CHAPTER 28
Brain Stimulation Therapies*
Psychiatry is now developing a third realm of treatment modalities, complementing the well-established realms of psychopharmacology (medications) and psychotherapy Different names are being bandied about to describe these treatments, ranging from neuromodulation to brain stimulation techniques to the hard-to-understand and cumbersome term nonpharmacological somatic treatments (the title of this chapter in a previous edition of this book). As a class, these methods involve focal electrical brain stimulation of some sort, varying widely in their invasiveness and methods of delivery. Table 28-1 lists most of the current methods. In this chapter, we review only those brain stimulation treatments that have been approved by the U.S. Food and Drug Administration (FDA) to treat the traditionally defined psychiatric disorders (electroconvulsive therapy [ECT], vagus nerve stimulation [VNS], transcranial magnetic stimulation [TMS]); that have FDA device exemption (deep brain stimulation [DBS]); or that have a large literature of human clinical trials (trans-cranial direct current stimulation [tDCS]) in psychiatric diseases. The brain stimulation methods are also being used to treat traditionally defined neurological disorders (e.g., DBS in dystonia or Parkinson's disease). We mention those uses in more traditional neurological disorders where appropriate, but a complete review of these areas is found in the references listed in Suggested Readings at the end of the chapter.
Additionally, several brain stimulation techniques are quite popular as research methods, but are not likely to be used therapeutically in the near future. Optogenetics is a method developed within the past 5 years in which individual cells are transfected with light-sensitive membrane ions and then stimulated with fiber-optic light (LaLumiere 2011). This allows for exquisite spatial, temporal, and even histological specificity but requires gene vector transfection and an invasive light source implanted into the brain. To our knowledge, this has not been tried yet in humans. Similarly, there is much excitement about the potential for using transcranial pulsed ultrasound. For reasons that are not clear but that might involve mechanoreceptors sensitive to movement on neuronal cell membranes, pulsed ultrasound can cause certain neurons to discharge (conventional, nonpulsed ultrasound does not cause neuronal firing). The electrical discharge of neurons is not due to damage or heating. Pulsed ultrasound has been demonstrated in rabbits and rodents but so far requires an open craniotomy to get the ultrasound past the skull (www.you-tube.com/watch?v=RGEP6iWLsvQ). Ultrasound in this form cannot penetrate through bone, and thus this technique has not been performed in humans, except during brain surgery (Bystritsky et al. 2011).
A fundamental concept for these techniques is that the brain is an electrochemical organ. All neurons transmit information and communicate with other cells through an electrical impulse (depolarization) traveling from a dendrite through the cell body out to the synapse. Because of the fascination with events happening chemically between neurons (psychopharmacology), psychiatrists have neglected or forgotten the important principle that the entire communication and action between neurons begins with an electrical impulse.
This renaissance in brain stimulation techniques would not be nearly as successful as it is without the revolution in brain imaging that has occurred over the past 20 years. Fundamentally, it is important to have hypotheses about where to stimulate. Thus, the results from brain imaging studies provide the needed knowledge base to hypothesize about where to apply the techniques. With some of the techniques, such as DBS, brain imaging also allows one to guide the individual placement. Although the regional neuroanatomy of depressive disorders, anxiety disorders, or psychotic disorders is not as well defined as, for example, Parkinson's disease, scientists have a much better understanding of the regions involved in affective regulation, anxiety, or hallucinations now than they did 20 years ago. Brain stimulation techniques largely build on this body of knowledge from neuroimaging in guiding where in the brain they are applied. Once an area of the brain is targeted, the various brain stimulation technologies could potentially "turn on," "turn off," or modulate that region. Although these are called "stimulation treatments," various characteristics (frequency, strength, timing of stimulation, etc.) could actually lead the treatments to reduce unwanted activity (rather than to stimulate or excite activity).
Finally, it is rare to use any of the brain stimulation methods alone in treatment settings. Most commonly they are used in combination with medications or talking therapy. In fact, a most interesting area of research is to investigate how to combine the different stimulation techniques with medications or talking therapy. It seems likely that some medications, more than others, will work in synergy with a brain stimulation treatment for a particular disorder (Sackeim et al. 2009). Additionally, it is likely that what a person is doing or thinking while being stimulated will ultimately matter in terms of clinical response (Vedeniapin et al. 2010). A particularly interesting line of research involves having patients crave a substance, and then applying TMS to try and modify the emotional memory of the craving. Similarly, researchers are having patients with posttraumatic stress disorder (PTSD) remember their index trauma and are then applying TMS during or immediately after the recall, again with the idea of modifying the ability to control the emotional response to the memory (Isserles et al. 2013).
|
Table 28-1. Overview of brain stimulation treatments |
||||
| Full name | Convulsive? | Stimulation site | Psychiatric disorders | FDA clinical use status |
|
Electroconvulsive therapy (ECT) |
Yes |
Cortical |
Depression, mania, catatonia |
Grandfathered FDA approval |
|
Focal electrically administered seizure therapy (FEAST) |
Yes |
Cortical |
Depression |
Experimental, all conditions |
|
Magnetic seizure therapy (MST) |
Yes |
Cortical |
Depression |
Experimental, all conditions |
|
Transcranial magnetic stimulation (TMS) or repetitive transcranial magnetic stimulation (rTMS) |
No |
Cortical |
Depression |
FDA approval of one device |
|
Vagus nerve stimulation (VNS) |
No |
Cervical cranial nerve |
Depression |
FDA approval for treatment-resistant depression |
|
Deep brain stimulation (DBS) |
No |
Subcortical |
Depression |
FDA approval for PD; HDE for OCD; pivotal trials in depression under way |
|
Transcranial direct current stimulation (tDCS) |
No |
Cortical |
Substance abuse, depression |
Experimental, all conditions |
|
Transcutaneous electrical nerve stimulation (TENS) |
No |
Peripheral nerve |
Pain |
FDA approval for pain conditions |
|
Functional magnetic resonance imaging with echo planar imaging |
No |
Unknown—subcortical? |
Depression |
Experimental, all conditions |
|
Transcranial Doppler ultrasound |
No |
Any |
Unknown |
Experimental, all conditions |
Note. FDA=U.S. Food and Drug Administration; HDE=humanitarian device exemption; OCD = obsessive-compulsive disorder; PD=Parkinson's disease.
ECT, the grandfather of the brain stimulation treatments, involves the deliberate induction of a generalized tonic-clonic seizure by electrical means. Contemporary ECT devices typically deliver bidirectional (alternating-current) brief-pulse square-wave stimulation through a pair of electrodes, which are applied externally to the patient's scalp. Given that the electricity must pass through the skin and scalp, a reasonable-sized charge must be applied to have a therapeutic effect. ECT electrodes can be placed bifrontally, bitemporally, or unilaterally (often done to minimize cognitive effects). Because of the risk of bodily harm from the convulsion, ECT is performed under general anesthesia, with the body paralyzed. As with other convulsive therapies that historically preceded ECT, the goal is to produce a seizure. The presence of seizure activity appears to be essential. Stimuli that are below the seizure threshold appear to be clinically ineffective. However, although the production of a seizure appears to be necessary, a seizure alone is not sufficient. Some forms of seizure induction are in fact clinically ineffective (Sackeim et al. 1993). Various psychiatric and neurological conditions respond favorably to ECT, although the majority of patients treated with ECT have a mood disorder, such as unipolar or bipolar depression, particularly when the depression is severe or accompanied by psychotic symptoms. Certain other conditions, such as mania, schizoaffective disorder, catatonia, neuroleptic malignant syndrome, Parkinson's disease, and intractable seizures, may respond to ECT as well. Schizophrenia has also been treated with ECT, although the results tend to be less favorable and often transient compared with those obtained in mood disorders. Those patients with schizophrenia who also have a prominent disturbance of mood probably respond best to ECT.
For a typical series or a course of ECT, treatments are usually given two to three times per week for 8-12 treatments. Treatments can be conducted in either an outpatient or an inpatient setting. This course may then be followed by maintenance treatment in the form of medication, additional ECT given at less frequent intervals, or both. A number of questions remain regarding the most effective methods for performing ECT, as well as its mechanism or mechanisms of action. The technique and skill of the ECT group likely matters. ECT as practiced in the general community has lower response rates (40%-60%) than the historical response rates (60%-80%) in the published literature from academic medical settings (Prudic et al. 2004). ECT is unfortunately associated with acute and sometimes more chronic memory loss (Sackeim et al. 2002). Because of these limitations and societal misperceptions, ECT is underused.
Data suggest that shorter pulse widths have fewer side effects than the fatter pulse widths used in traditional ECT (Sackeim et al. 2002). (Applying electricity after the neuron has depolarized is not needed and is perhaps cognitively harmful.) Sackeim et al. (2008) determined that the most efficient pulse width for an ECT pulse was about 0.25 milliseconds; they label this as "ultrabrief pulse width" and have shown that right unilateral ultrabrief pulse width ECT applied at a dose 6 times that needed to produce a seizure (seizure threshold) is as effective as older forms of ECT, with markedly fewer cognitive side effects. Note that it is not just the seizure that is needed for the antidepressant response; electrode placement and optimal stimulation parameters, such as pulse width and suprathreshold dose, enhance clinical profiles. Because of its improved cognitive side-effect profile and roughly similar efficacy, right unilateral ultrabrief pulse ECT is now widely used.
Although ECT is the most effective acute treatment for depression, it is disappointing that at 6 months, many ECT patients who have responded or remitted will relapse. For example, one study randomly assigned ECT remitters to placebo, nortriptyline, or nortriptyline plus lithium after patients had remitted (Sackeim et al. 2002). At 6 months, the relapse rate was 84% with placebo, 60% with nortriptyline, and 39% with nortriptyline plus lithium. Some psychiatrists administer maintenance ECT treatments every 3-5 weeks to prevent relapse, although few formal studies with maintenance ECT have been reported. Some psychiatrists have even suggested using ECT for quick and acute antidepressant relief in a patient, and then using other, more durable brain stimulation techniques, such as vagus nerve stimulation, for longer-term relapse prevention.
Harold Sackeim began work many years ago to build supercharged TMS devices capable of reliably producing seizures in humans, reasoning that a TMS-induced seizure would be more focal and efficient than an ECT-induced seizure and would spare much of the brain from receiving unneeded electricity (George and Wassermann 1994; Sackeim 1994). His technique, magnetic seizure therapy (MST), was shown to be feasible first in animals and later in patients (Lisanby et al. 2003). Patients have markedly less acute cognitive disruption from MST seizures than from traditional ECT. Whether MST has clinical antidepressant efficacy similar to that of conventional ECT is still not clear, and a multisite study is under way.
Conventional ECT involves biphasic pulses or even sine waves. This means that ECT is a form of alternating current, with either electrode producing the same effect. Alternating current offers limited control of intracerebral current paths. There is no direction to the current flow, and there are not positive or negative electrodes as with a battery (which uses direct current). Another variant of ECT involves delivering only monophasic pulses, or delivering direct current that cycles rapidly on and off and may create focal seizure activity. Researchers call this directional ECT focal electrically administered seizure therapy, or FEAST. By using a newer electrode configuration and unidirectional stimulation, this newer form of ECT can initiate seizures focally and specifically in the prefrontal cortex prior to secondary seizure generalization. Essentially, the area under the exiting anode on the right forehead becomes excited, and the seizure begins more focally, directly underneath the electrode. This approach has been effectively demonstrated in animal studies (Spellman et al. 2009). It is thought that seizure induction in prefrontal cortex is key for clinical efficacy, whereas seizure expression in medial temporal lobes is responsible for amnestic effects. FEAST hypothetically preserves treatment efficacy while reducing memory side effects. A proof of concept clinical trial in adult patients with depression has shown that the cognitive side effects are mild, even when compared with right unilateral ultrabrief pulse ECT, and that clinical effects are in the ballpark of conventional ECT (Nahas et al. 2013). More work is ongoing.
TMS is perhaps the most popular of the new techniques, because with TMS the skull does not need to be opened to focally stimulate, no seizure is needed, and to date there appear to be only limited side effects. Moreover, TMS can be used either as a research tool (to measure how excitable the brain is or to produce a temporary lesion) or as a therapy. TMS involves creating a powerful electrical current near the scalp. The electricity flowing in an electromagnetic coil on the scalp creates an extremely potent (near 1.5-Tesla) but brief (microseconds) magnetic field. The neat trick is that the TMS magnetic field enters the surface of the brain without interference from the skin, muscles, and bone. Although skin and bone act as resistors to impede electrical currents, magnetic fields pass unimpeded through the skull and soft tissue. In the brain, the magnetic pulse encounters nerve cells with resting potentials and induces electrical current to flow. Thus, electrical energy is converted to magnetic fields outside the brain, which then pass through the skull and are converted back into electrical currents in the brain (Bohning 2000). TMS is thus sometimes called electrodeless electrical stimulation.
The magnetic field acts as a trick to bridge the skull. Although magnetic fields do have biological effects on tissue, the vast majority of TMS effects likely derive not from the magnetic fields but rather from the induced electrical currents generated in the brain. TMS, with powerful but extremely brief magnetic fields, differs from constant low-field magnets. TMS directly electrically tickles the brain, while constant weak magnets do not induce currents.
The amount of electricity needed to actually cause someone's thumb to move with a TMS coil varies considerably across different individuals. Referred to as the motor threshold (MT), this can be determined with either measuring electromyography (EMG) of the thumb or index finger or by assessing visible movement or twitches in the intended muscles (Mishory et al. 2004; Pridmore et al. 1998). The motor threshold changes depending on whether muscles are resting or active (tensed), and can also change with sleep deprivation and various medications (Paulus et al. 2008). About 60% of the variance between individuals in their motor threshold is because of differences in the distance from the skull to the motor cortex (Kozel et al. 2000a).
The idea of using TMS, or something akin to it, to alter neural function dates back to at least the early 1900s. In 1902 Pollacsek and Beer, psychiatrists working down the street from Sigmund Freud in Vienna, filed a patent to treat depression and neuroses with an electromagnetic device that looks surprisingly like today's TMS machines (Beer 1902). The modern TMS era began in 1985 when Tony Barker and colleagues, working in Sheffield, England, created a focal electromagnetic device with sufficient power to induce currents in the spine (Barker et al. 1985). They quickly realized that their device could also directly and noninvasively stimulate the human brain. Their device could only stimulate the surface of the brain, however, because the magnetic field falls off sharply with distance from the coil. Several researchers, including one commercial company, are creating more powerful TMS devices that stimulate deeper in the brain (Roth et al. 2005). Unfortunately, it appears that the deeper one stimulates, the broader or less focal the field must be. Thus, it is not yet possible with TMS to stimulate both deep in the brain and locally, as with DBS (Deng et al. 2013).
A single pulse of TMS, applied over the motor cortex, produces a jerklike movement in the hand, arm, face, or leg, depending on where the coil is positioned. A single pulse applied over the back of the brain can produce a phosphene (seeing light without light actually entering the eye). However, that is about the extent of the immediate positive effects that single-pulse TMS can produce. TMS pulses applied in rhythmic succession are referred to as repetitive TMS (rTMS). rTMS can create behaviors that do not occur with single pulses, including the potential risk of causing an unintended seizure, particularly if the stimulation is conducted near the motor cortex. Over 20 seizures have occurred in the history of TMS use, out of an unclear total number of people stimulated but easily over 100,000 sessions (Rossi et al. 2009). Since market introduction of the Neuro-Star TMS Therapy system in October 2008, seven seizures have been reported with NeuroStar TMS Therapy, over a usage of more than 250,000 treatment sessions and over 8,000 patients. In five of the seven seizures, patients had concurrent use of medications that may have altered seizure threshold. The estimated risk of seizure under ordinary clinical use is approximately 1 in 30,000 treatments (0.003% of treatments) or 1 in 1,000 patients (0.1% of patients) (M. Demitrack, Neuronetics, personal communication, November 12, 2012). This risk is less than or comparable to the risk of seizure associated with antidepressant medications. All TMS seizures have occurred during stimulation, rather than later, and have been self-limited with no sequelae. rTMS seizures are more likely to occur with certain combinations of TMS intensity, frequency, duration, and interstimulus interval (Wassermann 1997).
Much research is under way to determine exactly which neurons TMS affects, and the cascade of neurobiological events that follow stimulation. Different factors, such as gyral anatomy (how the brain is shaped), the distance from the skull to the brain (brain atrophy), and the orientation of nerve fibers relative to coil, are all important.
One of the more interesting rTMS effects is that for brief periods of time, during stimulation, rTMS can block or inhibit a brain function. That is, rTMS over the motor area that controls speech can temporarily leave the patient speechless (motor aphasia), but only while the device is firing. Cognitive neuroscientists have used this knockout aspect or 'Temporary lesioning" ability of TMS to reexplore and test the large body of information gleaned from years of studying stroke patients. Additionally, two pulses of TMS in quick succession can provide information about the underlying excitability of a region of cortex. This diagnostic technique, called paired-pulse TMS, can demonstrate the behavior of local interneurons in the motor cortex and serve as an indirect measure of γ-aminobutyric acid (GABA) or glutamate (Heide et al. 2006).
Single nerve cells form themselves into functioning circuits over time through repeated discharges. Externally stimulating a single nerve cell with low-frequency electrical stimulation can cause long-term depression, where the efficiency of links between cells diminishes. High-frequency stimulation over time can cause the opposite effect, called long-term potentiation. These behaviors are thought to be involved in learning, memory, and dynamic brain changes associated with networks. A very exciting aspect of research with TMS, as well as the other brain stimulation techniques, is whether one can use external brain stimulation to change brain circuits over time in a manner analogous to long-term depression or long-term potentiation. Many TMS studies have now shown inhibition or excitation lasting for up to several hours beyond the time of stimulation (Di Lazzaro et al. 2005). The clinical implications here are profound. If one could use brain imaging to identify the faulty network in the brain, one could then use TMS or other techniques to change learning and memory, or to resculpt brain circuits. Some basic physiological studies indicate that a circuit can be changed only while the behavior is ongoing and the cells involved in the various neural pathways are acting as a circuit (Stanton and Sejnowsky 1989). It is an important question whether TMS should be delivered while patients are thinking about important topics or using a certain muscle. Thus, research is now focusing on combining TMS with modified forms of cognitive-behavioral therapy or physical medicine rehabilitation.
Animal and cellular studies with TMS reinforce that it is a powerful technique able to alter neuronal function. One stumbling block in using TMS in animals is that it is hard to make TMS coils that are the same relative size to most animals as to humans. Small coils simply explode. Thus, most animal TMS studies, especially small animal studies, have not really used focal TMS as in humans. Nevertheless, studies have shown that rTMS enhances apomorphine-induced stereotypy and reduces immobility in the Porsolt swim test (Fleischmann et al. 1996), as well as inducing electroconvulsive shock-like changes in rodent brain monoamines, β-adrenergic receptor binding, and immediate early gene induction (Ben-Sachar et al. 1997). In addition, researchers have found that TMS can induce neurogenesis (Pope and Keck 2001).
A critically important research area that will ultimately guide clinical parameters involves combining TMS with functional imaging to directly monitor TMS effects on the brain, and to thus understand the varying effects of different TMS parameters on brain function. Because TMS at different frequencies appears to have divergent effects on brain activity, combining TMS with functional brain imaging will better delineate not only the behavioral neuropsychology of various psychiatric syndromes but also some of the pathophysiological circuits in the brain. In contrast to imaging studies with ECT that have found that ECT shuts off global and regional activity following the seizure (Nobler et al. 2001), most studies using serial scans in depressed patients undergoing TMS have found increased activity in the cingulate and other limbic regions (Teneback et al. 1999). However, two studies have found divergent effects of TMS on regional activity in depressed patients, determined both by the frequency of stimulation and by the baseline state of the patient (Mitchel 2002). That is, for patients with global or focal hypometabolism, high-frequency prefrontal stimulation increases brain activity over time, with the opposite happening as well. Conversely, patients with focal hyperactivity have reduced activity over time following chronic daily low-frequency stimulation. However, these two small sample studies have numerous flaws. They simultaneously show the potential and the complexity surrounding the issue of how to use TMS to change activity in defined circuits. They also point out an obvious difference with ECT, where the net effect of the ECT seizure is to decrease prefrontal and global activity (Nobler et al. 2001).
When a neuron fires or discharges, different neurotransmitters are released in the synaptic cleft. Thus, the brain stimulation methods are in one view simply "focal pharmacology." These links between brain stimulation methods and traditional pharmacological views of the psychiatric illnesses have been highlighted by studies using radioligands. Baeken et al. (2011) examined the neuro-biological impact of 10 rTMS sessions applied to the left dorsolateral prefrontal cortex on postsynaptic serotonin type 2A (5-HT2A) receptor binding indices measured with 123I-5-I-R91150 single-photon emission computed tomography (SPECT). Compared with the control group, patients displayed significantly less bilateral dorsolateral prefrontal cortical and significantly higher left hippocampal baseline 5-HT2A receptor binding indices. Successful high-frequency rTMS treatment correlated positively with 5-HT2A receptor binding indices in the dorsolateral prefrontal cortex bilaterally and correlated negatively with right hippocampal 5-HT2A receptor uptake values. These results indicate that high-frequency rTMS treatment affects the serotonergic system within the prefrontal cortex as patients respond.
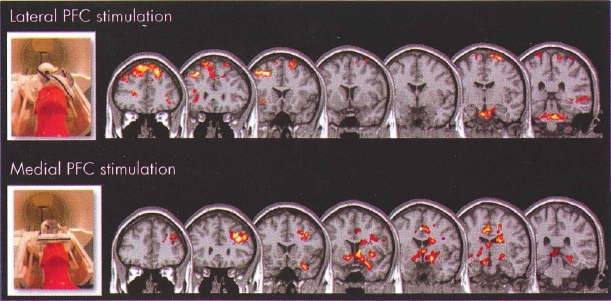
TMS can actually be performed within a magnetic resonance imaging (MRI) scanner, which is itself a huge magnet and is constantly on (Bohning 2000; Bohning et al. 1998). Work with this interleaved TMS/functional MRI technology has shown that prefrontal TMS at 80% motor threshold produces much less local and remote blood flow change than does TMS at 120% motor threshold (Na-has et al. 2001). Strafella, Paus, and their colleagues used positron emission tomography (PET) to show that prefrontal cortex TMS causes dopamine release in the caudate nucleus (Strafella et al. 2003) and has reciprocal activity with the anterior cingulate gyrus (Paus et al. 2001). Our group at the Medical University of South Carolina (George et al. 1999) and others in Scotland (Shajahan et al. 2002) and Australia (Mitchel 2002) have shown that lateral prefrontal TMS can cause changes in the anterior cingulate gyrus and other limbic regions in depressed patients. It is therefore clear that TMS delivered over the prefrontal cortex has immediate effects in important subcortical limbic regions. TMS over different aspects of the prefrontal cortex (lateral vs. medial) can produce different secondary activations (Figure 28-1). This highlights the notion that stimulating the cortex with TMS really is "opening a window" to different cortical-subcortical networks. The initial TMS effect on cortex and the secondary synaptic changes in other regions likely differ as a function of mood state, cortical excitability, and other factors that would change resting brain activity. Combining TMS with functional imaging will likely continue to be an important method for understanding TMS mechanisms of action. Combined TMS and imaging will likely also evolve to be an important neuroscience tool for researching brain connectivity (George and Bohning 2002).
Although more work is needed, certain brain regions have consistently been implicated in the pathogenesis of depression and mood regulation. These include the medial and dorsolateral prefrontal cortex, the cingulate gyrus, and other regions commonly referred to as limbic (amygdala, hippocampus, parahippocampus, septum, hypothalamus, limbic thalamus, insula) and paralimbic (anterior temporal pole, orbitofrontal cortex). A widely held theory over the last 20 years has been that depression results from a dysregulation of these prefrontal cortical and limbic regions (George et al. 1994, 1995a, 1996; Mayberg et al. 1999). The very first uses of TMS as an antidepressant were not influenced by this regional neuroanatomical literature, and stimulation was applied over the vertex (Beer 1902; Grisaru et al. 1994; Kolbinger et al. 1995). However, working within the prefrontal cortical limbic dysregulation framework outlined above, and realizing that theories of ECT action emphasize the role of prefrontal cortex effects (Nobler et al. 1994), George and colleagues performed the first open trial of daily prefrontal TMS as an antidepressant in 1995. This was followed immediately by a crossover double-blind study (George et al. 1995b, 1997). Their reasoning was that chronic, frequent, subconvulsive stimulation of the prefrontal cortex over several weeks might initiate a therapeutic cascade of events both in the prefrontal cortex and in connected limbic regions, thereby causing the dysregulated circuits to rebalance and normalize, alleviating depression symptoms (George and Wassermann 1994). The imaging evidence previously discussed now shows that this hunch was largely correct: prefrontal TMS sends direct information to important mood-regulating regions, such as the cingulate gyrus, orbitofrontal cortex, insula, and hippocampus. Thus, beginning with these prefrontal studies, modern TMS was specifically designed as a focal, nonconvulsive, circuit-based approach to therapy.
In 2008, the NeuroStar TMS Therapy System (Neuronetics, Malvern, Pennsylvania) received FDA clearance for the treatment of adult patients with major depressive disorder who have failed to receive satisfactory improvement from one prior antidepressant medication at or above the minimal effective dose and duration in the current episode. FDA clearance was based on a large, multisite, sham-controlled randomized study that showed that daily prefrontal TMS was a safe and effective treatment for certain patients with major depression. The observed effect sizes in both the original study population (N=301; O'Reardon et al. 2007) and the subset of patients who met the FDA-approved indication for use of the NeuroStar TMS Therapy System (N=164; Demitrack and Thase 2009) are of similar or greater magnitude than those observed with the majority of currently approved antidepressant medication treatments.
George et al. (2010), in a 190-patient multisite, randomized controlled trial (RCT; called OPT-TMS, short for the Optimization of TMS for the Treatment of Depression Trial) sponsored by the National Institute of Mental Health, demonstrated that rTMS, as drug-free monotherapy, produced statistically significant antidepressant effects with a remission rate four times that of sham therapy. This study provided industry-independent Class I evidence of safety and efficacy in a well-studied and carefully controlled cohort. Two additional publications resulted from this trial. McDonald et al. (2011) reported on an open-label extension phase of this trial. They found that 43 of 141 (30.5%) patients who enrolled in this phase of the study eventually met criteria for remission. Some patients took up to 6 weeks to fully remit (McDonald et al. 2011). Most recently, Mantovani et al. (2012) reported on the 3-month durability of the TMS antidepressant response in this trial. Of the 50 patients who remitted and agreed to participate in follow-up, at 3 months, 29 (58%) were classified as in remission (24-item Hamilton Rating Scale for Depression [Ham-D-24] score <10), 2 (4%) were classified as partial responders (reduction in Ham-D-24 score from baseline: >30% <50%), and 1 (2%) met criteria for relapse.

Figure 28-1= Transcranial magnetic stimulation (TMS) opens "cortical windows."
To view this figure in color, see Plate 9 in Color Gallery in middle of book.
These are group blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) activation maps using an optimized interleaved transcranial magnetic stimulation (TMS)/BOLD sequence in two cortical targets to examine the differential activation of lateral and medial neural circuits, which are known to be important in drug abuse or mood regulation, or both.
Interleaved TMS/BOLD imaging data were acquired for 10 healthy individuals who received TMS in two runs with the coil positioned over the (top) dorsolateral prefrontal cortex (DLPFC) and (bottom) medial prefrontal cortex (mPFC). DLPFC TMS was associated with a significant elevation of BOLD signal in multiple dorsal cortical areas, whereas mPFC TMS was associated with a significant elevation of BOLD signal in multiple medial and limbic subcortical areas. These preliminary data demonstrate that cortical TMS produces secondary effects deeper in the brain. TMS is thus opening cortical windows into different brain circuits, depending on the connectivity pattern of the cortical region being stimulated.
Source. Images derived from work by Dr. Colleen Hanlon and colleagues at the Medical University of South Carolina (Hanlon and Centerberry 2012; Li et al. 2004b). Used with permission.
These studies demonstrate that left prefrontal TMS daily for 3-6 weeks has antidepressant effects that are significantly greater than sham, and that these effects in the open-label extension phase trial are clinically meaningful (30% remission rate). Even more importantly, the outcomes are at least as robust as those for the next-best choice of antidepressant medication, the procedure was found to be safe and well tolerated with a low incidence of treatment discontinuation, and the therapeutic effects, once obtained, are reasonably durable.
Other recent studies highlight the effectiveness of TMS in modern clinical practice. The first was a recent publication that describes the findings of a multisite observational study in 307 real-world patients getting care with TMS in clinical practice settings (Carpenter et al. 2012). The individuals described in this report had received an average of two antidepressant treatments of adequate dose and duration without successful improvement in their current episode of depression and were markedly ill at enrollment. With an acute course of TMS treatments (average of 28 treatment sessions), the subjects' symptom severity ratings decreased significantly, regardless of the metric used for assessment. With categorical outcomes, 58% of the subjects were responders on the primary outcome measure, the Clinical Global Impression Severity scale, and 37% had reached remission, with similar findings on the secondary measures. This study of TMS outcomes, as delivered in real-world practices to care-seeking patients, adds additional evidence regarding the effectiveness of TMS in patients with treatment-resistant depression. This therapy appears to work in real-world settings and not only in rigorous clinical research trials. Unlike with many therapies in medicine, there does not appear to be an efficacy-effectiveness gap with prefrontal TMS for acute depression.
The second recent report, another observational study of how TMS works in real-world settings, was published by Connolly et al. (2012) from the University of Pennsylvania. These investigators reported data from the first 100 patients treated at their university-based TMS clinical service following FDA approval. Their cohort was also treatment resistant, with a mean of three failed adequate antidepressant trials in the current episode. Thirty-one individuals had received prior lifetime ECT, and 60% had a history of psychiatric hospitalization. At 6 weeks, the Clinical Global Impression Improvement scale response rate was 50% and the remission rate was 25%. The Ham-D response and remission rates were 41% and 35%, respectively. Forty-two patients (49%) entered 6 months of maintenance TMS treatment. Of these, 26 (62%) maintained their responder status at the last assessment during the maintenance treatment. TMS treatment was well tolerated, with a discontinuation rate of 3% in the acute treatment phase. No serious adverse events related to TMS were observed during acute or maintenance treatment. The authors concluded that adjunctive TMS was safe and effective in both acute and maintenance treatment of patients with treatment-resistant depression.
Although the literature suggests that prefrontal TMS has an antidepressant effect greater than sham and that the magnitude of this effect is in the range of other antidepressants, many issues are not resolved. Work done to date has shown substantial evidence that prefrontal TMS produces immediate (George et al. 1999; Li et al. 2002) and longer-term (Teneback et al. 1999) changes in mood-regulating circuits. Thus, the original hypothesis about its antidepressant mechanism of action is still the most likely explanation. What remains unclear is which specific prefrontal or other brain locations might be the best for treating depression, and whether this can be determined with a group algorithm or needs individual imaging guidance. For the most part, the coil has been positioned using the rule-based algorithm that researchers (including chapter coauthor M.S.G.) developed in early studies (George et al. 1995b) to find the prefrontal cortex. However, this method was shown to be imprecise in the particular prefrontal regions stimulated directly underneath the coil, depending largely on the subject's head size (Herwig et al. 2001). Additionally, most studies have stimulated patients at or above the patients' motor threshold. There is now increasing recognition that higher intensities of stimulation are needed to reach the prefrontal cortex, especially in elderly patients, in whom prefrontal atrophy may outpace that of the motor cortex, where the motor threshold is measured (Kozel et al. 2000b). A few case series suggest that weekly or monthly rTMS can be used as a maintenance treatment after someone has responded acutely (Li et al. 2004a; Nahas et al. 2000; O'Reardon et al. 2005).
Other active areas of research look at whether one can combine TMS with some behavioral therapy to increase efficacy (Vedeniapin et al. 2010), or whether one can deliver higher doses or density of treatment to speed response (Holtzheimer et al. 2010).
Another interesting development with TMS involves different coil designs (Deng et al. 2013; Huang et al. 2009). Most studies described in this chapter used a figure-eight coil, which is quite focal in terms of the field created in the brain (Roth et al. 1991). Abraham Zangen and his colleagues in Israel (Roth et al. 2005) have designed a series of different TMS coils that penetrate much deeper, and broader, into the brain than do traditional coils. The company Brainsway now manufactures these coils and has completed a multisite clinical trial of their coil in depression. A press release from that trial showed positive results compared with sham. On the basis of these trial results, the Brainsway coil received FDA approval for the treatment of depression in January 2013.
It is unclear whether and to what degree and in what conditions a deeper or broader TMS coil would have advantages over a more superficial coil. Theoretically, if one is not using individual structural or functional MRI scans to guide the TMS coil placement, a broader coil would have a higher probability of stimulating mood-regulating circuits than would a more precise coil. Metaphorically speaking, without a way of seeing the target, a shotgun fired into the woods has a higher probability of hitting prey than does a rifle. It simply covers a broader area. Are there specific prefrontal regions that each person uses to regulate his or her mood? If so, how could these regions be imaged most effectively? Or is regulation a function of broad areas of the cortex, in which case a broader, less focal coil might be more effective? More research is needed into this most important question with respect to using TMS to treat depression and other psychiatric disorders.
TMS has also been investigated as a possible treatment for a variety of neuropsychiatric disorders. In general, the published literature for these conditions is much less extensive than for TMS as an antidepressant, and therefore conclusions about the clinical significance of effects must remain tentative until large sample studies are conducted.
Several studies have used TMS to investigate corticospinal conductivity in schizophrenia (Feinsod et al. 1998). In the first study of motor function in schizophrenia using TMS, Puri et al. (1996) detected a significantly shorter latency of motor evoked potentials in nine unmedicated patients with schizophrenia compared with nine healthy subjects. Two other studies measuring motor evoked potential latency did not find a difference between medicated schizophrenia patients and normal control subjects (Davey and Puri 1997; Davey et al. 1997; Puri et al. 1996).
Hoffman et al. (1998) used repeated daily sessions of low-frequency TMS over the temporal lobes to treat hallucinations in patients with schizophrenia. Some but not all groups have replicated these effects. Further studies are needed (Cohen et al. 1999).
Several studies have shown promise in using TMS to treat obsessive-compulsive disorder (OCD). In the initial study in the field, Greenberg et al. (1997) found that a single session of right prefrontal rTMS decreased compulsive urges for 8 hours. Mood was also transiently improved, but there was no effect on anxiety or obsessions. Two other studies have examined possible therapeutic effects of rTMS in OCD. A double-blind study using right prefrontal slow (1-Hz) rTMS and a less focal coil failed to find statistically significant effects greater than sham (Alonso et al. 2001). In contrast, an open-label study in a group of 12 OCD patients refractory to standard treatments, who were randomly assigned to right or left prefrontal fast rTMS, found that clinically significant and sustained improvement was observed in one-third of patients (Sachdev et al. 2001). Finally, a group stimulated the supplemental motor area and found clinical improvements in OCD and Tourette's disorder (Mantovani et al. 2006). Clearly, further testing of TMS as a potential treatment for OCD is warranted. The improvements seen using TMS for OCD appear to be short lived, lasting only several days. Not uncommonly, patients with comorbid OCD and depression get treated for their depression, which improves and stabilizes in a well state; however, the OCD improvements are transient, and patients require daily or near-daily treatment indefinitely.
Using TMS to treat anxiety, particularly PTSD, is also an area of interest. McCann et al. (1998) reported that two patients with PTSD improved during open treatment with 1-Hz rTMS over the right frontal cortex. Grisaru et al. (1998) similarly stimulated 10 PTSD patients over the motor cortex and found decreased anxiety. Watts et al. (2012) treated 20 PTSD patients with either real or sham TMS to the right orbitofrontal region. Patients were treated daily for 2 weeks. Overall, there was a clinically and statistically significant reduction in PTSD symptoms measured by both the Clinician-Administered PTSD Scale (CAPS) and the PTSD Checklist (PCL). The active TMS group had a nearly 30-point reduction in CAPS scores (from 81 to 53; P<0.0001) and a 15-point reduction in PCL scores. However, the researchers did not have patients engage in exposure to fear stimuli (i.e., traumatic memories) during the TMS. Isserles et al. (2013) in Israel used the large deep TMS coil to treat 30 PTSD subjects in one of three arms: exposure therapy (ET) with sham TMS, combined ET with active TMS, and TMS alone. Only the ET with active TMS group had significant drops in CAPS scores. The combined ET and TMS group had a 44% response rate, with a 12% response rate in the TMS alone group and no responders in the ET-alone group.
Further work is needed, particularly concerning whether to have PTSD patients remember their trauma immediately before or during stimulation.
Mood-regulating centers in the brain overlap significantly with the neural pathways involved in pain regulation, especially the regions involved in determining whether a pain is really bothersome. Thus, some researchers have begun exploring whether TMS might have a therapeutic role in treating acute or chronic pain. There are exciting reports that TMS over either the prefrontal cortex or the motor cortex can acutely decrease pain in healthy adults or patients with chronic pain (Andre-Obadia et al. 2006; Pridmore and Oberoi 2000; Short et al. 2011). A recent RCT found that a single 20-minute session of left prefrontal rTMS given to patients in the recovery room following surgery reduced self-administered morphine use by 40% (Borckardt et al. 2006b). In the laboratory, a 20-minute dose of prefrontal TMS can also increase pain thresholds. This effect is blocked in healthy volunteers by pretreatment with naloxone, suggesting that opiate receptors play a necessary role in the antinociceptive effects of TMS (Taylor et al. 2012).
Overall, TMS is a promising new therapy, as well as a research tool. The bulk of clinical work to date has been in depression, where one TMS device has been FDA approved. TMS holds promise therapeutically in several other psychiatric disorders.
TMS is noninvasive; focal, largely limited to different cortical sites; and intermittent. VNS is in some sense the opposite of TMS because it is invasive, requiring surgical implantation of a device in the chest wall and a wire in the neck. The brain region stimulated always follows the same initial route: the vagus nerve in the neck. The VNS device is also a permanent implant that cannot be removed without surgery. VNS has been approved since 1997 as a treatment for epilepsy, and was approved by the FDA in 2005 for chronic use in patients with treatment-resistant depression.
It seems obvious to ask whether and to what degree stimulation of a cranial nerve has effects on brain function. Thus, throughout the past century, researchers have investigated whether stimulation of cranial nerves might have observable brain effects. Of all the cranial nerves (CNs), with the exception of CN I for olfaction, the vagus nerve (CN X) has been the most intriguing and arguably the most misunderstood. The vagus nerve helps regulate the body's autonomic functions, which are important in a variety of emotional tasks. For reasons that are unclear, most people are more familiar with the vagus nerve's efferent functions—that is, its role as the messenger for signals from the brain to the viscera. Traditionally, the vagus nerve has been considered a parasympathetic efferent nerve (controlling and regulating autonomic functions such as heart rate and gastric tone). The afferent role of the vagus has been underemphasized in the traditional literature. The vagus is actually a mixed nerve, composed of about 80% afferent sensory fibers carrying information to the brain from the head, neck, thorax, and abdomen (Foley and Dubois 1937).
Over the past 100 years, several researchers have convincingly demonstrated the extensive projections of the vagus nerve via its sensory afferent connections in the nucleus tractus solitarius to diverse brain regions (Zardetto-Smith and Gray 1990). Reasoning in part from this body of literature, Zabara (1992) discovered an anticonvulsant action of VNS on experimental seizures in dogs. Zabara hypothesized that VNS could prevent or control the motor and autonomic components of epilepsy. Penry and Dean (1990) and others ushered in the modern clinical application of VNS in 1988, using an implanted device to treat epilepsy.
Although the route of entry into the brain is constrained, VNS offers the potential for modulating and modifying function in many brain regions, through transsynaptic connections (George et al. 2000). The incoming sensory (afferent) connections of the vagus nerve provide direct projections to many of the brain regions implicated in neuropsychiatric disorders. These connections provide a basis for understanding how VNS might be a portal to the brain stem and connected limbic and cortical regions. These pathways likely account for the neuropsychiatric effects of VNS, and they invite additional theoretical considerations for potential research and clinical applications. Functional imaging studies in patients with implanted VNS devices have largely confirmed this important neuroanatomy of the vagus (Bohning et al. 2001; Chae et al. 2003; Conway et al. 2006; Henry et al. 1998).
The broad term vagus nerve stimulation refers to any technique used to stimulate the vagus nerve, including procedures used in animals where the vagus was accessed through the abdomen and diaphragm. However, for virtually all human studies, VNS refers to stimulation of the left cervical vagus nerve using a commercial device. There are groups studying whether one can stimulate the vagus nerve through a cutaneous branch in the earlobe (Busch et al. 2013; Stefan et al. 2012) or through noninvasive stimulation through the neck (Miner et al. 2012); however, these approaches have not been used in the clinical trials discussed in this section, which rely on the implanted device and stimulation of the cervical vagus.
Cervically implanted VNS has been commercially available for the treatment of resistant partial-onset seizures in Europe since 1994 and in the United States since 1997. Implanting a VNS device resembles implanting a cardiac pacemaker. In both VNS and cardiac pacemakers, a subcutaneous generator sends an electrical signal to an organ through an implanted electrode. With VNS, the electrical stimulation is delivered through the generator, an implantable, multiprogrammable, bipolar pulse generator (about the size of a pocket watch) that is implanted in the left chest wall to deliver electrical signals to the left vagus nerve through a bipolar lead. The electrode is wrapped around the vagus nerve in the neck and is connected to the generator subcutaneously.
The VNS implantation surgery is typically an outpatient procedure, most commonly but not exclusively performed by neurosurgeons. The VNS generator can be controlled by a personal computer or personal digital assistant connected to an infrared wand. As a safety feature, the VNS generator is designed to shut off in the presence of a constant magnetic field. Each patient is thus given a magnet that, when held over the pulse generator, turns off stimulation. When the magnet is removed, normal programmed stimulation resumes. This allows patients to control and temporarily eliminate stimulation-related side effects during important behaviors such as public speaking (voice tremor) or heavy exercising (mild shortness of breath).
VNS has been most extensively studied as a treatment for epilepsy. Two doubleblind studies have been conducted in patients with epilepsy, with a total of 313 treatment-resistant completers (Ben-Menachem et al. 1994; Handforth et al. 1998). In this difficult-to-treat group, the average decline in seizure frequency was about 25%-30% compared with baseline.
Most epilepsy patients with VNS have not been able to reduce or stop taking antiepileptic medications. Thus, VNS, as now delivered, has not been shown to be a substitute for anticonvulsant medications, although in some patients, the dosage levels or numbers of antiepileptic medications have been decreased with the addition of VNS. VNS is increasingly being used in children with epilepsy, in part because of its lack of negative cognitive effects, which are common with other anticonvulsants (Helmers et al. 2001).
There are several different programmable variables in determining how to deliver VNS. These use parameters include the pulse width of the electrical signal (130,250,500,750,1,000 microseconds), the intensity (0.25-4 mA is clinically tolerated), the frequency (1-145 Hz), the length of stimulation (7-270 seconds), and the length of time between trains of stimuli (0.2 seconds to 180 minutes). In general, the initial epilepsy use parameter settings were those found to stop seizures acutely in animal models. The initial human epilepsy studies compared efficacy in two groups, based on different use parameters, including a high-stimulation group (30 Hz, 30 seconds on, 5 minutes off, 500-microsecond pulse width) and a low-stimulation group (1 Hz, 30 seconds on, 90-180 minutes off, 130-microsecond pulse width). Most the VNS epilepsy efficacy and safety data come from trials with use parameters similar to those used in the high-stimulation group. Similarly, most of the data from other neuropsychiatric disorders (depression, anxiety) involve VNS at use parameters similar to those used in the initial epilepsy studies. It is difficult to imagine that these use parameters are the maximally effective choices, or that the same parameters work equally well in all conditions and all patients. Epilepsy physicians commonly switch nonresponding patients to use parameter settings that are different from their current settings. However, there has been no clear demonstration that changing settings improves efficacy. Further work is needed to understand the translational neurobiology of these use parameter choices, as well as the relationship of these choices to clinical symptoms.
The mood-stabilizing or antidepressant effects of anticonvulsant medications (e.g., carbamazepine) and devices (e.g., ECT) have a long history in psychiatry. In early 1998, there were several lines of evidence to suggest that VNS might have antidepressant effects. Anecdotal reports of mood improvement in VNS-implanted epilepsy patients, knowledge of vagus function and neuroanatomy, brain imaging studies, work in animals, and cerebrospinal fluid studies all supported an initial pilot clinical trial in treatment-resistant depression (George et al. 2000).
An initial open study of VNS for chronic or recurrent treatment-resistant depression involved four sites and 30 subjects (Rush et al. 2000), with a later extension of 30 more subjects to clarify the effect size and look for response predictors (Sackeim et al. 2001b). The study design involved selecting patients with treatment-resistant chronic or recurrent major depressive episode (unipolar or nonrapid cycling bipolar) and then adding VNS to a stable regimen of antidepressant medications or no antidepressant medications. No stimulation was given for the first 2 weeks following implantation, creating a single-blind placebo phase and allowing for surgical recovery. All patients met eligibility criteria by failing at least two adequate treatment trials in the current episode.
VNS therapy was provided for 10 weeks with medications held constant. Of 59 completers (1 patient improved during the surgical recovery period), the response rate was 30.5%. VNS was well tolerated in this group, with side effects similar to those encountered by epilepsy patients. The most common side effect, occurring in 36 of 60 patients (60%), was voice alteration or hoarseness, which was generally mild and related to the intensity of the output current. There were no adverse cognitive effects (Sackeim et al. 2001a). The only response predictor was prior antidepressant treatment resistance. VNS as used in this open study was more effective in depressed patients who were less treatment resistant.
These encouraging initial results served as the basis for a U.S. multisite double-blind trial of VNS for chronic or recurrent depression of low to moderate treatment resistance. In this trial, the active VNS group failed to show a statistically significant difference in acute response from the sham group (Rush et al. 2005). The response rates were 10% for sham and 15% for active VNS, a difference that was not significant given the small sample size (N=235). The longer-term response rates for VNS-implanted depression patients were encouraging (Nahas et al. 2005) and appear better than would be expected in this population (Rush et al. 2006). A parallel but nonrandomized comparison found that patients with VNS implants had better outcomes at 1 year than a group receiving treatment as usual (George et al. 2005). These data served as the basis for FDA approval of VNS for the chronic (not acute) treatment of chronic recurrent depression. Thus, VNS is FDA approved, although there is no doubleblind randomized controlled evidence for VNS as an antidepressant in patients with depression. There are, however, open (Flarden et al. 2000) and doubleblind (Eiger et al. 2000) studies showing that VNS has antidepressant effects in epilepsy patients with comorbid depression. A long-term European study found open-label results similar to those of the U.S. study (Schlaepfer et al. 2008b). After 3 months of VNS, response rates (>50% reduction in baseline scores) reached 37% and remission rates (Ham-D-28 score <10) 17%. Response rates increased to 53% after 1 year of VNS, and remission rates reached 33%. Response was defined as sustained if no relapse occurred during the first year of VNS after response onset; 44% of patients met these criteria. Median time to response was 9 months.
In summary, VNS for depression does not appear to be a useful acute treatment, but rather appears to help treatment-resistant patients chronically, with long-term improvements (Nahas et al. 2005). Unfortunately, only 30% of implanted patients have clinically meaningful improvements, and there are no proven methods of choosing before implantation who will later respond (Conway et al. 2012).
As reviewed in "Brief History" earlier in this section on VNS, the sensory afferent nerve fibers that VNS stimulates in the vagus travel to the brain and terminate in the nucleus tractus solitarius. These fibers are the primary means by which the brain receives information from the organs within the gut and diaphragm. From there, information travels to the locus coeruleus, the primary site of all norepinephrine fibers in the brain. Norepinephrine has long been considered to be a critical neurotransmitter system involved in the pathogenesis and regulation of anxiety. A device that directly stimulates this norepinephrine control site would likely have important effects on anxiety. A small clinical trial of VNS in anxiety patients found promising results (George et al. 2008).
Information about hunger and satiety from the stomach and small intestine travels through the vagus nerve to the brain. It seems plausible to ask whether vagus stimulation might change eating patterns and other forms of craving. Investigators have implanted bilateral subdiaphragmatic vagus stimulators in several normal-weight mongrel dogs. Over several months, chronic intermittent VNS in this manner resulted in substantial weight loss. Interestingly, there was no change in the dogs' metabolism. Rather, they took much longer to consume their food, and they even left food on their plate—something mongrel dogs rarely if ever do (Roslin and Kurian 2001). Although VNS had no overall effect on weight in the RCTs in epilepsy and depression, it is still possible that VNS might be used for the treatment of obesity. A recent laboratory study of
VNS in depressed patients found that patients had different food cravings in response to pictures of food when the device was firing compared with when it was turned off (Bodenlos et al. 2008).
Some information about pain, especially visceral pain, travels through the vagus nerve. A study showing changes in pain perception as a function of different VNS settings hints at the promise of using VNS for some form of pain modulation (Borckardt et al. 2005, 2006a).
VNS has an important role in the treatment of epilepsy. Although the only clinical effects that have been shown with double-blind studies are in epilepsy for seizure control and depression occurring in the setting of epilepsy, VNS is FDA approved for depression. There are many areas where more information would facilitate its adoption. Compared with talking therapy, medications, and even ECT, VNS requires a different approach to treating depression. Whereas other treatments can be begun and sampled and then easily abandoned if not effective, VNS, with the installation of an implant, requires careful consideration prior to initiating therapy. There is interest, but no data, about using a noninvasive method of VNS prior to a permanent implant to potentially increase the certainty that implanted patients would be responders. Additionally, because of the relatively large initial capital costs of implanting a VNS generator, data are needed to convince payers that VNS is cost-effective. An initial implantation fee of around $30,000 (device and surgery) is about equal to 1 week of hospitalization or a course of outpatient ECT. VNS would thus be cost-effective for those recurrent chronically ill patients for whom implantation results in clinical improvements that eliminate the need for hospitalization, ECT, or more frequent and aggressive outpatient medication management.
Another radical difference between VNS and medication treatments is that VNS facilitates almost 100% adherence. The device, once implanted, cycles on and off without problems for several years. Several studies have shown that even the best patients skip and forget medications, with resultant problems in their clinical course. VNS is therefore a most interesting approach to treating depression, but more information is critically needed.
The most invasive of all the brain stimulation techniques is DBS. An electrode is implanted deep within the brain and connected to a generator, located in the chest wall, which sends constant electrical current into the brain. Theoretically, DBS electrodes can be removed without destroying large amounts of the brain, and therefore DBS results in less morbidity and mortality than resective brain surgery. DBS of the internal globus pallidus or subthalamic nucleus is an accepted and established treatment for Parkinson's disease, especially for those patients who are medication intolerant or resistant (Kumar et al. 1998; Limousin et al. 1995, 1998). It also is used in patients with dystonia.
DBS was first used for treatment of Parkinson's disease tremor (Limousin et al. 1995) and more recently for dystonia (Halpem et al. 2007; Tisch et al. 2007). DBS for these movement disorders involves placing the electrodes at one of two different target locations: the subthalamic nucleus or the internal globus pallidus. In this use of DBS, the electrodes are turned on constantly at high frequency (>130 Hz). The neuropsychiatric use of DBS began with work for treatment-resistant OCD patients, with the electrodes implanted at the anterior limb of the internal capsule, bilaterally (Greenberg et al. 2008; Nuttin et al. 1999, 2003). This DBS placement followed the neurosurgical literature, which demonstrated that focal ablation of these communicating fibers sometimes resulted in therapeutic treatment. Although positive effects were found in OCD symptoms, mood improvements were also seen (Gabriels et al. 2003; Greenberg et al. 2000, 2003). These open case series results were presented by a DBS manufacturer (Medtronic) to the FDA for potential approval of a humanitarian device exemption (HDE) for treatment-resistant OCD. This HDE was granted in February 2009 for DBS to be used "in conjunction with medications for the treatment of chronic, treatment resistant adult OCD patients to aid in the management of the symptoms" (U.S. Food and Drug Administration 2009).
Expanding on the positive mood effects observed in patients with OCD, Malone et al. (2009) used DBS at the same target site (anterior limb of the internal capsule) in patients with treatment-resistant depression. Fifteen patients were implanted in an open-label fashion with the DBS electrodes as an adjunctive treatment. In this highly treatment-resistant cohort, mean Ham-D-24 scores dropped significantly over 6 months from 33 at entry to 17.5 at 6 months, and there was one case of emergent hypomania.
Following a different line of reasoning, another group implanted electrodes in the white matter fiber tracts next to the rostral anterior cingulate (CG25) (May-berg et al. 2005). In 20 treatment-resistant unipolar depressed patients, CG25 DBS produced a reduction in Ham-D score from 24.4 at entry to 12.6 at 12 months. Three patients had their devices removed before 12 months (Lozano et al. 2008; McNeely et al. 2008).
Following yet another line of reasoning (Schlaepfer et al. 2008a), another group investigated effects of bilateral high-frequency stimulation to the nucleus accumbens. Acute antianhedonic and antidepressant effects have been demonstrated (Schlaepfer et al. 2008a), and very recent results in 10 very treatment-resistant depressed patients demonstrated response in half of the patients (Kayser et al. 2009). One remitted patient committed suicide during a relapse. The same research group presented unpublished work on bilateral stimulation in the medial forebrain bundle of seven treatment-resistant depressed patients. Preliminary results suggest a more robust antidepressant response than that observed with nucleus accumbens or anterior cingulate cortex stimulation at lower currents (Schlaepfer 2012). Although these studies are small in sample size (ranging from 10 to 20 patients), their results are very important in light of the fact that the patients studied had failed all other potential treatments.
Another form of invasive brain stimulation is epidural cortical stimulation, which is based on cortical regulation of subcortical, limbic regions. In one pilot study, two anterior and two midlateral cortical stimulation leads were implanted in five treatment-resistant depressed patients. At the 7-month followup, there Was a 60% improvement in Ham-D scores and three patients remitted. One patient's left hemisphere leads were explanted 12 weeks postsurgery because of a scalp infection. Findings suggest that the possible targets of implanted brain stimulation may not solely be subcortical (Nahas et al. 2010).
There are case reports and ongoing studies of DBS in the treatment of Tourette's disorder, substance abuse, addictions, obesity, and schizophrenia.
In DBS, side effects are related either to the operation itself (e.g., bleeding, local infections at the implantation site or generator location) or to the stimulation (e.g., increase of mood, anxiety). Fortunately, the stereotactic operation technique has become safer with the help of improved structural neuroimaging. Rates of hemorrhage of DBS surgeries are between 0.2% and 5% (Greenberg et al. 2003; Kayser et al. 2009; Lozano et al. 2008).
Continued research in DBS for neuropsychiatric disorders is needed. Although DBS is less invasive than ablative surgery, it is the most invasive of the brain stimulation therapies. Therefore, clinicians need to be especially cautious in potential clinical use. Due to the high vulnerability of psychiatric patients, the lack of extensive short- and long-term data about effectiveness and adverse effects, and the haunting history of psychosurgery, the use of DBS for psychiatric indications remains controversial and bears several specific ethical concerns that have only rarely been addressed to date.
For the indications of OCD and chronic pain, DBS at appropriate targets is clinically indicated but should only be performed by well-trained neurosurgeons in well-screened patients who have been referred from a treating psychiatrist and who have been aggressively treated with other options that failed. Psychiatrists should be the clinicians making the recommendation and providing follow-up. In general, DBS patients must also receive a positive second opinion recommending DBS from a trained and certified psychiatrist who is not related to the research group or referring psychiatrist. As a general guideline, it is also appropriate to consider whether all other options have been tried, and to discuss the patient's potential reaction if the treatment does not work.
In the area of treatment-resistant depression, the literature is supportive but not yet sufficient to recommend clinical use. DBS for depression is not FDA approved and should be performed only in well-controlled research studies. Because the functional neuroanatomy of depression is not nearly as well known as that for Parkinson's disease or dystonia, a great deal of caution is needed in this area. Psychiatrists should not re-create the mistakes of the lobotomy years, where overenthusiastic adoption of an invasive technology ruined many lives and damaged the reputation of a field.
tDCS is a brain stimulation technique achieved through application of constant weak (typically <1 mA) electrical current through scalp electrodes. Unlike TMS, tDCS does not elicit action potentials in cortical neurons. Instead, the constant direct current of tDCS induces subthreshold changes in membrane potential that increase or decrease the ease with which an action potential may be triggered (Leung et al. 2009). These changes in cortical excitability were first documented using motor potentials evoked with TMS. Although tDCS is currently just an investigational tool, studies have shown that it may also have therapeutic applications.
Although the first formal use of modern tDCS is debatable, brain stimulation techniques that use similar principles have been employed for over two centuries. With the eighteenth century advent of the capacitor, known as a Leyden jar, experiments gradually began to support Luigi Galvani's concept of animal electricity over Alessandro Volta's concept of bimetallic electricity or Franz Mesmer's concept of animal magnetism. Coining the term galvanism, Galvani's precocious nephew Giovanni Aldini began exploring the effects of direct current on decapitated livestock and corpses. Shortly after his reanimation experiments, Aldini began applying galvanism to ameliorate "melancholy madness" in hospital patients. Although it is likely that Aldini also employed various waveforms other than constant direct current, his macabre methodology undoubtedly contributed to the development of tDCS-like techniques. One such technique was pioneered by Georges Duchenne de Boulogne, a neurologist who used a small generator and battery to examine the effects of direct current on muscle contraction and disorders such as muscular dystrophy (George 1994). When Duchenne's historic experiments failed to reveal clinical applications for direct current stimulation, interest in the technique waned. Outside of a few studies on "electrosleep therapy" in the early twentieth century, largely in Russia, tDCS was ignored until Walter Paulus and colleagues began investigating its effects on neurophysiology.
There are currently no FDA-approved therapeutic uses for tDCS. Nevertheless, tDCS remains an active area of research because of the ease with which it can be applied and the flexibility derived from its various potential electrode placements. A small current source and damp sponges (electrodes) are all that is required for tDCS administration. This portable, safe, inexpensive setup is particularly alluring because it may enable tDCS to be used in nontraditional ways, including in a patient's home or during therapeutic interventions such as cognitive-behavioral therapy or rehabilitation exercises. tDCS has the capacity to alter cortical excitability. The next step is to determine how physicians might use this form of noninvasive neuromodulation for therapeutic purposes.
Thus far, the most promising effects of tDCS have been found in rehabilitation studies. Although more RCTs are needed, there is emerging evidence that tDCS may enhance poststroke aphasia rehabilitation therapies. A few studies have begun to explore the circuitry changes induced by tDCS, but precise mechanisms remain largely unknown. There is also evidence to suggest that tDCS augments poststroke motor recovery, but these studies frequently feature small sample sizes, heterogenous outcome measures, and mixed results (Bastani and Jaberzadeh 2012). These limitations also apply to studies investigating tDCS as a treatment for conditions such as pain and depression (Kalu et al. 2012). Although tDCS shows some promise for many different clinical applications, insufficient evidence currently exists for any conclusions to be reached.
A number of unresolved issues need to be addressed before tDCS can be thoroughly evaluated as a therapeutic tool. One of the most fundamental issues with tDCS is the lack of clarity regarding the neurophysiological effects produced by each electrode. When tDCS is applied, direct current typically flows from energy source to anode to cathode. Although most studies have found that an-odal and cathodal stimulation normally increase and decrease cortical excitability, respectively, others have found circumstances under which these polarity effects may be reversed. More studies are needed to clarify how brain complexities such as neuronal morphology and network connectivity affect neuronal response to tDCS.
Another unresolved tDCS issue pertains to dose titration. Unlike TMS, which can be titrated based on motor threshold, tDCS is not typically tailored for each individual patient. It may be the case that experimental trials fail to find significant behavioral results because the dose of tDCS used in many individuals is insufficient for the induction of durable changes in neural excitability. Once tDCS doses can be titrated, then studies can begin to explore tDCS in a more systematic way.
Moreover, there are no dose finding studies or even systematic safety studies with tDCS. The current limit of 2 mA is largely based on consensus and the potential for scalp burns, but higher doses are possible and are probably safe.
Brain stimulation is a fertile and rapidly growing field, with additional brain stimulation techniques emerging almost monthly. In general, it is best to adopt an open but skeptical approach to these treatments. Although the techniques might work, rigorous RCTs are needed before adoption.
There are several different small portable stimulation methods, such as transcutaneous electrical nerve stimulator (TENS) units, that stimulate peripherally and purport to change brain function. These use biphasic, low-voltage current and selectable parameters such as pulse rate and pulse width to stimulate sensory nerves to block pain signals and theoretically alter brain function. Broad neuropsychiatric therapeutic claims about this class are bandied about on the Internet and in advertisements, but these claims are not based on rigorous RCT data. Some devices in this group are designed to stimulate the earlobe, which has complex afferent fibers (including branches of the vagus nerve). Some of these devices were in production when the legislation creating the devices branch of the FDA was approved, and they were "grandfathered" in, without clear evidence of efficacy.
The brain stimulation therapies are now an established part of the psychiatrist's toolbox. This is a rapidly expanding area that holds promise for even more advances in terms of treatments for neuropsychiatric conditions.
Key Clinical Points
* The authors' work with brain stimulation treatments has been supported over the past 5 years in part by research grants from NIH, DOD, VA, and NARSAD. The Brain Stimulation Laboratory has received grant funding from Brainsway, Cervel (Neurostim), Neosync, Neuronetics, Neuropace, MECTA, and St. Jude. Dr. George serves or has served as a paid consultant to several non-TMS device and pharmaceutical companies. He owns no equity in any device or pharmaceutical company. The other authors report no conflicts and have nothing to disclose.
Alonso P, Pujol J, Cardoner N, et al: Right prefrontal TMS in OCD: a double-blind, placebo-controlled study. Am J Psychiatry 158:1143-1145, 2001
Andre-Obadia N, Peyron R, Mertens P, et al: Transcranial magnetic stimulation for pain control: double-blind study of different frequencies against placebo, and correlation with motor cortex stimulation efficacy. Clin Neurophysiol 117:1536-1544, 2006
Baeken C, De Raedt R, Bossuyt A, et al: The impact of HF-rTMS treatment on serotonin A) receptors in unipolar melancholic depression. Brain Stimul 4:104-111, 2011
Barker AT, Jalinous R, Freeston IL: Noninvasive magnetic stimulation of the human motor cortex. Lancet 1:1106-1107, 1985
Bastani A, Jaberzadeh S: Does anodal transcranial direct current stimulation enhance excitability of the motor cortex and motor function in healthy individuals and subjects with stroke? a systematic review and meta-analysis. Clin Neurophysiol 123:644-657, 2012
Beer B: Uber das Auftretten einer objectiven Lichtempfindung in magnetischen Felde. Klinische Wochenzeitschrift 15:108-109, 1902
Ben-Menachem E, Manon-Espaillat R, Ristanovic R, et al: Vagus nerve stimulation for treatment of partial seizures, 1: a controlled study of effect on seizures. Epilepsia 35:616-626, 1994
Ben-Sachar D, Belmaker RH, Grisaru N, et al: Transcranial magnetic stimulation induces alterations in brain monoamines. J Neural Transm 104:191-197, 1997
Bodenlos JS, Borckardt JJ, George MS: Vagus nerve stimulation and food cravings: a response to Gibson and Mohiyeddini. Appetite 51:226-228, 2008
Bohning DE: Introduction and overview of TMS physics, in Transcranial Magnetic Stimulation in Neuropsychiatry. Edited by George MS, Belmaker RH. Washington, DC, American Psychiatric Press, 2000, pp 13-44
Bohning DE, Shastri A, Nahas Z, et al: Echo-planar BOLD fMRI of brain activation induced by concurrent transcranial magnetic stimulation (TMS). Invest Radiol 33:336-340, 1998
Bohning DE, Lomarev MP, Denslow S, et al: Feasibility of vagus nerve stimulation-synchronized blood oxygenation level-dependent functional MRI. Invest Radiol 36:470-479, 2001
Borckardt JJ, Kozel FA, Anderson B, et al: Vagus nerve stimulation affects pain perception in depressed adults. Pain Res Manag 10:9-14, 2005
Borckardt JJ, Anderson B, Kozel FA, et al: Acute and long-term VNS effects on pain perception in a case of treatment-resistant depression. Neurocase 12:216-220, 2006a
Borckardt JJ, Weinstein M, Reeves ST, et al: Postoperative left prefrontal repetitive transcranial magnetic stimulation (rTMS) reduces patient-controlled analgesia use. Anesthesiology 105:557-562, 2006b
Busch V, Zeman F, Heckel A, et al: The effect of transcutaneous vagus nerve stimulation on pain perception: an experimental study. Brain Stimul 6:202-209, 2013
Bystritsky A, Korb AS, Douglas PK, et al: A review of low-intensity focused ultrasound pulsation. Brain Stimul 4:125-136, 2011
Carpenter LL, Janicak PG, Aaronson ST, et al: Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety 29:587-596, 2012
Chae JH, Nahas Z, Lomarev M, et al: A review of functional neuroimaging studies of vagus nerve stimulation (VNS). J Psychiatr Res 37:443-455, 2003
Cohen E, Bernardo M, Masana J, et al: Repetitive transcranial magnetic stimulation in the treatment of chronic negative schizophrenia: a pilot study. J Neurol Neurosurg Psychiatry 67:129-130, 1999
Connolly RK, Helmer A, Cristancho MA, et al: Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J Clin Psychiatry 73:e567-e573, 2012
Conway CR, Sheline YI, Chibnall JT, et al: Cerebral blood flow changes during vagus nerve stimulation for depression. Psychiatry Res 146:179-184, 2006
Conway CR, Sheline YI, Chibnall JT, et al: Brain blood-flow change with acute vagus nerve stimulation in treatment-refractory major depressive disorder. Brain Stimul 5:163-171, 2012
Davey NJ, Puri BK: On electromyographic responses to transcranial magnetic stimulation of the motor cortex in schizophrenia. J Neurol Neurosurg Psychiatry 63:468-473, 1997
Davey NJ, Puri BK, Lewis HS, et al: Effects of antipsychotic medication on electromyographic responses to transcranial magnetic stimulation of the motor cortex in schizophrenia. J Neurol Neurosurg Psychiatry 63:468-473, 1997
Demitrack MA, Thase ME: Clinical significance of transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant depression: synthesis of recent data. Psychopharmacol Bull 42:5-38, 2009
Deng ZD, Lisanby SH, Peterchev AV: Electric field depth-focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul 6(1):1-13, 2013
Di Lazzaro V, Pilato F, Saturno E, et al: Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol 565:945-950, 2005
Eiger G, Hoppe C, Falkai P, et al: Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res 42:203-210, 2000
Feinsod M, Sreinin B, Chistyhakov A, et al: Preliminary evidence for a beneficial effect of low-frequency, repetitive transcranial magnetic stimulation in patients with major depression and schizophrenia. Depress Anxiety 7:65-68, 1998
Fleischmann A, Sternheim A, Etgen AM, et al: Transcranial magnetic stimulation downregulates beta-adrenoreceptors in rat cortex. J Neural Transm 103:1361-1366, 1996
Foley JO, Dubois F: Quantitative studies of the vagus nerve in the cat, I: the ratio of sensory and motor studies. J Comp Neurol 67:49-67, 1937
Gabriels L, Cosyns P, Nuttin B, et al: Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: psychopathological and neuropsychological outcome in three cases. Acta Psychiatr Scand 107:275-282, 2003
George MS: Reanimating the face: early writings by Duchenne and Darwin on the neurology of facial emotion expression. J Hist Neurosci 3:21-33, 1994
George MS, Bohning DE: Measuring brain connectivity with functional imaging and transcranial magnetic stimulation (TMS), in Neuropsychopharmacology: Fifth Generation of Progress. Edited by Desimone B. New York, Lippincott, Williams & Wilkins, 2002, pp 393-410
George MS, Wassermann EM: Rapid-rate transcranial magnetic stimulation (rTMS) and ECT. Convuls Ther 10:251-253, 1994
George MS, Ketter TA, Post RM: Prefrontal cortex dysfunction in clinical depression. Depression 2:59-72, 1994
George MS, Post RM, Ketter TA, et al: Neural mechanisms of mood disorders, in Current Review of Mood Disorders. Edited by Rush AJ. Philadelphia, PA, Current Medicine, 1995a
George MS, Wassermann EM, Williams WA, et al: Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuro Report 6:1853-1856, 1995b
George MS, Ketter TA, Post RM: What functional imaging studies have revealed about the brain basis of mood and emotion, in Advances in Biological Psychiatry. Edited by Panksepp J. Greenwich, CT, JAI Press, 1996, pp 63-113
George MS, Wassermann EM, Williams WE, et al: Mood improvements following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: a placebo-controlled crossover trial. Am J Psychiatry 154:1752-1756, 1997
George MS, Stallings LE, Speer AM, et al: Prefrontal repetitive transcranial magnetic stimulation (rTMS) changes relative perfusion locally and remotely. Hum Psychopharmacol 14:161-170, 1999
George MS, Sackeim HA, Rush AJ, et al: Vagus nerve stimulation: a new tool for brain research and therapy. Biol Psychiatry 47:287-295, 2000
George MS, Rush AJ, Marangell LB, et al: A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol Psychiatry 58:364-373, 2005
George MS, Ward HE, Ninan PT, et al: A pilot study of vagus nerve stimulation (VNS) for treatment-resistant anxiety disorders. Brain Stimul 1:112-121, 2008
George MS, Lisanby SH, Avery D, et al: Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry 67:507-516, 2010
Greenberg BD, George MS, Dearing J, et al: Effect of prefrontal repetitive transcranial magnetic stimulation (rTMS) in obsessive-compulsive disorder: a preliminary study. Am J Psychiatry 154:867-869, 1997
Greenberg BD, Murphy DL, Rasmussen SA: Neuroanatomically based approaches to obsessive-compulsive disorder: neurosurgery and transcranial magnetic stimulation. Psychiatr Clin North Am 23:671-686, 2000
Greenberg BD, Price LH, Rauch SL, et al: Neurosurgery for intractable obsessive-compulsive disorder and depression: critical issues. Neurosurg Clin North Am 14:199-212, 2003
Greenberg BD, Gabriels LA, Malone DA Jr, et al: Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry 15:64-79, 2008
Grisaru N, Yarovslavksy U, Abarbanel J, et al: Transcranial magnetic stimulation in depression and schizophrenia. Eur Neuropsychopharmacol 4:287-288, 1994
Grisaru N, Amir M, Cohen H, et al: Effect of transcranial magnetic stimulation in post-traumatic stress disorder: a preliminary study. Biol Psychiatry 44:52-55, 1998
Halpem C, Hurtig H, Jaggi J, et al: Deep brain stimulation in neurologic disorders. Parkinsonism Relat Disord 13:1-16, 2007
Handforth A, Degiorgio CM, Schachter SC, et al: Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology 51:48-55, 1998
Hanlon C, Centerberry M: The use of brain imaging to elucidate neuronal circuit changes in cocaine addiction. Subst Abuse Rehabil 3:115-128, 2012
Harden CL, Pulver MC, Ravdin LD, et al: A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav 1:93-99, 2000
Heide G, Witte OW, Ziemann U: Physiology of modulation of motor cortex excitability by low-frequency suprathreshold repetitive transcranial magnetic stimulation. Exp Brain Res 171:26-34, 2006
Helmers SL, Wheless JW, Frost M, et al: Vagus nerve stimulation therapy in pediatric patients with refractory epilepsy: retrospective study. J Child Neurol 16:843-848, 2001
Henry TR, Bakay RA, Votaw JR, et al: Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy, I: acute effects at high and low levels of stimulation. Epilepsia 39:983-990.1998
Herwig U, Padberg F, Unger J, et al: Transcranial magnetic stimulation in therapy studies: examination of the reliability of "standard" coil positioning by neuronavigation. Biol Psychiatry 50:58-61, 2001
Hoffman R, Boutros N, Berman R, et al: Transcranial magnetic stimulation and hallucinated "voices." Biol Psychiatry 46:130-132.1998
Holtzheimer PE III, McDonald WM, Mufti M, et al: Accelerated repetitive transcranial magnetic stimulation for treatment-resistant depression. Depress Anxiety 27:960-963, 2010
Huang Y, Sommer M, Thickbroom GW, et al: Consensus: new methodologies for brain stimulation. Brain Stimul 2:2-13, 2009
Isserles M, Shalev AY, Roth Y, et al: Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder: a pilot study. Brain Stimul 6(3):377-383, 2013
Kalu UG, Sexton CE, Loo CK, et al: Transcranial direct current stimulation in the treatment of major depression: a metaanalysis. Psychol Med 42:1791-1800, 2012
Kayser S, Bewernick B, Axmacher N, et al: Magnetic seizure therapy of treatment-resistant depression in a patient with bipolar disorder. J ECT 25:137-140, 2009
Kolbinger HM, Hoflich G, Hufnagel A, et al: Transcranial magnetic stimulation (TMS) in the treatment of major depression: pilot study. Hum Psychopharmacol 10:305-310, 1995
Kozel FA, Nahas Z, Debrux C, et al: How coil-cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neuro-sci 12:376-384, 2000a
Kozel FA, Nahas Z, Debrux C, et al: How the distance from coil to cortex relates to age, motor threshold and possibly the antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci 12:376-384, 2000b
Kumar R, Fozano AM, Kim YJ, et al: Doubleblind evaluation of subthalamic nucleus deep brain stimulation in advanced Parkinson's disease. Neurology 51:850-855, 1998
LaLumiere RT: A new technique for controlling the brain: optogenetics and its potential for use in research and the clinic. Brain Stimul 4:1-6, 2011
Leung A, Donohue M, Xu R, et al: rTMS for suppressing neuropathic pain: a metaanalysis. J Pain 10:1205-1216, 2009
Li X, Teneback CC, Nahas Z, et al: Lamotrigine inhibits the functional magnetic resonance imaging response to transcranial magnetic stimulation in healthy adults (abstract). Biol Psychiatry 51(suppl):S67, 2002
Li X, Nahas Z, Anderson B, et al: Can left prefrontal rTMS be used as a maintenance treatment for bipolar depression? Depress Anxiety 20:98-100, 2004a
Li X, Teneback CC, Nahas Z, et al: Interleaved transcranial magnetic stimulation/functional MRI confirms that lamotrigine inhibits cortical excitability in healthy young men. Neuropsychopharmacology 29:1395-1407, 2004b
Limousin P, Poliak P, Benazzouz A, et al: Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet 345:91-95, 1995
Limousin P, Krack P, Poliak P, et al: Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med 339:1105-1111, 1998
Lisanby SH, Moscrip T, Morales O, et al: Neurophysiological characterization of magnetic seizure therapy (MST) in nonhuman primates. Suppl Clin Neurophysiol 56:81-99, 2003
Lozano AM, Mayberg HS, Giacobbe P, et al: Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry 64:461-467, 2008
Malone DA Jr, Dougherty DD, Rezai AR, et al: Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry 65:267-275, 2009
Mantovani A, Lisanby SH, Pieraccini F, et al: Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive-compulsive disorder (OCD) and Tourette's syndrome (TS). Int J Neuro-psychopharmacol 9:95-100, 2006
Mantovani A, Pavlicova M, Avery D, et al: Long-term efficacy of repeated daily prefrontal transcranial magnetic stimulation (TMS) in treatment-resistant depression. Depress Anxiety 29:883-890, 2012
Mayberg HS, Liotti M, Brannan SK, et al: Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 156:675-682, 1999
Mayberg HS, Lozano AM, Voon V, et al: Deep brain stimulation for treatment-resistant depression. Neuron 45:651-660, 2005
McCann UD, Kimbrell TA, Morgan CM, et al: Repetitive transcranial magnetic stimulation for posttraumatic stress disorder (letter). Arch Gen Psychiatry 55:276-279, 1998
McDonald WM, Durkalski V, Ball ER, et al: Improving the antidepressant efficacy of transcranial magnetic stimulation: maximizing the number of stimulations and treatment location in treatment-resistant depression. Depress Anxiety 28:973-980, 2011
McNeely HE, Mayberg HS, Lozano AM, et al: Neuropsychological impact of Cg25 deep brain stimulation for treatment-resistant depression: preliminary results over 12 months. J Nerv Ment Dis 196:405M10, 2008
Miner JR, Lewis LM, Mosnaim GS, et al: Leasibility of percutaneous vagus nerve stimulation for the treatment of acute asthma exacerbations. Acad Emerg Med 19:421-429, 2012
Mishory A, Molnar C, Koola J, et al: The maximum-likelihood strategy for determining transcranial magnetic stimulation motor threshold, using parameter estimation by sequential testing is faster than conventional methods with similar precision. J ECT 20:160-165, 2004
Mitchel P: 15 Hz and 1 Hz TMS have different acute effects on cerebral blood flow in depressed patients. Int J Neuropsychopharmacol 5:S7-S.08.02, 2002
Nahas Z, Oliver NC, Johnson M, et al: Feasibility and efficacy of left prefrontal rTMS as a maintenance antidepressant (abstract). Biol Psychiatry 47 (suppl):S156-S157, 2000
Nahas Z, Lomarev M, Roberts DR, et al: Unilateral left prefrontal transcranial magnetic stimulation (TMS) produces intensity-dependent bilateral effects as measured by interleaved BOLD fMRI. Biol Psychiatry 50:712-720, 2001
Nahas Z, Marangell LB, Husain MM, et al: Two-year outcome of vagus nerve stimulation (VNS) therapy for major depressive episodes. J Clin Psychiatry 66:1097-1104, 2005
Nahas Z, Anderson BS, Borckardt J, et al: Bilateral epidural prefrontal cortical stimulation for treatment-resistant depression. Biol Psychiatry 67:101-109, 2010
Nahas Z, Short B, Burns C, et al: A feasibility study of a new method for electrically producing seizures in man: focal electrically administered seizure therapy (FEAST). Brain Stimul 6:403-408, 2013
Nobler MS, Sackeim HA, Prohovnik I, et al: Regional cerebral blood flow in mood disorders, III: treatment and clinical response. Arch Gen Psychiatry 51:884—897, 1994
Nobler MS, Oquendo MA, Kegeles LS, et al: Decreased regional brain metabolism after ECT. Am J Psychiatry 158:305-308, 2001
Nuttin B, Cosyns P, Demeulemeester H, et al: Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet 354:1526, 1999
Nuttin B, Gabriels LA, Cosyns PR, et al: Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery 52:1263-1272, 2003
O'Reardon JP, Blumner KH, Peshek AD, et al: Long-term maintenance therapy for major depressive disorder with rTMS. J Clin Psychiatry 66:1524-1528, 2005
O'Reardon JP, Solvason HB, Janicak PG, et al: Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry 62:1208-1216, 2007
Paulus W, Classen J, Cohen LG, et al: State of the art: pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimul 1:151-163, 2008
Paus T, Castro-Alamancos MA, Petrides M: Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. Eur J Neurosci 14:1405-1411, 2001
Penry JK, Dean JC: Prevention of intractable partial seizures by intermittent vagal nerve stimulation in humans: preliminary results. Epilepsy 3T.S40-S43, 1990
Pope A, Keck ME: Transcranial magnetic stimulation as a therapeutic tool in psychiatry: what do we know about neuro-biological mechanisms? J Psychiatr Res 35:193-215, 2001
Pridmore S, Oberoi G: Transcranial magnetic stimulation applications and potential use in chronic pain: studies in waiting. J Neurol Sci 182:1-4, 2000
Pridmore S, Fernandes Filho JA, Nahas Z, et al: Motor threshold in transcranial magnetic stimulation: a comparison of a neurophysiological method and a visualization of movement method. J ECT 14:25-27, 1998
Prudic J, Olfson M, Marcus SC, et al: Effectiveness of electroconvulsive therapy in community settings (see comment). Biol Psychiatry 55:301-312, 2004
Puri BK, Davey NJ, Ellaway PH, et al: An investigation of motor function in schizophrenia using transcranial magnetic stimulation of the motor cortex. Br J Psychiatry 169:690-695, 1996
Roslin M, Kurian M: The use of electrical stimulation of the vagus nerve to treat morbid obesity. Epilepsy Behav 2:S11-S16, 2001
Rossi S, Hallett M, Rossini PM, et al: Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120:2008-2039, 2009
Roth BJ, Cohen LG, Hallett M: The electric field induced during magnetic stimulation. Electroencephalogr Clin Neurophysiol Suppl 43:268-278, 1991
Roth Y, Zangen A, Voller B, et al: Transcranial magnetic stimulation of deep brain regions: evidence for efficacy of the H-coil. Clin Neurophysiol 116:775-779, 2005
Rush AJ, George MS, Sackeim HA, et al: Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry 47:276-286, 2000
Rush AJ, Marangell LB, Sackeim HA, et al: Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry 58:347-354, 2005
Rush AJ, Trivedi MH, Wisniewski SR, et al: Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med 354:1231-1242, 2006
Sachdev PS, McBride R, Loo CK, et al: Right versus left prefrontal transcranial magnetic stimulation for obsessive-compulsive disorder: a preliminary investigation. J Clin Psychiatry 62:981-984, 2001
Sackeim HA: Magnetic stimulation therapy and ECT. Convuls Ther 10:255-258, 1994
Sackeim HA, Prudic J, Devanand DP, et al: Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy (see comments). N Engl J Med 328:839-846, 1993
Sackeim HA, Keilp JG, Rush AJ, et al: The effects of vagus nerve stimulation on cognitive performance in patients with treatment-resistant depression. Neuropsychiatry Neuropsychol Behav Neurol 14:53-62, 2001a
Sackeim HA, Rush AJ, George MS, et al: Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 25:713-728, 2001b
Sackeim HA, Haskett RF, Mulsant BH, et al: Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. JAMA 285:1299-1307, 2002
Sackeim HA, Prudic J, Nobler MS, et al: Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul 1:71-83, 2008
Sackeim HA, Dillingham EM, Prudic J, et al: Effect of concomitant pharmacotherapy on electroconvulsive therapy outcomes: short-term efficacy and adverse effects. Arch Gen Psychiatry 66:729-737, 2009
Schlaepfer TE: Deep brain stimulation to structures of the reward system: hypotheses and results (abstract 694). Presented at the 67th Annual Scientific Convention & Program of the Society for Biological Psychiatry, Philadelphia, PA, May 3-5, 2012
Schlaepfer TE, Cohen MX, Frick C, et al: Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 33:368-377, 2008a
Schlaepfer TE, Frick C, Zobel A, et al: Vagus nerve stimulation for depression: efficacy and safety in a European study. Psychol Med 38:651-661, 2008b
Shajahan PM, Glabus MF, Steele JD, et al: Left dorsolateral repetitive transcranial magnetic stimulation affects cortical excitability and functional connectivity, but does not impair cognition in major depression. Prog Neuropsychopharmacol Biol Psychiatry 26:945-954, 2002
Short EB, Borckardt JJ, Anderson BS, et al: Ten sessions of adjunctive left prefrontal rTMS significantly reduces fibromyalgia pain: a randomized, controlled pilot study. Pain 152:2477-2484, 2011
Spellman T, Peterchev AV, Lisanby SH: Focal electrically administered seizure therapy: a novel form of ECT illustrates the roles of current directionality, polarity, and electrode configuration in seizure induction. Neuropsychopharmacology 34:2002-2010, 2009
Stanton PK, Sejnowsky TJ: Associative long-term depression in the hippocampus induced by hebbian covariance. Nature 339:215-218, 1989
Stefan H, Kreiselmeyer G, Kerling F, et al: Transcutaneous vagus nerve stimulation (t-VNS) in pharmacoresistant epilepsies: a proof of concept trial. Epilepsia 53:ell5-ell8, 2012
Strafella AP, Paus T, Fraraccio M, et al: Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain 126:2609-2615, 2003
Taylor JJ, Borckardt JJ, George MS: Endogenous opioids mediate left dorsolateral prefrontal cortex rTMS-induced analgesia. Pain 153:1219-1225, 2012
Teneback CC, Nahas Z, Speer AM, et al: Two weeks of daily left prefrontal rTMS changes prefrontal cortex and paralimbic activity in depression. J Neuropsychiatry Clin Neurosci 11:426-435, 1999
Tisch S, Rothwell JC, Limousin P, et al: The physiological effects of pallidal deep brain stimulation in dystonia. IEEE Trans Neural Syst Rehabil Eng 15:166-172, 2007
U.S. Food and Drug Administration: Approval Order: Reclaim™ Deep Brain Stimulation for Obsessive Compulsive Disorder (OCD) Therapy—H050003. February 19, 2009. Available at: http://www.fda.gov/MedicalDevices/Prod-uctsandMedicalProcedures/Device-ApprovalsandClearances/Recently-ApprovedDevices/ucml25520.htm. Accessed August 4, 2013.
Vedeniapin A, Cheng L, George MS: Feasibility of simultaneous cognitive behavioral therapy and left prefrontal rTMS for treatment resistant depression. Brain Stimul 3:207-210, 2010
Wassermann EM: Report on risk and safety of repetitive transcranial magnetic stimulation (rTMS): suggested guidelines from the International Workshop on Risk and Safety of rTMS (June 1996). Electroencephalogr Clin Neurol 108:1-16, 1997
Watts BV, Landon B, Groft A, et al: A sham controlled study of repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Brain Stimul 5:38-43, 2012
Zabara J: Inhibition of experimental seizures in canines by repetitive vagal stimulation. Epilepsia 33:1005-1012, 1992
Zardetto-Smith AM, Gray TS: Organization of peptidergic and catecholaminergic efferents from the nucleus of the solitary tract to the rat amygdala. Brain Res Bull 25:875-887, 1990
George MS: Stimulating the brain. Sci Am 289:66-73, 2003
Higgins ES, George MS: Brain Stimulation Therapies for Clinicians. Washington, DC, American Psychiatric Publishing, 2008
Sackeim HA, George MS: Brain Stimulation: Basic, Translational and Clinical Research in Neuromodulation: why a new journal? Brain Stimul 1:4-6, 2008
Schlaepfer TE, George MS, Mayberg H: WFSBP Guidelines on Brain Stimulation Treatments in Psychiatry. World J Biol Psychiatry 11:2-18, 2010
George MS, Belmaker RH: Transcranial Magnetic Stimulation in Clinical Psychiatry. Washington, DC, American Psychiatric Publishing, 2006
George MS, Post RM: Daily left prefrontal repetitive transcranial magnetic stimulation for acute treatment of medication-resistant depression. Am J Psychiatry 168:356-364, 2011
George MS, Lisanby SH, Avery D, et al: Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry 67:507-516, 2010
Henry TR: Therapeutic mechanisms of vagus nerve stimulation. Neurology 59:S15-S20, 2002
Brain Stimulation Lab at Medical University of South Carolina: http://academic-departments.musc.edu/psychiatry/research/bsl
Brain Stimulation Journal Home Page: www.brainstimjrnl.com/home