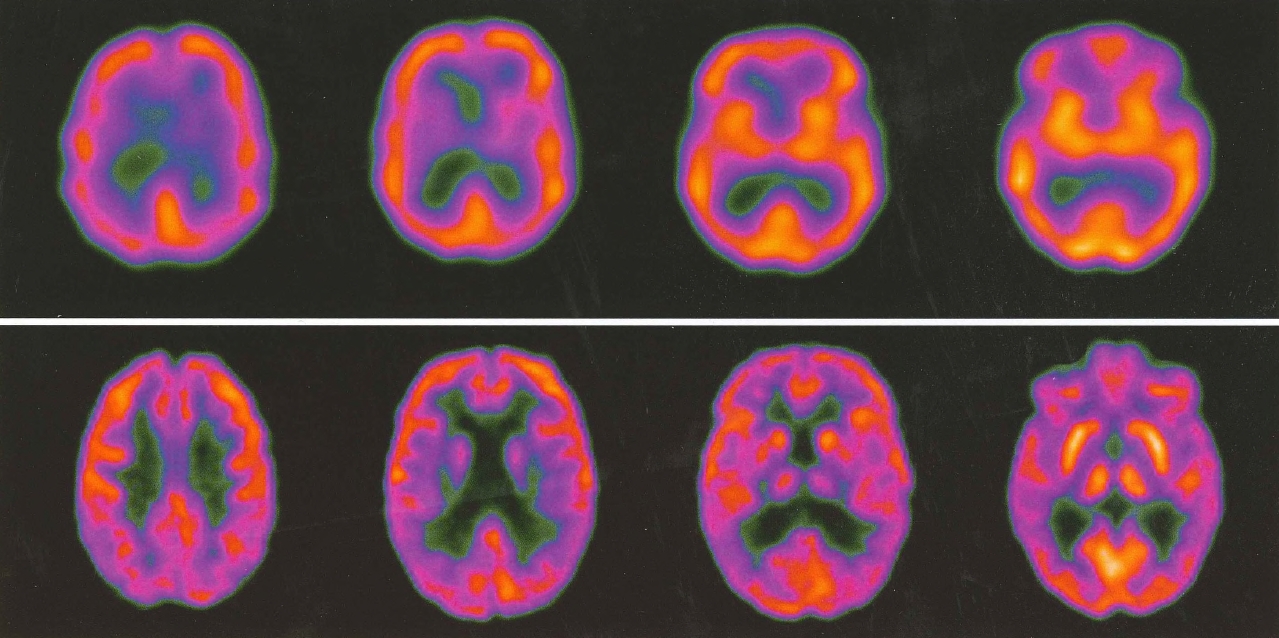
Figure 4-6. Side-by-side comparison of single-photon emission computed tomography (SPECT) versus positron emission tomography (PET).
To view this figure in color, see Plate 3 in Color Gallery in middle of book.
SPECT (top row) and PET images from two patients with clinically similar degrees of mild cognitive impairment. The PET scan demonstrates parietal changes, suggesting that this patient is at greater risk of developing Alzheimer's disease. The PET scan also demonstrates much better resolution than the SPECT scan.
Source. Images courtesy of Paul E. Schulz, M.D., Department of Neurology, Baylor College of Medicine, Houston, Texas.
Recently, a great deal of research has been done regarding the use of radiolabeled neuroreceptor ligands in SPECT and PET imaging to study the distribution and density of neuroreceptors in the brains of patients with psychiatric disorders. Many experimental neurochemical targets have been investigated with SPECT and PET radioligands, including dopamine transporters, postsynaptic dopamine receptors (D1 and D2), several serotonin receptors (5-HT1A and 5-HT2A) and transporters, γ-aminobutyric acid-A receptors, acetylcholine receptors, and histamine receptors. The outcome variable of this technique is the binding potential (or binding) of the radiotracer or the receptor/transporter availability, which is equivalent to the product of receptor/transporter density and affinity of the radiotracer for the receptor/transporter (Parvas et al. 2011; Vyas et al. 2013). PET can also be used to quantify the concentration of enzymes such as monoamine oxidase types A and B in the human brain (Fowler et al. 2005).
Substance-related disorder and addiction research, for example, has substantially benefited from the use of neuroreceptor imaging. [11C]raclopride has been used to measure D2 receptor availability and to measure changes in extracellular dopamine (Volkow et al. 1994), and [11C] cocaine has been used to measure pharmacokinetics and distribution of cocaine in the human brain and also to assess dopamine transporter availability and blockade by stimulant drugs (Volkow et al. 1997b).
Functional MRI (fMRI) measures the level of oxygenation in brain tissue to map the neuroanatomical activation that occurs with various challenges. Several fMRI techniques have been developed, but the most widely used one is the blood oxygenation level-dependent (BOLD) technique. BOLD fMRI is based on the magnetic susceptibility of blood, whose hemoglobin fluctuates between a paramagnetic, deoxygenated state in resting state blood and an isomagnetic, oxygenated state. Deoxyhemoglobin acts as an endogenous contrast agent. Increased neuronal activity in response to a sensorimotor, cognitive, or behavioral challenge results in an increase in regional cerebral blood flow and a subsequent decrease in regional deoxyhemoglobin concentration. Oxygen saturation changes in blood due to cognitive challenge or sensory stimuli result in a corresponding change in T2-weighted magnetic resonance signal intensity, thus allowing neuronal activation to be mapped neuroanatomically through the BOLD signal. fMRI images are obtained when the subject is at rest and when the subject is engaged in a sensorimotor or cognitive task and then compared to determine changes in regional cerebral blood flow. Structural MRI images can be obtained simultaneously, and these images can be interleaved with the fMRI images to more precisely pinpoint neuroanatomical locations of regional activation.
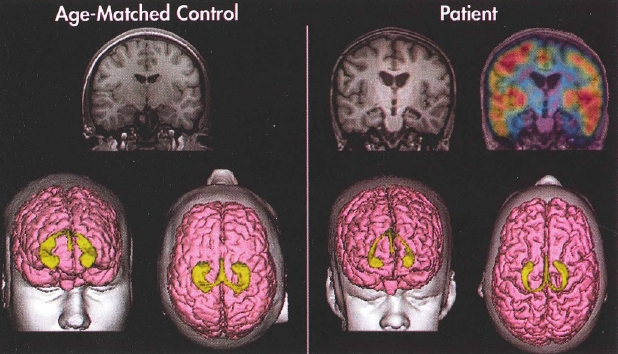
Figure 4-7. Structural magnetic resonance imaging (MRI) and positron emission tomography (PET) imaging of a healthy control subject and a patient with traumatic brain injury.
To view this figure in color, see Plate 4 in Color Gallery in middle of book.
Coronal slices (MRI) and three-dimensional reconstruction of the cortical surface (pink) and hippocampi (yellow) of a typically developing adolescent male (left) and an adolescent male with traumatic brain injury (right). Note the significant cortical and hippocampal atrophy in the patient as compared with the age-matched control. The top right image portrays PET findings overlaid on the MRI. PET reveals significant bilateral metabolic defects in the patient's mesial temporal areas as indicated by the absence of "warm" colors. Red represents areas of the greatest metabolic activity, followed by orange, yellow, green, blue, and violet.
Source. Images courtesy of Erin Bigler, Ph.D., University of Utah, Salt Lake City, Utah.
fMRI has many advantages compared with other functional imaging techniques in that it provides superior spatial and temporal resolution in relation to PET and SPECT, is minimally invasive, and does not involve exposure to harmful ionizing radiation. It is being used extensively in research to understand the neurocircuitry involved in psychotic disorders, mood and anxiety disorders, substance-related disorders, and cognitive and developmental disorders. Furthermore, the effects of psychotropic medications are being studied via fMRI, with the hope of understanding the regional brain effects of acute and chronic treatment with these medications. Despite the insights into structure-function relations that fMRI has revealed, it is not yet used as a diagnostic or treatment modality. This probably has to do with difficulties standardizing stimuli and determining stimuli that differentiate between different disorders.
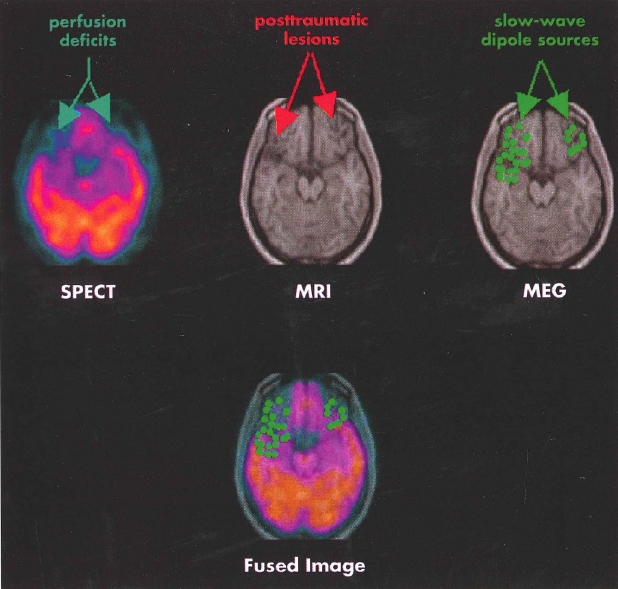
Figure 4-8. Single photon emission computed tomography (SPECT), structural magnetic resonance imaging (MRI), and magnetoencephalography (MEG) imaging of a patient with traumatic brain injury.
To view this figure in color, see Plate 5 in Color Gallery in middle of book.
Findings from multiple neuroimaging modalities in a patient with traumatic brain injury reveal structural and functional deficits in the inferior frontal and temporal regions, common sites of focal injury in head trauma. Functional imaging reveals even more extensive defects in perfusion (SPECT, left) and dipole abnormality (MEG, right) than the areas of focal injury evident on structural MRI (center). The fused image (bottom) displays the results of the SPECT and MEG overlaid on the MRI.
Source. Images courtesy of Erin Bigler, Ph.D., University of Utah, Salt Lake City, Utah.
More recently, the use of fMRI at rest (often termed "resting-state fMRI") has enabled researchers to investigate functional connectivity of the human brain (Rosazza and Minati 2011). Measures of resting functional connectivity have been shown to be reproducible and consistent across laboratories (Tomasi and Volkow 2010) and to be sensitive to a number of conditions in the brain, including drug addiction (Gu et al. 2010), traumatic brain injury, posttraumatic stress disorder, social anxiety disorder (Fouche et al. 2013), bipolar disorder (Liu et al. 2012; Whalley et al. 2012), schizophrenia (Guller et al. 2012), and other psychiatric disorders.
Magnetoencephalography (MEG) measures extracranial magnetic signals generated by the positive ionic flow of cortical pyramidal cells in the brain. These extracranial neuromagnetic fields are about 1(T8 to 1CT9 of Earth's magnetic field (Reite et al. 1999). To be identified, they require expensive superconducting technology as well as magnetic shielding to screen out competing magnetic fields from the Earth, sun, and environment. MEG is noninvasive, does not entail exposure to ionizing radiation, and has excellent spatial and temporal resolution. It is currently being studied in several psychiatric disorders and has been used to localize epileptiform activity by co-registration with structural MRI data. It has also been used in conjunction with evoked potentials to presurgically map auditory and somatosensory cortical areas to be avoided during neurosurgical procedures (Hund et al. 1997). MEG is also being used to study possible cortical reorganization, cerebral lateralization, and auditory sensory memory abnormalities in patients with psychotic disorders.
Structural and functional neuroimaging of psychiatric disorders has exploded in recent decades, given the many new and powerful imaging techniques that are now available. Although these marked advances have led to few conclusive findings at the present time about the pathophysiology and workings of the mysterious human brain, significant work is under way to utilize automated or semiautomated classification algorithms and recent analysis techniques to better identify patterns in psychiatric disease (Bansal et al. 2012) and to better translate recent research findings into clinical practice (Keedwell and Linden 2013). A comprehensive discussion of the research findings to date in the neuroimaging of the major psychiatric disorders is beyond the scope of this chapter. However, Table 4-15 summarizes the structural and functional neuroimaging findings in selected psychiatric disorders that may be of interest to the psychiatric clinician.
Laboratory assessment and imaging studies are important to the evaluation of the psychiatric patient. Their influence and scope, although of limited clinical use in the past, have the potential to increase tremendously as promising new modalities become more widely available and demonstrate ever-increasing clinical possibilities.
Judicious choice of laboratory testing, guided by a complete psychiatric assessment—including a thorough medical and psychiatric history, review of systems, and physical examination—may often uncover an unsuspected medical or neurological etiology underlying primarily psychiatric symptomatology. Likewise, structural and functional neuroimaging are powerful tools that can provide evidence of tangible abnormalities that might underlie psychiatric symptoms. Ideally, through advances in neuroimaging and laboratory testing, promising genetic and biological markers will be discovered and will attain a level of clinical utility so that a new and important dimension may be added to the uses of laboratory testing: the identification, biological treatment, and ultimately prevention of psychiatric illnesses.
|
Table 4-15. Summary of neuroimaging findings in selected psychiatric disorders |
|
| Disorder/imaging modality | Findings |
Schizophrenia spectrum and other related psychotic disorders |
|
|
Structural imaging studies |
Ventricular enlargement; abnormalities in medial temporal lobe structures and superior temporal gyrus (Shenton et al. 2001; Shepherd et al. 2012). Majority of studies report frontal lobe abnormalities (prefrontal gray matter and orbitofrontal regions) (Shenton et al. 2001) and anterior cingulate (Shepherd et al. 2012). Subcortical abnormalities involving cavum septum pellucidum, basal ganglia, corpus callosum, and thalamus (Shenton et al. 2001; Shepherd et al. 2012). Ventricular enlargement and decreased gray matter volumes in first-episode schizophrenic patients (Lim et al. 1996; McDonald et al. 2006). White matter: Most DTI studies have found fractional anisotropy reductions in various brain regions, including the left-frontal and temporal white matter (Ellison-Wright and Bullmore 2009; Kubicki et al. 2007). |
|
Functional imaging studies |
PET shows relative hypometabolism in the prefrontal cortex (Buchsbaum et al. 1982; Tamminga et al. 1992). Metabolic abnormalities of limbic areas (temporal lobe, anterior cingulum) (Nordahl et al. 2001; Tamminga et al. 1992). fMRI: Meta-analysis of 41 executive-function studies in schizophrenia revealed consistently reduced activation in the left dorsolateral prefrontal cortex, rostral/dorsal anterior cingulate cortex, left thalamus, and inferior/posterior cortical areas. Increased activation was observed in several midline cortical areas (Minzenberg et al. 2009). |
|
Magnetic resonance spectroscopy |
Decreased NAA levels in frontal, temporal, and thalamic regions (Bertolino et al. 1998; Yurgelun-Todd et al. 1996). Antipsychotic medications associated with selective increase in NAA in dorsolateral prefrontal cortex (Bertolino et al. 2001). |
Mood disorders (depressive disorders and bipolar and related disorders) |
|
|
Structural imaging studies |
Abnormal signal hyperintensities in frontal cortex and basal ganglia (Videbech 1997). Bipolar: Volume increases in the globus pallidus and putamen have also been reported (Arnone et al. 2009; Kempton et al. 2008). Ventricular enlargement and increased sulcal prominence in patients with bipolar disorder and unipolar depression (Elkis et al. 1995), which may be more prominent in those with psychotic features (Strasser et al. 2005). Volume losses in medial thalamic or hypothalamic areas that form the walls of the third ventricle have been presumed to underlie this enlargement. Flippocampal atrophy has also been found (Rossi et al. 2012). Bipolar: Reductions in right prefrontal and temporal lobe gray matter (Selvaraj et al. 2012). Depression: Volume reductions in prefrontal and anterior cingulate cortices and also in subcortical structures such as caudate nucleus and putamen (Bora et al. 2012). |
|
Functional imaging studies |
Depression: Hypometabolism in limbic and dorsolateral prefrontal cortical regions, but hypermetabolism of ventrolateral frontal cortex (Brody et al. 2001; Ketter et al. 1996). |
|
Magnetic resonance spectroscopy |
Depression: Increased choline levels in basal ganglia and anterior cingulate (Renshaw et al. 2001; Soares et al. 1999). |
Anxiety disorders and obsessive-compulsive and related disorders |
|
|
Structural imaging studies |
OCD: Unclear findings; no volume differences in striatal or ventricular regions (Aylward et al. 1996). |
|
Functional imaging studies |
Hypermetabolism in the orbitofrontal cortex and anterior cingulum (Holzschneider and Mulert 2011; Swedo et al. 1989). Successful treatment of OCD associated with decreased metabolism in orbitofrontal cortex, anterior cingulum, and caudate nucleus (Baxter et al. 1992; Swedo et al. 1992). |
Posttraumatic stress disorder |
|
|
Structural imaging studies |
Smaller hippocampal volumes, smaller frontal volumes (Pitman et al. 2012). Reduced cingulate, caudate, and insula volumes, even in subthreshold PTSD in veterans (Herringa et al. 2012). |
|
Functional imaging studies |
Altered activity in the amygdala, vmPFC, and dACC, as well as in the hippocampus and insular cortex in individuals with PTSD. A recent meta-analysis of 79 functional PTSD neuroimaging studies found that the mid-ACC, dACC, and bilateral amygdala were the most hyperactivated regions, whereas the vmPFC and inferior frontal gyrus were the most hypoactivated regions (Pitman et al. 2012). One PET study found a reduction in GABA type A receptor binding throughout the cortex, hippocampus, and thalamus, in veterans with PTSD compared to veterans without PTSD. |
Note. ACC=anterior cingulate cortex; dACC=dorsal anterior cingulate cortex; DTI= diffusion tensor imaging; fMRI = functional magnetic resonance imaging; GABA=γ-aminobutyric acid; NAA=N-acetyl aspartate; OCD=obsessive-compulsive disorder; PET=positron emission tomography; PTSD=posttraumatic stress disorder; vmPFC=ventromedial prefrontal cortex.
Key Clinical Points
Agzarian MJ, Chryssidis S, Davies RP, et al: Use of routine computed tomography brain scanning of psychiatry patients. Australas Radiol 50:27-28, 2006
Albon E, Tsourapas A, Frew E, et al: Structural neuroimaging in psychosis: a systematic review and economic evaluation. Health Technol Assess 12:iii-iv, ix-163, 2008
Amen DG, Highum D, Licata R, et al: Specific ways brain SPECT imaging enhances clinical psychiatric practice. J Psychoactive Drugs 44:96-106, 2012
American Academy of Neurology: American Academy of Neurology practice guidelines for dementia. Continuum 13: Appendix A, 2007
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. Arlington, VA, American Psychiatric Association, 2013
American Psychiatric Association Task Force on the Use of Laboratory Tests in Psychiatry: Tricyclic antidepressants: blood level measurements and clinical outcome. An APA Task Force report. Am J Psychiatry 142:155-162, 1985
Amin M, Wang J: Routine laboratory testing to evaluate for medical illness in psychiatric patients in the emergency department is largely unrevealing. West J Emerg Med 10:97-100, 2009
Anfinson TJ, Kathol RG: Screening laboratory evaluation in psychiatric patients: a review. Gen Hosp Psychiatry 14:248-257, 1992
Anfinson TJ, Stoudemire A: Laboratory and neuroendocrine assessment in medical-psychiatric patients, in Psychiatric Care of the Medical Patient, 2nd Edition. Edited by Stoudemire AS, Fogel BS, Greenberg D. New York, Oxford University Press, 2000, pp 119-145
Arnone D, Cavanagh J, Gerber D, et al: Magnetic resonance imaging studies in bipolar disorder and schizophrenia: metaanalysis. Br J Psychiatry 195:194-201, 2009
Aylward EH, Harris GL, Hoehn-Saric R, et al: Normal caudate nucleus in obsessive-compulsive disorder assessed by quantitative neuroimaging. Arch Gen Psychiatry 53:577-584, 1996
Bansal R, Staib LH, Laine AF, et al: Anatomical brain images alone can accurately diagnose chronic neuropsychiatric illnesses. PloS One 7:e50698, 2012
Baxter LR Jr, Schwartz JM, Bergman KS, et al: Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Arch Gen Psychiatry 49:681-689, 1992
Berkemeier KL, Nipper ML, Williams JM: Is the chest x-ray an appropriate screening exam for ER patients with AMS? Emerg Radiol 15:421-425, 2008
Bernardo M, Palao DJ, Arauxo A, et al: Monitoring plasma level of haloperidol in schizophrenia. Hosp Community Psychiatry 44:115-118, 1993
Bertolino A, Callicott JH, Elman I, et al: Regionally specific neuronal pathology in untreated patients with schizophrenia: a proton magnetic resonance spectroscopic imaging study. Biol Psychiatry 43:641-648, 1998
Bertolino A, Callicott JH, Mattay VS, et al: The effect of treatment with antipsychotic drugs on brain N-acetylaspartate measures in patients with schizophrenia. Biol Psychiatry 49:39-46, 2001
Bonne O, Gilboa A, Louzoun Y, et al: Cerebral blood flow in chronic symptomatic mild traumatic brain injury. Psychiatry Res 124:141-152, 2003
Bora E, Harrison BJ, Davey CG, et al: Metaanalysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorders. Psychol Med 42:671-681, 2012
Boutros N, Struve F: Electrophysiological testing, in Neuropsychiatric Assessment. Edited by Yudofsky SC, Kim HF (Review of Psychiatry Series; Oldham JM and Riba MB, series eds). Washington, DC, American Psychiatric Publishing, 2004, pp 69-104
Brody AL, Barsom MW, Bota RG, et al: Prefrontal-subcortical and limbic circuit mediation of major depressive disorder. Semin Clin Neuropsychiatry 6:102-112, 2001
Buchsbaum MS, Ingvar DH, Kessler RC, et al: Cerebral glucography with positron tomography: use in normal subjects and in patients with schizophrenia. Arch Gen Psychiatry 39:251-259, 1982
Carroll BJ: Problems with diagnostic criteria for depression. J Clin Psychiatry 45:14-18, 1984
Curry S: The strategy and value of neuroleptic drug monitoring. J Clin Psychopharmacol 5:263-271, 1985
de Leon J: Psychopharmacological treatment based on individual drug metabolism: CYP2D6 poor metabolizers. CNS Spectr 11:8-12, 2006 16384813
de Leon J, Armstrong S, Cozza KL: Clinical guidelines for psychiatrists for the use of pharmacogenetic testing for CYP450 2D6 and CYP450 2C19. Psychosomatics 47:75-85, 2006
Elkis H, Friedman L, Wise A, et al: Metaanalyses of studies of ventricular enlargement and cortical sulcal prominence in mood disorders: comparisons with controls or patients with schizophrenia. Arch Gen Psychiatry 52:735-746, 1995
Ellison-Wright I, Bullmore E: Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res 108:3-10, 2009
Engel J Jr: The epilepsies, in Cecil Textbook of Medicine, 19th Edition, Vol 2. Edited by Wyngaarden JB, Smith LH, Bennett JC. Philadelphia, PA, WB Saunders, 1992, pp 2202-2213
Erhart SM, Young AS, Marder SR, et al: Clinical utility of magnetic resonance imaging radiographs for suspected organic syndromes in adult psychiatry. J Clin Psychiatry 66:968-973, 2005
Fowler JS, Logan J, Volkow ND, et al. Translational neuroimaging: positron emission tomography studies of monoamine oxidase. Mol Imaging Biol 7:377-387, 2005
Fouche JP, van Der Wee NJ, Roelofs K, et al: Recent advances in the brain imaging of social anxiety disorder. Hum Psychopharmacol 28:102-105, 2013
Gu H, Salmeron BJ, Ross TJ, et al: Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage 53:593-601, 2010
Guller Y, Tononi G, Postle BR: Conserved functional connectivity but impaired effective connectivity of thalamocortical circuitry in schizophrenia. Brain Connect 2:311-319, 2012
Henry TR, van Heertum RL: Positron emission tomography and single photon emission computed tomography in epilepsy care. Semin Nucl Med 33:88-104, 2003
Herringa R, Phillips M, Almeida J, et al: Post-traumatic stress symptoms correlate with smaller subgenual cingulate, caudate, and insula volumes in unmedicated combat veterans. Psychiatry Res 203:139-145, 2012
Hollister LE: Electrocardiographic screening in psychiatric patients. J Clin Psychiatry 56:26-29, 1995
Hollister LE, Shah NN: Structural brain scanning in psychiatric patients: a further look. J Clin Psychiatry 57:241-244, 1996
Holzschneider K, Mulert C: Neuroimaging in anxiety disorders. Dialogues Clin Neurosci 13:453-461, 2011
Honig A, Tan ES, Weenink A, et al: Utility of a symptom checklist for detecting physical disease in chronic psychiatric patients. Hosp Community Psychiatry 42:531-533, 1991
Hughes JR, John ER: Conventional and quantitative electroencephalography in psychiatry. J Neuropsychiatry Clin Neurosci 11:190-208, 1999
Hund M, Rezai AR, Kronberg E, et al: Magnetoencephalographic mapping: basic of a new functional risk profile in the selection of patients with cortical brain lesions. Neurosurgery 40:936-942, discussion 942-943, 1997
Hyman SE, Arana GW: Handbook of Psychiatric Drug Therapy, 2nd Edition. Boston, MA, Little, Brown, 1991
Ingelman-Sundberg M, Oscarson M, McLellan RA: Polymorphic human cytochrome P450 enzymes: an opportunity for individualized drug treatment. Trends Pharmacol Sci 20:342-349, 1999
Keedwell PA, Linden DE: Integrative neuroimaging in mood disorders. Curr Opin Psychiatry 26:27-32, 2013
Kempton MJ, Geddes JR, Ettinger U, et al: Meta-analysis database, and metaregression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry 65:1017-1032, 2008
Ketter TA, George MS, Kimbrell TA, et al: Functional brain imaging, limbic function, and affective disorders. The Neuroscientist 2:55-65, 1996
Kirchheiner J, Brosen K, Dahl ML, et al: CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand 104:173-192, 2001
Kubicki M, McCarly R, Westin CF: A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res 41:15-30, 2007
Lim KO, Tew W, Kushner M, et al: Cortical gray matter volume deficit in patients with first-episode schizophrenia. Am J Psychiatry 153:1548-1553, 1996
Liu CH, Ma X, Li F, et al: Regional homogeneity within the default mode network in bipolar depression: a resting-state functional magnetic resonance imaging study. PloS One 7:e48181, 2012
Lukens TW, Wolf SJ, Edlow JA, et al: Clinical policy: critical issues in the diagnosis and management of the adult psychiatric patient in the emergency department. Ann Emerg Med 47:79-99, 2006
Marangell LB, Martinez JM, Silver JM, et al: Concise Guide to Psychopharmacology. Washington, DC, American Psychiatric Publishing, 2002
McDonald C, Marshall N, Sham PC, et al: Regional brain morphometry in patients with schizophrenia or bipolar disorder and their affected relatives. Am J Psychiatry 163:478-87, 2006
Minzenberg MJ, Laird AR, Thelen S, et al: Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 66:811-822, 2009
Moles JK, Franchina JJ, Sforza PP: Increasing the clinical yield of computerized tomography for psychiatric patients. Gen Hosp Psychiatry 20:282-291, 1998
Mookhoek EJ, Sterrenburg-vdNieuwegiessen IM: Screening for somatic disease in elderly psychiatric patients. Gen Hosp Psychiatry 20:102-107, 1998
Mrazek DA: Incorporating pharmacogenetics into clinical practice: reality of a new tool in psychiatry. The context of genetic testing in clinical psychiatric practice. CNS Spectr 11(3, suppl 3):3-1, 2006
Nordahl TE, Carter CS, Salo RE, et al: Anterior cingulate metabolism correlates with Stroop errors in paranoid schizophrenia patients. Neuropsychopharmacology 25:139-148, 2001
Olshaker JS, Browne B, Jerrard DA, et al: Medical clearance and screening of psychiatric patients in the emergency department. Acad Emerg Med 4:124-128, 1997
Parvas MA, Alia-Klein N, Woicik PA, et al: Neuroimaging for drug addiction and related behaviors. Rev Neurosci 22:609-624, 2011
Pasternak O, Westin CF, Bouix S, et al: Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J Neurosci 28:17365-17372, 2012
Petersen RC, Thomas RG, Grundman M, et al: Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 352:2379-2388, 2005
Pitman RK, Rasmusson AM, Koenen KC, et al: Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 13:769-787, 2012
Rauch SL, Renshaw PF: Clinical neuroimaging in psychiatry. Harv Rev Psychiatry 2:297-312, 1995
Reite M, Teale P, Rojas DC: Magnetoencephalography: applications in psychiatry. Biol Psychiatry 45:1553-1563, 1999
Renshaw PF, Parow AM, Hirashima F, et al: Multinuclear magnetic resonance spectroscopy studies of brain purines in major depression. Am J Psychiatry 158:2048-2055, 2001
Ringholz GR: Differential diagnosis. Lecture presented at Current Neurology conference, Houston, TX, November 2001
Rosazza C, Minati L: Resting-state brain networks: literature review and clinical applications. Neurol Sci 32:773-785, 2011
Rossi R, Lanfredi M, Pievani M, et al: Volumetric and topographic differences in hippocampal subdivisions in borderline personality and bipolar disorders. Psychiatry Res 203:132-138, 2012
Sachse C, Brockmoller J, Bauer S, et al: Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet 60:284-295, 1997
Sadock BJ, Sadock VA: Laboratory tests in psychiatry, in Kaplan and Sadock's Synopsis of Psychiatry, 10th Edition. Baltimore, MD, Lippincott Williams & Wilkins, 2007, pp 255-267
Sagar M, Seensalu R, Tybring G, et al: CYP 2C19 genotype and phenotype determined with omeprazole in patients with acid-related disorders with and without Helicobacter pylori infection. Scand J Gastroenterol 33:1034-1038, 1998
Selvaraj S, Arnone D, Job D, et al: Grey matter differences in bipolar disorder: a meta-analysis of voxel-based morphometry studies. Bipolar Disord 14:135-145, 2012
Sheline Y, Kehr C: Cost and utility of routine admission laboratory testing for psychiatric inpatients. Gen Hosp Psychiatry 12:329-334, 1990
Shenton ME, Dickey CC, Frumin M, et al: A review of MRI findings in schizophrenia. Schizophr Res 49:1-52, 2001
Shepherd AM, Laurens KR, Matheson SL, et al: Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev 36:1342-1356, 2012
Soares JC, Boada F, Spencer S, et al: NAA and choline measures in the anterior cingulate of bipolar disorder patients (abstract). Biol Psychiatry 45 (suppl):119S, 1999
Strasser HC, Lilyestrom J, Ashby ER, et al: Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biol Psychiatry 57:633-639, 2005
Streetman DS: Metabolic basis of drug interactions in the intensive care unit. Crit Care Nurs Q 22:1-13, 2000
Swedo SE, Schapiro MB, Grady CL, et al: Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Arch Gen Psychiatry 46:518-523, 1989
Swedo SE, Pietrini P, Leonard HL, et al: Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder: revisualization during pharmacotherapy. Arch Gen Psychiatry 49:690-694, 1992
Taber KH, Pierpaoli C, Rose SE, et al: The future for diffusion tensor imaging in neuropsychiatry. J Neuropsychiatry Clin Neurosci 14:1-5, 2002
Tamminga CA, Thaker GK, Buchanan R, et al: Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry 49:522-530, 1992
Tomasi D, Volkow ND: Functional connectivity density mapping. Proc Natl Acad Sci USA 107:9885-9890, 2010
Videbech P: MRI findings in patients with affective disorder: a meta-analysis. Acta Psychiatr Scand 96:157-168, 1997
Volkow ND, Wang GJ, Fowler JS, et al: Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse 16:255-262, 1994
Volkow ND, Rosen B, Farde L: Imaging the living human brain: magnetic resonance imaging and positron emission tomography. Proc Natl Acad Sci USA 94:2787-2788, 1997a
Volkow ND, Wang GJ, Fischman MW, et al: Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature 386:827-830, 1997b Vyas NS, Patel NH, Herscovitch P, et al: Recent developments in neurochemical imaging in schizophrenia: an update. Curr Med Chem 20(3) :351-356, 2013
Wallach J: Interpretation of Diagnostic Tests.
Boston, MA, Little, Brown, 1992
Wallach J: Interpretation of Diagnostic Tests, 7th Edition. Philadelphia, PA, Lippincott Williams & Wilkins, 2000
Whalley HC, Papmeyer M, Sprooten E, et al: Review if functional magnetic resonance imaging studies comparing bipolar disorder and schizophrenia. Bipolar Disord 14:411-431, 2012
Yurgelun-Todd DA, Renshaw PF, Gruber SA, et al: Proton magnetic resonance spectroscopy of the temporal lobes in schizophrenics and normal controls. Schizophr Res 19:55-59, 1996
Baumann P, Hiemke C, Ulrich S, et al: The AGNP-TDM Expert Group consensus guidelines: therapeutic drug monitoring in psychiatry. Pharmacopsychiatry 37:243-265, 2004
Cabeza R, Nyberg L: Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12:1-47, 2000
de Leon J, Armstrong SC, Cozza KL: Clinical guidelines for psychiatrists for the use of pharmacogenetic testing for CYP450 2D6 and CYP450 2C19. Psychosomatics 47:75-85, 2006
Jacobson SA: Laboratory Medicine in Psychiatry and Behavioral Science. Washington, DC, American Psychiatric Publishing, 2012
Linden DE: The challenges and promise of neuroimaging in psychiatry. Neuron 12:8-22, 2012
Yudofsky SC, Kim HF (eds): Neuropsychiatric Assessment (Review of Psychiatry Series; Oldham JM and Riba MB, series eds). Washington, DC, American Psychiatric Publishing, 2004