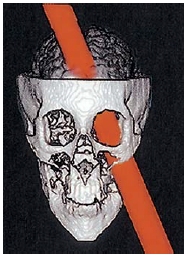
FIGURE 13.1 This computer reconstruction shows how the tamping iron passed through Phineas Gage’s brain. The iron entered just below the left eye and exited from the top. It destroyed much of the medial region of the prefrontal cortex.

|
The one thing that unites all human beings, regardless of age, gender, religion, economic status, or ethnic background, is that, deep down inside, we all believe that we are above-average drivers. ~ Dave Barry |
Chapter 13
Social Cognition
OUTLINE
Anatomical Substrates of Social Cognition
Deficits
Socrates’ Imperative: Know Thyself
Theory of Mind: Understanding the Mental States of Others
Social Knowledge
WHEN PRESENTED WITH the perfect storm of high speed and an immovable solid object, the brain, sitting within its bony confines, is protected by the skull’s armor only to a limited degree. Unfortunately for patient M.R., who crashed his motor- cycle into an inanimate object, the result was extensive damage to his orbitofrontal cortex (OFC), the portion of the frontal lobes that rests behind the eye orbits. Evolutionary history also contributed to this injury. The human skull has evolved sockets, cavities for the eyes surrounded by jagged bony ridges, that provide protective support for the eyeball and its appendages. In the aftermath of the sort of high-speed collision that is associated with modern-day vehicles, however, these ridges can become essentially like a set of knives slicing away brain tissue (see Figure 3.9). M.R.’s injury is commonly known as a coup-contra-coup injury. It occurs when impact causes the brain first to bounce against the back of the skull and then rebound. Coup-contra-coup injuries are especially pronounced in the orbitofrontal cortex because of the jagged ridges around the eye sockets.
Surprisingly, despite his extensive brain damage, M.R. does well on standard neuropsychological tests of memory, motor, and language skills. Why is it surprising? Because even in a casual conversation with him, something about his behavior is amiss: It is socially inappropriate, a common result of orbitofrontal damage like M.R.’s (Beer et al., 2003). Patients with this type of damage might choose to discuss personal topics with a complete stranger or talk endlessly about topics that clearly bore their conversation partner. Although you may have had the latter experience during a recent date (possibly giving you a hint about your date’s OFC function), no doubt you were not as bored as you might be when talking with M.R., who would readily provide a detailed account of each and every cut he recently used to trim a bonsai tree. Orbitofrontal patients might greet a stranger with a hug, sit a little too close for comfort, or stare just a little too long. Other changes often associated with this type of lesion include less inhibition, lower tolerance of frustration, increased aggression, immaturity, apathy, and emotional coldness.
Cases of orbitofrontal damage are certainly not new in the history of neuroscience. The most famous case, familiar to most neuroscience students, occurred in June of 1848. Phineas Gage, the foreman of a railroad construction crew, made a mistake that would forever change his life. One of Gage’s responsibilities was to set up controlled explosions to blast through rock so that railroad tracks could be laid over a smooth surface. For each explosion, Gage made a small hole in the rock and filled it with explosive powder and a fuse. He then covered the explosive powder with sand and patted it down with a tamping iron. On that day, however, he failed to notice that some of the explosive powder was uncovered. The iron set off a spark that ignited the exposed powder. The explosion made the tamping iron blast off into space like a rocket. Unfortunately, Gage was standing in its path. The tamping iron passed through his skull, entering just below the left eye and exiting at the top of his head, and created a large hole in his orbitofrontal cortex (Figure 13.1).
Amazingly, Gage remained conscious and seemed quite alert. He even greeted the town physician, Dr. Harlow. Though his physical wounds healed after a few months, Gage was never the same. His friends said he was “‘No longer Gage’” (MacMillan, 2000, p. 13). Harlow described the postinjury Gage as “irreverent, indulging in the grossest profanity (which was not previously his custom), manifesting little deference to his fellows, impatient of restraint or advice when it conflicts with his desires.” Another physician noted that Gage’s “society was intolerable to decent people” (MacMillan, 1986).

FIGURE 13.1 This computer reconstruction shows how the tamping iron passed through Phineas Gage’s brain. The iron entered just below the left eye and exited from the top. It destroyed much of the medial region of the prefrontal cortex.
Although Gage had been a respected citizen, exemplary worker, and well-liked man, he became a different man after his injury. His employers with the railroad soon fired him. Many fantastic stories have been told about Gage’s life after he healed from his injury. Some of these stories suggest that Gage was never able to hold a steady job and even traveled with Barnum’s freak show for a time. Malcolm MacMillan at Deakin University in Australia (2000) has spent years investigating Gage’s life. He has uncovered documentation reporting that Gage spent most of his postinjury life employed as a stagecoach driver both in the United States and in Chile. Gage eventually moved to San Francisco to live with his mother, and he died there. Although his preinjury life had been filled with promise, he never again held a job as prestigious as railroad foreman.
Perhaps the most famous modern Gage-like patient is E.V.R. (Eslinger & Damasio, 1985). He lost most of his ventromedial prefrontal cortex (VMPFC), which included his OFC, when it was resected to remove a large tumor—a meningioma that extended bilaterally. After surgery, E.V.R. too was not his old self. Unable to maintain his profession or his family, he ended up bankrupt and divorced. Like M.R., he also tested normally on his neuropsychological tests despite huge changes in his social functioning and decision-making abilities. Over the years, more patients with similar lesions have been identified and studied. Typical findings in such patients include blunted affect, poor frustration tolerance, impaired goal-directed behavior, inappropriate social conduct and lack of insight into these changes (Barrash et al., 2000), impaired autonomic response to emotional pictures and emotional memories (Damasio, 1990), and diminished regret.
Only lately have scientists begun to tackle the problem of why some kinds of brain damage impair social behavior while sparing other complex cognitive abilities. As recently as 2002, media headlines boasted, “Man speared in head survives because spear passed through a place in the brain that is non-functional.” Much like Phineas Gage, this man survived having his orbitofrontal cortex pierced. Although damage to this region does not impair performance on many cognitive tests, the dysfunctional social behavior of patients like M.R., E.V.R., and Phineas Gage make it clear that the orbitofrontal cortex is anything but nonfunctional.
Humans are party animals. We have taken sociality to a level unheard of in the animal world by helping and cooperating in a reciprocal way with other people, both relatives and those unrelated to us. Compared to those of other animals, something is different about our brains that allows us to be so social. Social cognitive neuroscience is a new field that aims to tackle the problem of understanding how brain function supports the cognitive processes underlying social behavior. It differs from cognitive neuroscience in that it emphasizes that situations or contexts determine how we think or act (Ochsner, 2007), and those situations usually involve other people. Social interactions are an essential aspect of being human. Obviously, for a social interaction, it takes at least two to tango. Through interactions involving romantic partners, friends, family, and coworkers—even, quite frequently, strangers—we form a sense of self and also develop impressions of other people.
For us to get the tango straight, we have to understand both partners. This chapter discusses social cognitive neuroscience research concerning the neural representation of self, other people, and social knowledge and procedures. We begin with a bit about the anatomical structures that are involved in self–other processing and discuss autism, a developmental disorder, that results in social deficits. Next we turn to you—or rather, the sense of self and how you get to know yourself. Then we investigate how you get to know others. We consider whether learning about others and learning about ourselves are similar processes that involve the same neural substrates. Understanding ourselves and other people, however, is only part of successfully navigating our social worlds. We also need to learn social rules and use them to guide our behavior. How do we make decisions that are guided by social knowledge? What can the brain tell us about the psychological functions that might be involved in this process? The answers to these questions will give us insight into our everyday experiences. Note that social responses, including facial expressions, social group evaluation, and racial stereotyping—which are all considered to be social cognitive neuroscience topics—were covered in Chapter 10, which focused on emotion.
TAKE-HOME MESSAGES
Anatomical Substrates of Social Cognition
Does the processing of information about others and about ourselves happen in separate brain regions, overlapping regions, or all in the same place? Welcome to an active debate! When identifying brain regions that are concerned with self-referential processing—such as when you think about your personal traits, beliefs and desires, your past, and so forth—we encounter an interesting problem. Even though philosophers, theologians, clergy, and scientists have batted about the concept of self for thousands of years, no all-encompassing definition of self exists. What’s more, a lot of evidence suggests there is no single brain region we can point to and say, “This is where the self is located.” Increasingly, it looks like the self is a pastiche: It is made up of separable processes, full of separable content from a vast supply of sources, both from within and without the brain and the body. Lose a process, and you lose a part of your old self and turn into a new one, who may be quite different. Phineas Gage’s old friends may have considered him as no longer his old self, but he did have a self—just one that was different.
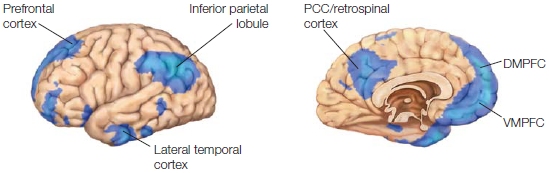
Regions of the prefrontal cortex (PFC) are a primary focus in this chapter. The PFC is the anterior aspect of the frontal lobe (see the Anatomical Orientation box). The lateral aspect of the PFC is divided into the dorsolateral prefrontal cortex (DLPFC) and the ventrolateral prefrontal cortex (VLPFC). The medial regions that we are concerned with are the orbitofrontal cortex (OFC) and the ventromedial prefrontal cortex (VMPFC). The regions that have been implicated in self-referential processing are the DLPFC and VMPFC, posterior cingulate cortex (PCC), and the medial and lateral parietal cortex. Subjective feelings also contribute to our sense of self and are mediated by all those regions that we outlined in Chapter 10 (Emotion), including the OFC, anterior cingulate cortex (ACC), and insula, as well as areas not limited to the cortex, including the autonomic nervous system (ANS), hypothalamic-pituitary-adrenal axis (HPA), and endocrine systems that regulate bodily states, emotion, and reactivity. Because memory is also part of self-referential processing, the temporal lobe is involved. When we try to understand others, various brain networks are activated that, depending on the task, can include the amygdala and its interconnections with the superior temporal sulcus (STS), the medial prefrontal cortex and OFC, ACC, and fusiform face area (FFA), regions associated with mirror neuron systems, the temporal poles, temporoparietal junction (TPJ), and the medial parietal cortex.
Deficits
As we saw in the chapter opener, damage to the orbitofrontal cortex may result in socially inappropriate behavior. Some people, who are diagnosed with autism spectrum disorders (ASD), also exhibit social deficits. These are pervasive but highly varied developmental disorders associated with impaired social interaction, among other symptoms. They include autism, Asperger’s syndrome, childhood disintegrative disorder, Rett syndrome, and pervasive developmental disorders not otherwise specified.
Individuals with autism tend to show little interest in other individuals or social interactions. Instead, they focus on their internal thoughts or on inanimate external stimuli. They may prefer routine activities and may become upset if these routines are interrupted. For example, seeing the table set in an unusual way, getting a new school bus driver, or having a change in plans can be upsetting. Instead of seeking out social interaction, people with autism may prefer to engage in repetitive behavior by themselves, such as repeatedly flicking a string back and forth. Rather than seeking out a hug, they may comfort themselves by rocking their bodies or twisting their hands and fingers. They may also be hypersensitive to sensory stimuli.
ANATOMICAL ORIENTATION
Anatomy of social cognition

Simon Baron-Cohen of Cambridge University (Baron-Cohen et al., 1985) has proposed that individuals with autism direct their attention away from other people because of deficiencies in the ability to understand the mental states of others. Chapter 6 described people with prosopagnosia, patients who become “face-blind” because they cannot identify people on the basis of facial information. Drawing on this notion, Baron-Cohen coined the term mindblindness to reflect the inability of children with autism to properly represent the mental states of others (Baron-Cohen, 1995). The mindblindness associated with autism extends to impaired use of nonverbal cues (such as facial expressions) to reason about another person’s internal states. A large body of research shows that people with autism are impaired on a variety of tasks that require the use of facial perception for social judgments (e.g., Baron-Cohen, 1995; Klin et al., 1999; Weeks & Hobson, 1987).
People with autism have difficulty identifying emotion and mental states from facial expressions, and they do not use this information in the same way that healthy control participants do. When asked to sort a set of facial pictures, most children organize them according to the emotional expressions on the faces. In other words, they put pictures of people expressing happiness in one pile, pictures of people expressing sadness in another pile, and so on. In contrast, children with autism are more likely to sort these pictures on the basis of physical features such as clothing. Recall from Chapter 10 that Ralph Adolphs and his colleagues investigated the facial perception abilities of patients with amygdala lesions by using computer software that presented small pieces of facial expressions at a time. They also conducted a study in which people with autism performed these procedures and found that they do not attend to eye gaze as much as normally developing and developed controls do (Spezio et al., 2007). For other disorders that affect a wide range of brain regions involved in social cognition, see “Milestones in Cognitive Neuroscience: Psychiatric Disorders and the Frontal Lobes.”
TAKE-HOME MESSAGES
Socrates’ Imperative: Know Thyself
Socrates emphasized the importance of “knowing thyself.” How exactly do we do that? We develop our self-knowledge (e.g., information about our characteristics, desires, and thoughts) through self-perception processes designed to gather information about the self. Because the self is simultaneously the perceiver and the perceived, self-perception is a unique social cognitive process. In other words, when we think about ourselves, the self is doing the thinking and the self is also the subject of our thoughts—the ultimate in subjective appraisals. Consider also that knowing oneself involves the physical you, your body as you (Is that my arm? Do I have blue eyes? Am I strong?), and the essence of you, which is more the story of your character, memories, experiences, and so forth (Am I loyal? Where was I born? Do I enjoy traveling?). In addition, we must distinguish ourselves from others: Our sense of self relies partially on seeing the difference between our self-knowledge and the knowledge we have about other people’s characteristics, desires, and thoughts. For example, you might be one of those unusual individuals who prefers a snake for a pet, but you can readily acknowledge that most people would prefer a dog. Your individual preferences help define what makes you unique from other people. When you wince as your friend twists her ankle, you may share her pain, but you know that she is the one feeling it and not you. The big questions in social cognitive neuroscience center on what neural and psychological mechanisms support the processing of information about the self and about other people, whether these mechanisms are the same or different, and how the brain differentiates between self and other.
In this section, we look at how people represent and gather information about themselves and what the brain can tell us about the nature of self-perception. For instance, do we really want to know all sides of ourselves, or just the good things? If we want to focus on the positive, how does the brain help us do that?
Self-Referential Processing
Where were you born? Where was Napoleon born? We all know that we remember some information better than other information. It is a safe bet that you know where you were born, but when it comes to Napoleon, perhaps not. If you have visited his birthplace in Ajaccio on the island of Corsica, you are more likely to remember that information than if you had never been there. According to Fergus Craik and Robert Lockhart’s levels-of-processing model of memory (1972), the depth of processing profoundly affects the storage of information. Craik and Lockhart found that information processed in a more meaningful way is remembered better than information processed more superficially. For example, in tests they performed, participants were much more likely to remember a list of words when they considered their meaning rather than when they considered their font. A few years after Craik and Lockhart’s study, other research groups extended these ideas about memory. Two labs discovered independently that people remember significantly more information when it is processed in relation to themselves than when they process it in other ways (Markus, 1977; T. B. Rogers et al., 1977). For example, people are more likely to remember the adjective happy if they have to judge how well it describes themselves than if they have to judge how well it describes the president of the United States (Figure 13.2). This is true even if they do not know that they will be asked to remember the adjectives when judging their descriptiveness. The enhanced memory for information processed in relation to the self is known as the self-reference effect.
Two hypotheses have been considered about why memory is better for information processed in relation to the self. One suggests that the self is a unique cognitive structure with unique mnemonic or organizational elements that promote processing in a way that is distinct from all other cognitive structures (T. B. Rogers et al., 1977). The other hypothesis bursts the bubble on a special self and suggests that we simply have more knowledge about the self, and this encourages more elaborate coding of information that relates to the self (Klein & Kihlstrom, 1986). From this latter perspective, the greater depth of processing might result because participants have to consider the adjective in relation to the wealth of stored information about the self. In contrast, their more superficial judgment of whether the word happy has two syllables is considered only in relation to a single dimension that they may have stored about that word. While numerous behavioral studies have been conducted to examine these hypotheses, it was several imaging studies that revealed the neural systems that underlie the self-reference effect.
MILESTONES IN COGNITIVE NEUROSCIENCE
Psychiatric Disorders and the Frontal Lobes
Psychiatric disorders such as schizophrenia and depression represent a widespread breakdown in mental function. Problems faced by patients suffering from these disorders affect almost all aspects of their behavior. Most likely their problems are not linked to a simple physiological mechanism. Rather, the disorders are thought to arise from a delicate interplay of physiological mechanisms that reflect endogenous dispositions and a person’s idiosyncratic experiences.
One of the most promising aspects of cognitive neuroscience is that it may offer new insights concerning the functional deficits associated with severe psychiatric disorders. Simple neuropsychological descriptions do not adequately account for these disorders. Schizophrenia cannot be thought of as a temporal lobe or frontal lobe problem; it arises as a disturbance in cognitive systems that span cortical and subcortical systems. For example, some imaging studies (Figure 1) have shown that schizophrenics have an underactive frontal cortex, especially in lateral regions. Losing their working memory and inhibitory capabilities renders them more reliant on activity in the posterior cortex. They may be easier to distract, and hence fail to inhibit irrelevant representations such as those related to persistent hallucinations.
Depressed patients, on the other hand, tend to exhibit a profile of overactivity in prefrontal regions associated with working memory and in areas linked to the generation of affective memories. For these people, representations persist for a long time and have more effect. A situation that a normal person might find neutral, or at most mildly aggravating, becomes amplified and often highly unpleasant. The depressed patient cannot let a situation go; the representation of a thought or obsession persists, sustained by input from inappropriate somatic markers.
From a cognitive neuroscience perspective, we can make sense of the outcome of one of the great debacles of neurosurgery: frontal lobotomies for treating psychiatric disorders (Valenstein, 1986). Before the use of drug therapies in the 1950s and 1960s, mental institutions were overflowing with desperate patients and doctors, eager to try any procedure that promised relief. In the 1930s, Egas Moniz, a renowned Portuguese neurologist who had developed cerebral angiography in 1927, introduced a psychosurgical procedure for treating patients with severe schizophrenia and obsessive-compulsive disorder.

FIGURE 1 Positron emission tomography (PET) reveals abnormal patterns of blood flow in patients with psychiatric disorders.
(a) Schizophrenic patients show hypometabolism in the prefrontal cortex. This abnormality is especially marked during tasks that produce increased blood flow in this area in healthy participants. In this study, participants were involved in a continuous auditory discrimination task. Compared to the control participants (top), uptake of the tracer is much lower in schizophrenic patients (bottom). Metabolic rates are represented from low to high, respectively, by black, purple, blue, green, yellow, red, and white. The lower metabolic rates in the mid-prefrontal cortex (top of slice) of the schizophrenic patient are readily apparent. (b) Blood flow at rest was measured in control participants and patients with depression. Colored areas indicate regions of increased blood flow in the depressed patients. These areas are centered in the lateral prefrontal cortex in the left hemisphere.
Moniz’s inspiration came from an international scientific conference at which two American researchers had reported the effects of frontal lobectomy in chimpanzees. One animal appeared to have undergone a personality change. Before the operation, the chimp was uncooperative and threw temper tantrums. After removal of most of her frontal lobes, the animal was cheerful and participated in experimental tests without hesitation. Moniz reasoned that the procedure might bring relief to severely agitated patients—a well-intended thought, given the lack of alternatives.
Removing large amounts of tissue from the frontal lobes seemed excessive. Instead, Moniz decided to isolate the prefrontal cortex from the rest of the brain by severing the white matter’s connecting fibers. In his early efforts, he applied toxic levels of alcohol through holes in the skull’s lateral surface. Later, he switched to the procedure of lowering a leukotome (a plunger with an extractable blade) into the brain to sever fibers in targeted regions.
Walter Freeman at Georgetown University refined this procedure. He developed a simple technique that did not require a surgeon. The patient was first given an anesthetic consisting of a severe electrical shock. While the patient was unconscious, usually for 15 minutes, the surgeon performed the lobotomy by jabbing an ice pick through the bone above each eye and wiggling it back and forth. To promote the benefits of this miracle cure, Freeman set off on a barnstorming trip. He took with him a portable kit containing his electroshock apparatus, ice picks, and a small hammer (Figure 2). The public and scientific community were welcoming. Thousands of procedures were performed over the next few decades, and for his work, Moniz received the Nobel Prize in Physiology or Medicine in 1949.
Thanks to hindsight, we now recognize the abject failings of the lobotomy craze. The few outcome studies that were done revealed that the discharge rate from mental institutions was no greater for lobotomy patients than it was for control participants. Scant concern was given to the patients selected; the procedure had minimal effect on schizophrenics but drastically altered patients with affective disorders like depression or severe neurosis, who felt much less anxious, impulsive, and depressed. But these feelings brought new problems that rendered these patients incapable of functioning outside the institutional setting. They were now withdrawn and underactive, lacking in affect or responsiveness. The benefits, if any, were experienced by attendants, who rejoiced that the patients were docile and easy to manage. As with Phineas Gage, the patients’ personalities had been transformed.
These differential outcomes make sense in light of metabolic studies. Lobotomies targeted the prefrontal cortex, a region already underactive in schizophrenia. Thus we might expect little effect on schizophrenics, or maybe new problems for those with overly dominant posterior brain function. For affective disorders, though, lobotomies isolated an overactive region. Moreover, the primary foci were medial regions, so the procedure may have eliminated behaviors associated with exaggerated emotionality but turned patients into unfeeling zombies.
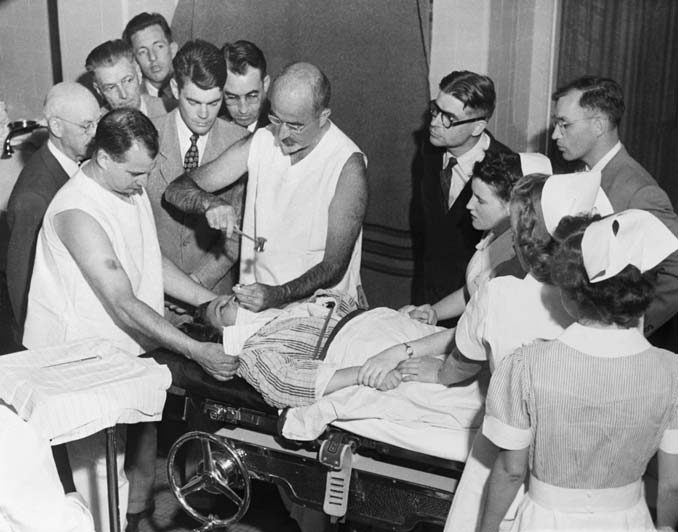
FIGURE 2 Walter Freeman, in 1949 at Western State Hospital, performing frontal lobotomy using the nonsurgical procedure he developed using an ice-pick-like instrument.
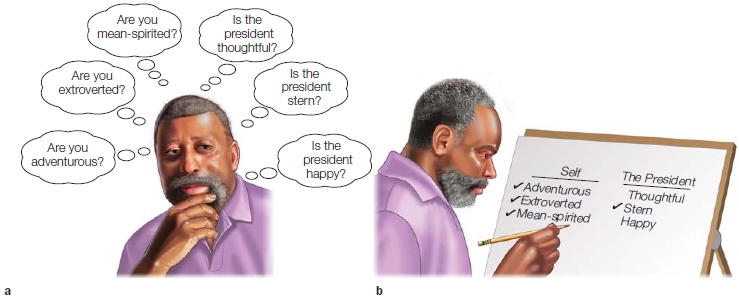
FIGURE 13.2 A typical self-referential processing experiment.
(a) Participants answer a series of questions about their own personality traits as well as the personality traits of someone else. (b) Then they are asked which of the trait words they can remember.
If the self is a special cognitive structure characterized by unique information processing, then distinct neural regions should be activated in relation to the self-reference effect. William Kelley and his colleagues (2002) at Dartmouth College conducted one of the first fMRI studies to test this hypothesis. Participants judged personality adjectives in one of three experimental conditions: in relation to the self (“Does this trait describe you?”); in relation to another person (“Does this trait describe George Bush?”—the president at the time the study was conducted); or in relation to its printed format (“Is this word presented in uppercase letters?”). As found in numerous other studies of the selfreference effect, participants were most likely to remember words from the self condition and least likely to remember words from the printed-format condition.
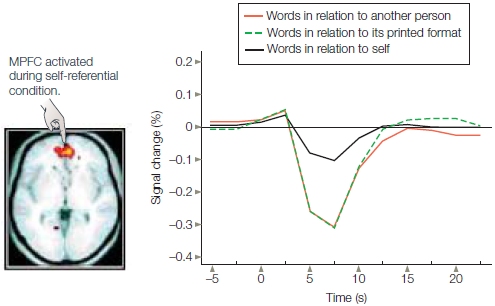
Was there unique brain activity, then, when participants were making judgments in the self condition? The medial prefrontal cortex (MPFC) was differentially activated in the self condition compared to the other two conditions (Figure 13.3). Later studies found that the level of activity in the MPFC predicted which items would be remembered on the surprise memory test (Macrae et al., 2004). The relation between MPFC and self-reference also extends to instances where participants have to view themselves through another person’s eyes. A similar region of MPFC is activated when people are asked to judge whether another individual would use particular adjectives to describe them (Ochsner et al., 2005).
|
FIGURE 13.3 Medial prefrontal cortex (MPFC) activity is associated with |
|
Although much of this research has been conducted with functional MRI, event-related potential (ERP) studies provide convergent results. Self-referential processing produces positive-moving shifts in ERPs that emerge from a midline location consistent with the location of medial prefrontal cortex (Magno & Allan, 2007). These studies suggest that self-referential processing is more strongly associated with medial prefrontal cortex function than is the processing of information about people we do not know personally, such as the president of the United States.
Self-Descriptive Personality Traits
The self-reference effect on memory is just one example of the unique effect of the self on cognition. Another process that is unique to self-perception has to do with self-descriptive personality traits. For instance, when you are deciding about whether a trait is self-descriptive (Are you physically strong?), you use a different source of information compared to when you are deciding whether another person possesses that trait (Is Antonio strong?). In other words, people have a uniquely strong memory for traits that they judge in relation to themselves, and they also have a unique way of deciding whether the trait is self-descriptive. Specifically, when we decide whether an adjective is self-descriptive, we rely on self-perceptions that are summaries of our personality traits rather than considering various episodes in our lives. In contrast, when making judgments of other individuals, we often focus on specific instances in which the person might have exhibited behaviors associated with the adjective.
Stanley Klein and his colleagues at the University of California, Santa Barbara (1992), arrived at this finding when they asked whether self-description judgments rely on recall of specific autobiographical episodes. How did they figure this out? Participants were shown a personality adjective on a computer screen and either rated it for self-descriptiveness (e.g., “Are you generous?”) or defined it (“What does generous mean?”). As a control, participants were shown a blank screen with no adjectives. After completing the initial task, participants were asked to describe a particular instance from their lives when they exhibited the personality characteristic. During this descriptive task, researchers recorded the time it took to perform the task. In the control condition, participants were asked to describe an episode when they exhibited a trait that they had not been asked about, for example, “Give an example of when you were stubborn.” If self-descriptions rely on looking through episodic memory for examples, participants should have been faster to recall an episode when they exhibited the personality characteristic that they had already been asked about, having just cruised through their episodic memory bank to make the self-descriptive judgment. What were the results? No differences were found between the self-judgment, definition, and control conditions. This result suggests that our judgments about self-characteristics are not linked to recall of specific past behaviors.
If this conclusion is correct, then we should be able to maintain a sense of self even if we are robbed of autobiographical memories across our lives. Can we do this? The ability to maintain a sense of self in the absence of specific autobiographical memories has been demonstrated in case studies of patients with dense amnesia (Klein et al., 2002; Tulving, 1993). Consider two patients who developed retrograde and anterograde amnesia (see Chapter 9). Patient D.B.’s memory problems developed after a heart attack as a result of the transient loss of oxygen to the brain—a condition known as hypoxia. Patient K.C. was in a motorcycle accident and sustained brain damage that resulted in amnesia. Neither of these patients could recall a single thing they had done or experienced in their entire life, yet both could accurately describe their own personality. For example, D.B. and K.C.’s personality judgments were consistent with judgments provided by their family members.
Possibly, however, this behavior reflects the preservation of more general social knowledge rather than the preservation of trait self-knowledge. This is seen in patients with Korsakoff’s syndrome, who have a profound inability to recall events. In one study, such patients were shown two pictures of men and told a biographical story of each. One man’s story was about a good guy; the other man’s was about a bad guy. One month later, most of the patients preferred the picture of the man whose story revealed him to be a good guy, although they did not recall any of the biographical in- formation about him (M. K. Johnson et al., 1985).
Klein and his colleagues made sure to address this question. They asked patient D.B. to rate his daughter’s personality traits by using the same test that he so accurately completed about himself. His responses and those of his daughter varied wildly, while those of control patients and their children did not. Although D.B. was unable to retrieve accurate trait information about his daughter, he had no trouble recalling information about himself (Klein et al., 2002). These results provide additional support for the suggestion that semantic trait self-knowledge exists outside of general semantic knowledge. They also suggest that at least some of the mechanisms of self-referential processing rely on neural systems distinct from the neural systems used to process information about other people.
Indeed, Klein stumbled across something interesting when doing a review of research on self-based knowledge (Klein & Lax, 2010): Trait-based semantic knowledge about the self is remarkably robust against a host of neural insults and damage. In this regard it is unlike other types of semantic knowledge, even other types of semantic knowledge about the self (you may not know your birthday or recognize yourself in the mirror, but you still know that you are persistent). Klein’s observation suggests that semantic trait knowledge about oneself is a special type of self-knowledge and that the self is not a single unified entity. The conclusion is that rather than being centered in one unique cognitive structure, the self is distributed across multiple systems. In fact, several different systems for self-knowledge have been identified, and they can be isolated functionally from each other. For example, there is a system for episodic memories of your own life (I had a great time hiking in South Dakota), another for semantic knowledge of the facts of your life (I am half Norwegian), one for a sense of personal agency (I am the agent that causes my arm to lift up), another for the ability to recognize your body in the mirror, in photos, and just looking down at your feet (That’s me, alright!), and many more systems mediating other types of self-knowledge.
TAKE-HOME MESSAGES
Self-Reference as a Baseline Mode of Brain Function
As we have seen in many previous chapters, during fMRI studies participants are given a task to perform. Between tasks, typically they are asked to rest. Imagine yourself lying in a “magnet” with nothing to do and being told to rest. Your mind does not turn off like a TV screen; you start thinking about the weekend, summer break, your friends, your dinner, the paper you have to write, something. And usually that something is all about you or something or someone connected to you in some way. Can studying the brain tell us anything about why self-referential processing is so prevalent? Some research suggests that the medial prefrontal cortex, the region associated with self-referential processing, has unique physiological properties that may permit self-referential processing to occur even when we are not actively trying to think about ourselves. This notion emerged as it gradually dawned on researchers that although participants inside the MRI machine were supposedly at rest, activity in specific brain regions was noticeably increasing. In fact, this activity was as vigorous as activity in other regions when individuals were performing mental tasks, such as math problems. The brain at rest apparently was not “off.” When participants were quizzed about what they were thinking during their “rest periods,” the typical answer related to self-referential processing (Gusnard et al., 2001; Gusnard & Raichle, 2001).
Obviously, even when you are resting quietly and not thinking about something in particular, blood continues to circulate to your brain as it uses oxygen. In fact, a network of brain regions, including the MPFC, has metabolic rates that are higher “at rest.” These circulatory and metabolic demands are costly because they take blood and oxygen away from other organs. Why would the brain consume so much of the body’s energy when it is not engaged in a specific cognitive task? Raichle, Gusnard, and their colleagues argue that when we are at rest cognitively speaking, our brains continue to engage in a number of psychological processes that describe a default mode of brain function (Gusnard & Raichle, 2001). They have named the brain regions that support these processes the default network (Raichle et al., 2001). The default network consists of the MPFC, precuneus, TPJ, medial temporal lobe, lateral parietal cortex, and posterior cingulate cortex (Figure 13.4). The researchers hypothesized that the higher metabolic rate in the medial prefrontal cortex reflects self-referential processing, such as thinking about what we might be getting ready to do or evaluating our current condition. Thus, they concluded, the default network is there to ensure that we always have some idea of what is going on around us. This is called the sentinel hypothesis.
|
FIGURE 13.4 The Default Network. |
|
The default network is most active when tasks direct our attention away from external stimuli, and we are inwardly focused. This makes sense, because there are no primary sensory or motor regions connected to the default network. For instance, the default network is strongly active when we are engaged in self-reflective thought and judgment assessments that depend on social and emotional content. The default network is connected to the medial temporal lobe memory system, which explains why we often consider the past in these default ramblings. The default network is deactivated while performing active tasks. Thus, when you want to detach yourself from ruminating about your own plight, whether it is brought on by sadness, anger, or depression, you can do so by performing an active task, such as learning a new skill. The great Antarctic explorer Sir Ernest Shackleton knew this instinctively. In his book, South, he describes the ordeal that he and his men went through when their ship was sunk and they were stranded on the pack ice just off the Antarctic coast in 1915. At one point he relates,
Then I took out to replace the cook [with] one of the men who had expressed a desire to lie down and die. The task of keeping the galley fire alight was both difficult and strenuous, and it took his thoughts away from the chances of immediate dissolution. In fact, I found him a little later gravely concerned over the drying of a naturally not over-clean pair of socks which were hung up in close proximity to our evening milk. Occupation had brough his thoughts back to the ordinary cares of life (Shackleton, 2004, p. 136).
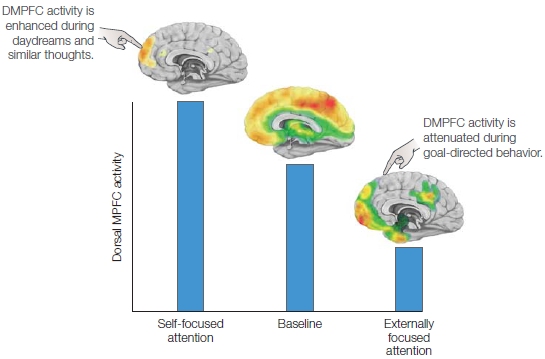
Interestingly, however, while performing active tasks that involve self-referential judgments, the MPFC deactivates less than it does for other types of tasks (Figure 13.5). Given that we generally think about ourselves when we are left to daydream, a self-referential task would not significantly change activation in the MPFC because it chronically engages in self-referential thinking, even during the rest or baseline condition. In the self-reference studies described earlier, the president and printed-format conditions direct cognitive resources away from self-referential thinking, and therefore the MPFC shows a strong deactivation relative to baseline.
Since the default network was first described, however, multiple studies have found that various tasks activate a set of regions remarkably similar to the default network. These include autobiographical memory tasks, tasks envisioning the self in the future or navigating to a different location, and tasks that evaluate personal moral dilemmas (e.g., would it be morally acceptable for you to push one person off a sinking boat to save five others?). Furthermore, similar regions of the brain are activated when we think about the beliefs and intentions of other people—that is, their mental states (known as theory of mind, which we discuss elsewhere in this chapter). Thus, the default network appears to do more than solely self-referential processing. Can you spot the common thread, or common cognitive process, running through all of these tasks?
|
FIGURE 13.5 Activity in the dorsal medial prefrontal cortex increases during tasks that involve self-referential mental activity or self-focused attention and decreases during tasks that involve externally focused attention. This finding is consistent with the observation that during goal-directed behaviors, self-focused attention decreases, and also indicates that at baseline, there should be some degree of self-referential mental activity engaging this region, a suggestion which has been supported by functional imaging data. |
|
All of these tasks have a similar core process. Although differing in content and goal, each task requires the participants to envision themselves in situations other than the here and now—that is, to adopt an alternative perspective (Buckner & Carroll, 2007; J. P. Mitchell, 2009). For example, imagine what you might think and feel if you had to change a flat tire in a rainstorm, without a raincoat, on the way to an important interview. Alternatively, how would you feel if you won a trip to Barcelona, or if you had to decide whether to push someone off a boat in order to save five other people? Each of these scenarios requires you to focus on thoughts that have no relation to the stimuli in your current environment. This type of cognitive process is exactly what we need to be able to infer the mental states of others, such as trying to imagine how your friend felt when he jumped up in the end zone and caught a seemingly impossible pass that won his team a ticket to the Orange Bowl. We need to step out of our shoes and into someone else’s. As this account suggests, the processes that give rise to our understanding of other people’s minds overlap with the processes that support speculations about our own activities. Jason Mitchell at Harvard University has suggested that the high resting activity measured in the default network may indicate that the human mind naturally prefers simulated realities over the immediate external environment (Tamir & Mitchell, 2011). Next time someone tells you to enjoy the moment instead of dreaming about the future, you can reply, “Dude, I’m high on my default network.” Mitchell has proposed that the deactivation of such regions may indicate that these virtual scenarios have been set aside temporarily in order to orient to the actual, concrete world around us.
If the brain is already making a set of default psychological computations, then what are the implications for brain activation when we deviate from the default state and actively try to think about something else? Why do the brain regions that seem to be involved with social cognition “switch off” while other regions come online to perform nonsocial tasks? You might say, “Well, to cut your metabolic costs.” This response seems logical, but in reality the task-related changes in local blood flow are insignificant. They are so small that during periods of transient task performance, metabolic rationing isn’t worth the effort. In fact, deactivations can occur far from locations of increased metabolism or even in their absence (Gusnard & Raichle, 2001; Raichle et al., 2001). J. P. Mitchell (2011) has proposed that the elevated activity of MPFC, TPJ, and medial parietal cortex interferes with nonsocial forms of thought. If our default mode is always prepared for social interaction, and it doesn’t quiet down when we engage in a task that involves objects governed by external forces, it could be rather incapacitating. Consider what it would be like if every time you popped a piece of bread into the toaster, you considered the feelings and thoughts of the bread (Does it want to be toasted?), or of the toaster (Would it rather be broiling than toasting?). What if your ancestors had gone into default mode while gazing at the rock they were poised to throw at the animal about to pounce on their toddler?
Mitchell suggests that the solution to this cognitive problem may require interrupting the spontaneous mental processes that otherwise induce a readiness for social thought. That is, we humans are naturally predisposed to think about mental states, but to interact appropriately with nonsocial aspects of our environment, we have to turn down those natural tendencies. We aren’t always successful at doing this, for example, when we get mad at our disabled car and accuse it of intentionally ruining our interview. We are also notoriously poor at this shift when it comes to animate objects other than humans: We frequently project human thoughts and intentions onto various animals.
Self-Perception as a Motivated Process
The studies described in the preceding discussion examined a number of ways that we process information about the self. They do not, however, address the question of how accurately we process this information. Judgments about the self are somewhat unique because, although the richest possible database is available, this process is often inaccurate. A wide range of behavioral studies have shown that people often have unrealistically positive self-perceptions (S. E. Taylor & Brown, 1988). Among high school students, 70 % rank themselves as above average in leadership ability, while 93 % of college professors believe that they are above average at their work (reviewed in Gilovich, 1991). More than 50 % of people believe they are above average in intelligence, physical attractiveness, and a host of other positive characteristics—as humorist Garrison Keillor’s description of his fictitious hometown, Lake Wobegon, attests: “Where all the women are strong, all the men are good-looking, and all the children are above average.” This view through rose-colored glasses extends to our expectations in life. People believe they are more likely than others to experience positive future events, such as winning the lottery, and less likely than others to experience negative future events, such as getting a divorce.
How does the brain allow us to maintain these positive illusions about ourselves? Chapter 6 described how optical illusions arise from higher order visual areas. Although research on self-perceptual biases is still unfolding, the results suggest that distinct higher order prefrontal regions allow people to focus selectively on positive aspects of themselves while preventing them from deviating too far from reality.
Two studies suggest that the most ventral portion of the anterior cingulate cortex is responsible for focusing attention on positive information about the self. An fMRI study conducted at Dartmouth College by Joseph Moran and his colleagues (2006) asked participants to make a series of self-descriptive judgments just like those in the self-reference studies. As expected from research on positive biases in self-perception, the participants tended to select more positive adjectives and fewer negative adjectives as self-descriptive. Differences in activity in the ventral anterior cingulate cortex were associated with making judgments about positive adjectives compared to negative adjectives, and this was particularly true for adjectives considered to be self-descriptive (Figure 13.6). Another fMRI study found that a similar region of anterior cingulate cortex was activated differentially when participants imagined experiencing a positive event in the future as compared to a negative event (Sharot et al., 2007). These studies suggest that the anterior cingulate cortex is important for distinguishing positive self-relevant information from negative self-relevant information. Marking information as positive versus negative may permit people to focus more on the positive.
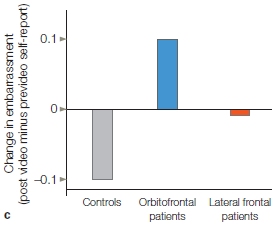
Although self-perceptions are sometimes biased in a positive direction, on average, self-perceptions are not delusional or completely detached from reality. Accurate self-perception is essential for appropriate social behavior. For example, people must have some insight into their behavior to make sure they are following social norms and avoiding social mistakes. Patients with damage to the orbitofrontal cortex (like M.R. at the beginning of the chapter) tend to have unrealistically positive self-views along with inappropriate social behavior. Jennifer Beer wondered whether patients’ behavior was inappropriate because they lacked insight into their own behavior or because they were unaware of the social norms. To explore this question, she videotaped healthy control participants, patients who had damage to the orbitofrontal cortex, and patients with lateral prefrontal cortex damage while they engaged in a structured social interaction with a stranger (Beer et al., 2006). In this interaction, the stranger made conversation with the participants by asking them a series of questions. Unlike the other two groups, patients with orbitofrontal damage tended to bring up impolite conversation topics. After the interview, the participants rated how appropriate their answers had been considering that they had been talking to a stranger. Patients with orbitofrontal damage believed they had performed very well on the social interaction task. When they were shown the videotaped interview, however, these patients become embarrassed by their social mistakes (Figure 13.7). This study suggests that the orbitofrontal cortex is important for spontaneous, accurate self-perceptions, and that rather than being unaware of social norms, patients with orbitofrontal damage demonstrate lack of insight. We will return to the orbitofrontal cortex later in the chapter.
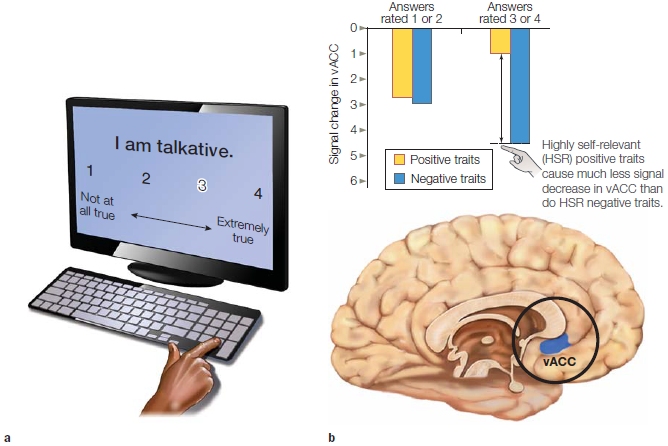
FIGURE 13.6 Neural activity in relation to judging positive information about the self.
(a) Participants rated the self-descriptiveness of a variety of personality traits. (b) Less deactivation in the anterior cingulate was associated with rating positive personality traits in comparison to negative personality traits. vACC is ventral anterior cingulate cortex.

|

|
|
FIGURE 13.7 Study of self-insight in patients with orbitofrontal damage. |
Predicting Our Future Mental State
How do we predict our own mental states? Do we consider actual experiences and predict from there, or do we use a set of rules that output a prediction? What if you were asked to choose between spending a year alone in a space station on Mars or alone in a submarine under the polar ice cap? This is a choice between scenarios that nobody has experienced, and thus, there are no general rules about how to choose. When participants had to make predictions about their mental states in novel scenarios, fMRI revealed that the ventral region of the MPFC was consistently engaged. It was also found that people’s preferences for one novel situation over another are stable over time (reviewed in J. P. Mitchell, 2009). This insight suggested to Mitchell that when we make these types of predictions, we begin by simulating the experience and then predicting which one we would like better.
Studies of patients with damage to the VMPFC support the notion that the VMPFC subserves predictions about an individual’s own likes and dislikes. In one study (Fellows & Farah, 2007), three groups were examined: patients with damage principally involving the orbitofrontal and/or the ventral portion of the medial wall of the frontal lobe, patients with damage to the dorsolateral PFC, and healthy controls. Each participant was asked which of two actors, foods, or colors they preferred. For instance, “Do you prefer Ben Affleck or Matthew Broderick?” When controls or patients with dorsal lateral PFC damage chose Affleck over Broderick, but Broderick over Tom Cruise, their preferences remained stable; they said they liked Ben more than Tom. Not so with patients who had damage to their VMPFC. Their preferences were inconsistent—they might choose Ben over Matthew and Matthew over Tom, but then choose Tom over Ben.
If you were offered either $20 today or a guaranteed $23 next week, which would you pick? Oddly enough, most people pick the $20. In general, people tend to make shortsighted decisions, even when they can foresee the consequences and understand that they would be better off with a different choice. Why do we do this? Activity in brain regions associated with introspective self-reference (such as the VMPFC) are more engaged when predicting how much a person would enjoy an event in the present compared to when judging future events (J. P. Mitchell et al., 2011). Not only that, but by looking at the magnitude of VMPFC reduction, researchers could predict the extent to which participants would make shortsighted monetary decisions several weeks later. The more the VMPFC was activated when predicting future events, the less shortsighted decisions were made. If you happen to be one of the few people who can delay the payoff, most likely your VMPFC engages better than most when thinking about the future. Considering the previous finding that the VMPFC contributes to the ability to simulate future events from a first-person perspective, Mitchell proposes that an individual’s shortsighted decisions may result in part from a failure to fully imagine the subjective experience of a future self.
TAKE-HOME MESSAGES
Theory of Mind: Understanding the Mental States of Others
Although self-perception and awareness are important features of human cognition, we are also eager to interact with and understand the behavior of other individuals. In contrast to our self-perceptions, which have privileged access to our rich autobiographical memories, unexpressed mental states, and internal physiological signals, our perceptions of other people are made without direct access to their mental and physiological states. Instead, we have access only to the verbal and nonverbal cues they exhibit, and from those we infer what others are thinking and how they feel. Our inferences may not always be right, but we are pretty good at it. How good are we? William Ickes has made a study of this feature, and he concludes that we are as good as it is good for us to be. Evolutionary pressures have calibrated our accuracy to the level that is high enough to allow us to deal well with others, but not so high that we weigh everyone else’s interest equal to our own, thus putting our genetic future at risk. Empathic accuracy refers to a perceiver’s accuracy in inferring a target person’s thoughts and feelings. Total strangers achieve an empathic accuracy score of about 20 %, but among close friends it is about 30 % of the time; between spouses, empathic accuracy is 30–35 % (see Ickes’s commentary in Zaki & Ochsner, 2011).
During our evolution as social animals, humans developed the ability to infer the current mental state of others—their intentions, thoughts, feelings, beliefs, and desires. Understanding the mental states of other people is critical for successful performance across a wide range of social activities, such as cooperating, empathizing, and accurately anticipating behavior. Most important, understanding the intentions of others is the basis of human cooperation (Moll & Tomasello, 2007).
This ability to infer the mental states of other people is known as theory of mind, a term coined by David Premack and Guy Woodruff of the University of Pennsylvania (1978). After working with chimpanzees for several years, they began to speculate about what might account for differences in cognition across species. They suggested that chimpanzees might be capable of inferring information about the mental states of other chimpanzees. This idea initiated an avalanche of research looking for evidence to support it. Although considerable debate continues on the competence of social cognition in nonhuman species (Call & Tomasello, 2008; Herrmann et al., 2007), the work of Premack and Woodruff sparked a deep interest in theory-of-mind research in humans. Theory of mind, also known as mentalizing, has received a considerable amount of attention in the developmental psychology literature and, more recently, in cognitive neuroscience studies.
Developmental Milestones
Curiosity about others appears at birth and is a primary source of motivation throughout life. For example, infants prefer to look at a human face rather than other objects. Research using ERP has found that even 4-month-old infants exhibit early evoked gamma activity at occipital channels and a late gamma burst over right prefrontal cortex channels in response to direct eye contact. These findings suggest that infants are quick to process information about faces and use neural structures similar to those found in adults (Grossmann et al., 2007). In adulthood, we continue to focus on the social aspects of our environment. Numerous studies have shown that humans spend on average 80 % of their waking time in the company of others, and 80–90 % of conversations are spent talking about ourselves and gossiping about other people (Emler, 1994).
Much of the behavioral work on theory of mind has examined how this ability develops over a person’s life span. Many tasks have been created to understand how theory of mind works. For several years, the Sally– Anne False-Belief Task (which we describe a bit later in this chapter) was the essential test in determining the presence or absence of theory of mind. Children didn’t reliably pass this test until they were about age 4. It eventually dawned on researchers, however, that this task was too difficult for young children and that it was more than just a false-belief task. It could be that later developing abilities, such as inhibition and problem solving, were confounding the results, whereas theory of mind could develop earlier than age 4 or even be innate. Changing the tasks revealed that infants younger than 4 years demonstrate the ability.
When an adult is looking for an object but doesn’t know where it is, 12-month-old babies who know the object’s location will point to where it is. When the adult does know the location, however, the babies do not point to it (Liszkowski et al., 2008), demonstrating that they understand the goals and intentions of the adult. Fifteen-month-old babies show “surprise” when someone searches in a container for a toy that had been placed there in their absence (Onishi & Baillargeon, 2005), suggesting that they understand that the person did not know the toy had been placed there. At 17 months, children understand that another person has a false belief (Southgate et al., 2010). At about age 3 or 4, children recognize that their physical vantage point gives them an individual perspective on the world that is different from the physical vantage point of other people. By 5 or 6 years of age, children appreciate that their mental states are distinct from the mental states of other people. Specifically, they are aware that two people can have different beliefs about the state of the world. At about 6 or 7 years of age, children can appreciate when the literal meanings of words communicate only part of the speaker’s intention, or that the actual intention may be quite different from the literal meaning of what is said. For example, they can understand irony and differentiate between a joke and a lie. At about 9 to 11 years of age, children are able to simultaneously represent more than one person’s mental state, and to discern when one person hurts another person’s feelings. They are ready to be teenagers.
This is how things stood until recently, when Hungarian developmental psychologists Agnes Kovacs, Erno Teglas, and Ansgar Endress (2010) came up with a new task and a radical hypothesis. Dave Premack happily points out that “their ideas constitute the first significant novelty in ToM in at least ten years” (Premack, in press). The researchers propose that theory of mind is innate and automatic. They reasoned that if this is so, then computing the mental states of others should be spontaneous, and the mere presence of another should automatically trigger the computation of their beliefs, even when performing a task in which those beliefs are irrelevant. They designed a visual detection task to test this idea.
The participants in the study by Kovacs and colleagues were adults. They were shown several animated movie scenarios that started with an agent placing a ball on a table in front of an opaque screen. The ball then rolled behind the screen. Next, one of four possible scenarios occurred:
In the first two instances, when the agent returns, he will have a true belief about the location of the ball. In the latter two examples, when the agent returns, he will have a false belief about the ball’s location. Participants, however, observed the ball in all four scenarios and know where it is. At the end of the film, the screen was lowered, and either the ball was there or it was not (independent of what the film had shown). The participants’ task was to press a button as soon as they detected the ball. The time it took for them to push the button—their reaction time (RT)—was measured. Notice that the agent’s beliefs were irrelevant to the task. The researchers predicted that reaction times should be faster when participants and agents thought the ball was behind the screen (and it was) compared to a baseline condition when neither the participant nor the agent thought the ball was there (but it was). The baseline scenario should produce the slowest RT.
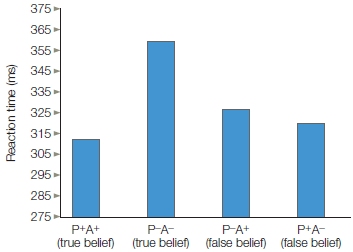
FIGURE 13.8 False-belief task.
Participant’s reaction time is influenced by agent’s belief, even though it is irrelevant. P = participant, A = agent.
Indeed, when the participants and the agents thought the ball was there, and it was, their RT was faster compared to the baseline condition. It was also faster when the participant alone believed it was there. What do you think happened when the participant did not believe it was there but the agent did? Their RTs were also faster than the baseline condition. The agent’s belief, though inconsistent with the participant’s belief, influenced the participant’s RT as much as his very own belief did (Figure 13.8). Thus it appears that adults track the beliefs of others automatically, but is this behavior acquired or innate? Do young infants also do this? The experiment was redesigned for 7-month-olds, this time using a violation of expectation task. The same results were found, suggesting that theory of mind is innate and that the mere presence of another automatically triggers belief computations. In addition, the researchers proposed that the mechanisms for computing someone else’s beliefs might be part of a core human-specific “social sense” that was essential for the evolution of human societies. What are those mechanisms?
Mechanisms for Inferring Other People’s Thoughts
Social cognitive neuroscientists are interested in how the brain supports our ability to make inferences about what other people are thinking, how we read their nonverbal cues, and how we understand the relation between the two. To infer the thoughts of others, the perceiver must translate what is observable (the behavior of another) into an inference about what is unobservable—his psychological state. Several theories have been proposed about how we accomplish this feat. One, known as simulation theory, or the more recently suggested term experience sharing system theory (ESS; see Zaki & Ochsner, 2011), proposes that we observe someone else’s behavior, imitate it, have a physiological response that we feel, and then infer that the other is feeling the same way. This process may occur unconsciously, involving a mirroring system similar to the mirror neuron systems involved with goal-directed actions and action understanding (discussed in Chapter 8). Alternatively, sometimes we can infer feelings by consciously “stepping into someone else’s shoes.” We often infer another person’s mental state, however, even when we can’t see them, or they are smiling on the outside but hurting on the inside, or they are saying one thing but intending another. That is, more than behavioral observation and imitation are at work here. Theory theory, or the newly suggested and perhaps clearer term, mental state attribution system theory (MSAS; Zaki & Ochsner, 2011), proposes that we may build a theory about the mental states of others from what we know about them. That knowledge involves memory about others, the situation they are in, their family, their culture, and so forth.
As is often true when hypothesizing about complex processes, the evidence suggests that both mechanisms, behavior reading and mind reading, are at work. And each behavior is associated with its own network of brain regions.
Simulation theory Recall that within the default network, MPFC activation is associated with the perception of both self and other people. Why would a common brain region be involved in both processes? One possibility is that a common brain region is recruited for both kinds of tasks, because a common psychological function can be used to perform both kinds of tasks. For example, people may draw on their self-representations to make inferences about another person. Simulation theory (or experience sharing) suggests that some aspects of inferring the thoughts of others, especially motor actions and emotions that can be mimicked, are based on an ability to put ourselves in the shoes of another person by using our own minds to simulate what might be going on in the mind of someone else (Harris, 1992; Figure 13.9). Such shared representations are considered by some to be the cornerstone of social cognition (Sebanz et al., 2006). How is the process of simulation reflected in brain activity?

FIGURE 13.9 Simulation theory.
People make inferences about the actions of others using their own expectations based on experiences from their own lives.
Medial prefrontal cortex: Similar and close others. The theory of simulation suggests an intrinsic relation between the perception of self and the perception of others. Therefore, the reason for the MPFC’s involvement in both types of perception may be that the perception of self is sometimes used to accomplish the perception of others. For example, in one fMRI study (J. P. Mitchell et al., 2006), scientists hypothesized that a similar region would be engaged when thinking about ourselves and a similar person, but it would not be activated when thinking about a person dissimilar to us. The researchers had participants read descriptions of two people: One person shared similar political views with the participants, and the other held the opposite political views. Next, the researchers measured the participants’ brain activity while answering questions about their own preferences as well as when speculating about the preferences of the person with similar views and the one with dissimilar views. A ventral subregion of the MPFC was found to increase its activity for self-perceptions and perceptions of the similar person, whereas a different, more dorsal region of the MPFC was significantly activated for perceptions of the dissimilar person. These activation patterns in the MPFC have been held up as evidence that participants may have reasoned that their own preferences would predict the preferences of someone like them but would not be informative for speculating about the preferences of someone dissimilar to themselves. Other studies have since shown a variable pattern of activation between the ventral and dorsal regions: It is dependent not on similarity per se, but on the level of relatedness between the two people based on familiarity, closeness, emotional importance, warmth, competence and knowledge, and so forth.
For instance, Kevin Ochsner and Jennifer Beer showed that a similar region of the MPFC was activated for self-perception as well as perception of a current romantic partner (Ochsner et al., 2005). This effect was not driven by perceived similarities between the self and the romantic partner. The researchers suggest that this activation likely represents commonalities in the complexity or emotional nature of information stored about ourselves and romantic partners. Studies like this one suggest that the MPFC is important for thinking about the self and other people when a common psychological process underlies the thought processes. Sometimes we may use ourselves as a way of understanding someone we do not know well, but who appears to be related to us in some way. At other times, these processes may be linked because we create incredibly rich stores of information about ourselves as well as others we are close to.
Empathy Understanding the mental state of another involves more than understanding their beliefs, goals, and intentions. It also involves understanding their emotions. Empathy, our capacity to understand and respond to the unique experiences of another person (Decety & Jackson, 2004), epitomizes the strong relation between self-perception and the perception of others. To respond appropriately to another, we need the ability to accurately detect the emotional information being transmitted by that other person. Though the details regarding the process of empathy are debatable, it is generally agreed that the first step is to take the other person’s perspective: We must momentarily create within ourselves the other person’s internal state in our effort to understand it. What brain mechanisms permit us to share the experience of another person?
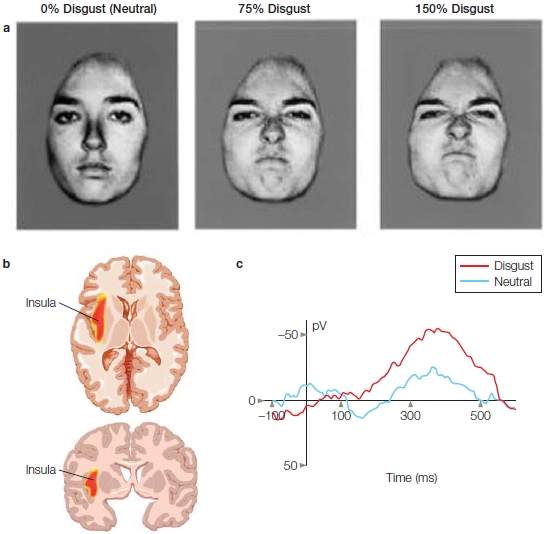
The perception–action model of empathy assumes that perceiving another person’s state of mind automatically activates the same mental state in the observer, triggering somatic and autonomic responses. This model fits with the idea that we are able to understand a mental state by sharing it. Given the role of mirror neurons in imitation and action recognition (see Chapter 8), it has been proposed that mirror neurons may be a critical physiological mechanism that allows us to have the same representation of another’s internal state within our own bodies. This mechanism is sometimes referred to as embodied simulation. For it to occur, some connection needs to be made with the structures for emotional processing. Evidence for such a connection was found in the primate brain, where the mirror neuron system and the limbic system are anatomically connected by the insula, suggesting that a large-scale network could be at the heart of the ability to empathize. As we mentioned in Chapter 10, a large body of research suggests that the brain regions supporting our emotional states are also activated when we perceive these emotional states in other people. For example, in humans, a series of experiments has found that the experience of disgust and the perception of facial expressions of disgust activate similar regions within the anterior insula. In fact, the magnitude of insula activation when observing facial expressions of disgust increases with the intensity of the other person’s facial expression of disgust (Phillips et al., 1997; Figure 13.10). A subsequent fMRI study found that when people inhaled odorants that produce a feeling of disgust, the same sites in the anterior insula, and to a lesser extent the anterior cingulate cortex, were engaged as when they observed facial expressions of disgust (Wicker et al., 2003).
Consistent with these fMRI studies is one using depth electrodes that found some neurons in the anterior insula were fired when these patients viewed disgusted facial expressions (Krolak-Salmon et al., 2003). Finally, a singlepatient case study of insula damage provides additional support for mirror neurons in the insula. After sustaining a lesion to the insula, this patient lost the ability to recognize disgust (Adolphs et al., 2003). Together, these studies suggest that the insula is important for experiencing disgust as well as for perceiving it in others.
|
FIGURE 13.10 Exploring the neural regions responsive to disgust. |
|
In a pain study conducted by Tania Singer and her colleagues at University College London, fMRI revealed that the insula and anterior cingulate are activated when experiencing physical pain in oneself as well as when perceiving physical pain in others (T. Singer et al., 2004). The researchers examined brain activity when participants received painful stimulation through an electrode on their hand or saw the painful stimulation delivered through an electrode to a romantic partner’s hand (Figure 13.11). Although the experience of pain activated a larger network of brain structures, both the experience of pain and the perception of a loved one’s pain activated the anterior insula, adjacent frontal operculum, and anterior cingulate. Furthermore, participants who scored high on a questionnaire that measured their degree of empathy showed the greatest activation in the insula and anterior cingulate when perceiving pain in their romantic partners.
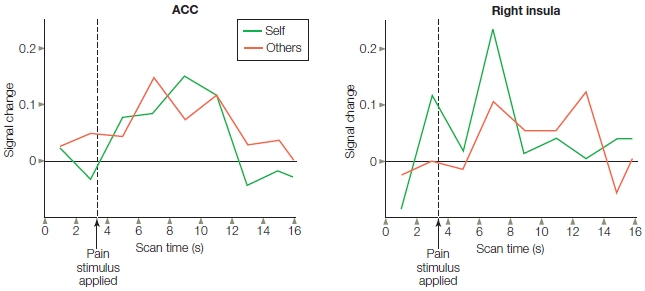
FIGURE 13.11 Study of empathy for pain.
Each participant watched as a partner’s hand received a shock through a set of electrodes. Brain activity was very similar for one’s own pain and the pain of the partner, and the degree of brain activation was correlated with empathy.
Additional evidence for shared activation comes from rare cases of patients who have had portions of their cingulate removed. Single-unit recordings have shown that the same neuron in the anterior cingulate fired both when the person was experiencing a painful stimulus and while anticipating or observing one (Hutchison et al., 1999).
The somatosensory cortex also appears to have a mirroring system. It is engaged when experiencing and observing painful touch (Avenanti et al., 2005) or nonpainful touch (Keysers et al., 2004, 2010). Consistent with these studies is an extensive study of lesion patients. Patients with damage to the somatosensory cortex were significantly impaired in their ability to identify another person’s emotional state when compared to patients who had damage to other brain regions (Adolphs et al., 2000).
Together, these studies suggest that some regions of the brain become engaged when individuals experience an internal state and when they observe someone else experiencing that state. That sounds a lot like the kind of activity observed in mirror neurons.
How do we know who was feeling what? If the same brain regions are activated when we experience something or when we observe someone else having the same experience, how do we know who is feeling what? The answer is that we don’t know, but a recent study has produced some interesting findings. Ryan Murray and his colleagues (2012) performed a meta-analysis of 23 fMRI and 2 PET studies that compared self-relevant processing against processing of close others and of public figures. The objective of the meta-analysis was to identify self-specific activations as well as activations that may permit differentiating between evaluation of close others and evaluation of people we have no connection with. Recall from Chapter 10 that the insula processes stimuli that arise from the body and mediates the conscious awareness of the physiological condition of the body (known as interoceptive awareness). The insula also performs other functions, such as affective evaluation (e.g., as in our previous discussion of disgust). Murray and colleagues found that the anterior insula is activated when appraising and processing information about the self as well as when appraising and processing close others, but not when appraising and processing public figures. Based on this finding, these researchers suggest that when we appraise ourselves and close others, we share a conscious mental representation that is internal, visceral, and actually felt physiologically. Known as embodied awareness, this mental representation affects each person’s emotional perspective. This result would support the idea that we garner knowledge of close others based on our embodied experience of those people.
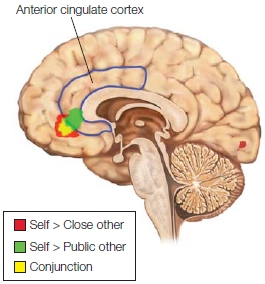
FIGURE 13.12 Regions activated when performing a task relevant to public figures were more dorsal than, and significantly dissociated from, activations associated with tasks monitoring both close others and self. Activation for public figures was mostly in the left superior frontal gyrus, while activation for close other centered in the left VMPFC. Self activation was found in the right VMPFC.
Self-specific processing was found in regions of the vACC and dACC that were not active when appraising close others and public figures (Figure 13.12). The dACC has been described as an effortful, goal-directed mechanism for allocating and regulating attention; it also responds to self-related stimuli and engages in self-reflection and action monitoring (Schmitz & Johnson, 2007). Murray and his colleagues further suggest that, acting as an affective and cognitive evaluation and monitoring unit, certain regions of dACC and vACC specialize in self-specific processing by selecting representations and mental attributes that fit an individual’s own personality. From these, representations which fit that person’s self-concept are constructed.
They also found that within the MPFC were differential activations for self, close other, and public other. Activation for the self was clustered primarily in the right VMPFC; activation for close other was clustered primarily in the left VMPFC, including some shared activation differentially engaging the VMPFC according to the level of relatedness. Activation for public other was significantly dissociated from both these regions, demonstrating greater dorsal MPFC activation in the left superior frontal gyrus. Thus it appears that activations across different regions of the brain differentiate who is feeling what.
Modulation of empathic responses. After recognizing the distinction between ourselves and the other person, we somehow need to monitor our response. For instance, a doctor or dentist needs to understand that his patient is in pain, but neither he nor his patient wants him to be incapacitated by sharing it; the patient wants him to go about the business of relieving it. Jean Decety (reviewed in Decety, 2011) and his colleagues have proposed a model that includes stimulus-driven processing of affective sharing (discussed earlier, in the section about inferring other people’s thoughts) and goal-directed processing. In this model, the perceiver’s motivation, intentions, and self-regulation influence the extent of an empathic experience, as well as the likelihood of behavior that benefits others.
One example of evidence for goal-directed regulation was an inventive experiment conducted by Decety and his colleagues in Taiwan. They hypothesized that regions typically associated with perceptions of physical pain would not be activated in acupuncturists, whose jobs require them to detach themselves from the painful aspect of administering acupuncture and instead focus on the long-term benefit to the patient (Cheng et al., 2007). To investigate this hypothesis, the researchers observed the brain activity of professional acupuncturists versus that of laypeople while they watched video clips depicting body parts receiving nonpainful stimulation (touch with cotton swab) or painful stimulation (acupuncture needles). Consistent with previous research, the study found that regions associated with the experience of pain, including the insula, anterior cingulate, and somatosensory cortex, were activated in nonexperts. In the acupuncturists, by contrast, these regions were not significantly activated—but regions associated with mental state attribution about others (discussed in the next section), such as the MPFC and rTPJ, were activated. Regions underpinning executive functions, self-regulation (dorsolateral and medial prefrontal cortex), and executive attention (precentral, superior parietal, and temporoparietal junction) also were activated. These findings suggest that activation of the mirror neuron system can be modulated by a goal-directed process that enhances flexible responses.
The researchers went on to study these acupuncturists by using ERPs (Decety et al., 2010), looking for the point when regulation of information processing occurs. Control participants had an early N100 differentiation between pain and no-pain conditions over the frontal area, and a late-positive potential around 300–800 ms over the centroparietal regions. Neither of these effects were detected in the physicians. It appears that in these physicians, emotional regulation occurs very early in the stimulus-driven processing of the perception of pain in others.
Tania Singer has studied whether fairness in social relations also affects empathy. That is, if you perceived someone as unfair, would you feel less empathy for them? For instance, would you feel the same when seeing a child trip and fall as when seeing the mugger who just grabbed your wallet trip and fall? In Singer’s study (T. Singer et al., 2006), male and female participants played a card game (involving cash) with two confederates, one who cheated and the other who did not. Then she used fMRI to measure the participants’ brain activity while they watched the confederates experiencing pain. Although both sexes had activation in the empathy-associated brain regions (frontoinsular and ACC) when watching the fair confederate receive pain, the empathy-induced activations in males were reduced significantly when seeing the cheater in pain. These reductions were actually accompanied by increased activation in the ventral striatum and nucleus accumbens, which are reward-associated areas. The males actually enjoyed seeing the cheater in pain. The degree of activation in the reward area correlated with an expressed desire for revenge, as indicated on a questionnaire that participants completed after the experiment. Singer points out that these findings suggest a neural foundation for social preferences: People value the gain positively if someone has gained something fairly, but not if it was gained unfairly. People (at least men) like cooperating with fair opponents, but they like punishing unfair ones.
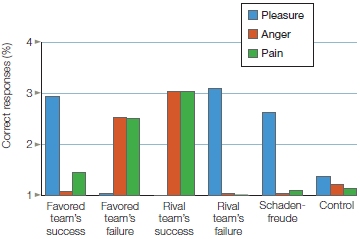
FIGURE 13.13 The bars indicate the average ratings for pleasure, anger, and pain for the success or failure of favored or rival teams.
What about sports rivalries? Mina Cikara wondered if the modulation of empathy seen on a personal level also applied at the group level (Cikara et al., 2011). For instance, when you watch a game between your favorite team (us) and a rival (them), what happens when you see your rivals fail? Do you feel good? How about when the opposing team scores? For her study, Cikara recruited avid fans of rival baseball teams: the Boston Red Sox and New York Yankees. While undergoing fMRI, participants viewed simulated figures representing the Red Sox or Yankees making baseball plays. In some plays the favored player was successful, and in others the rival was successful. Participants also viewed some control scenarios in which a player from a neutral team made plays against either the Red Sox or Yankees, or against another neutral team. After each play, participants rated the feelings of anger, pain, or pleasure they experienced while watching that play (Figure 13.13). Two weeks later, the participants filled out a questionnaire that asked them to rate the likelihood that they would heckle, insult, throw food, threaten, shove, or hit a rival fan (i.e., either a Yankee or Red Sox fan) or hit an Orioles fan (the team that played in the control games).
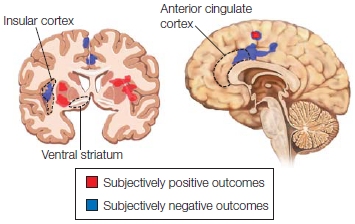
FIGURE 13.14 Viewing subjectively positive outcomes engaged the ventral system.
A subjectively positive outcome was one in which a favored team was successful or a rival team failed against a favored team. In this case, activations were seen in the ventral striatum, along with the left middle frontal and superior frontal gyrus, left insula, bilateral caudate, and SMA. A subjectively negative outcome was the opposite and activated the ACC, SMA, and the right insula.
Viewing subjectively positive plays (when the rival team failed) increased the response in the ventral striatum, whereas failure of the favored team and success of the rival team activated the ACC and insula (Figure 13.14) and correlated with the pain rating (Figure 13.15). Note that seeing an animated hypothetical baseball play elicited the same pain response in a diehard baseball fan as when participants (in previous studies) watched a close other undergo a painful experience! As in the Singer study discussed earlier, the ventral striatum reward effect correlated with the self-reported likelihood of aggression against the fan of the rival team. Thus, the response to a rival groups’ misfortune is neural activation associated with pleasure (aka schadenfreude—enjoyment of others’ troubles), which is correlated with endorsing harm against those groups.
|
FIGURE 13.15 Brain activity correlated with pleasure and pain ratings. |

|
Neural Correlates of Mental State Attribution
Sometimes mental states don’t match their observable cues. Consider a situation in which you ask someone out on a date. She declines, smiles, and tells you that she has a prior engagement. Now what do you do? How do you know whether she truly has other plans, or whether she is just making a plausible excuse and smiling to be kind? Her true preference may be that she wants to go out, but can’t. In that case, you can venture another request. But her smile may be misleading, and she would be annoyed if you pursued her further. Our daily lives are filled with instances in which people hide their true thoughts and feelings. In more extreme cases, our ability to recognize the mismatch between outward behavior and inner intentions is useful for recognizing people who should not be trusted.
Researchers find it challenging to design tasks that can identify which brain regions are involved with inferring mental states from unobservable cues, what exactly they are doing, and how they relate to each other. Much of the research has borrowed paradigms used in studying developmental milestones of children as they gain the ability to infer other people’s thoughts. These studies often proceed by asking participants to make inferences about the beliefs, knowledge, intentions, and emotions of others, based on written narratives or pictures.
Regions that are commonly engaged in a variety of tasks while participants are making inferences about the thoughts and beliefs of others include the medial prefrontal cortex (MPFC), temporoparietal junction (TPJ), superior temporal sulcus (STS), and the temporal poles. Let’s look at what these regions are up to in these tasks.
The medial prefrontal cortex Jason Mitchell and colleagues (2004) compared brain activity of participants engaged in two conditions: forming an impression of another person, and a sequencing task. Participants viewed pictures of people paired with statements about their personality (Figure 13.16a), such as, “At the party, he was the first to start dancing on the table.” A cue indicated how the participants should think about the faces and statements. In the impression formation task, the cue prompted participants to make an inference about the personality of the person in the picture. In the sequencing task, the cue prompted participants to remember the order in which specific statements were presented in relation to a particular face. Both conditions required participants to think about other people, but only the impression formation task required them to think about the internal states of those people. The impression formation task engaged the MPFC much more than the sequencing task did (Figure 13.16b).
The results of this study suggest that MPFC activation plays a strong role in forming impressions about the internal states of other people, but not in thinking about other types of information regarding another person. Thus, they suggest that social cognition relies on a distinct set of mental processes. Subsequent studies have shown that the relation between the MPFC and impression formation is specific to animate beings, such as dogs (J. P. Mitchell et al., 2005), but it is not present when individuals form impressions of inanimate objects. Together, these studies indicate that the MPFC is important for reasoning about the intangible mental states of other beings. As we discussed earlier in the section titled “Self-Reference as a Baseline Mode of Brain Function,” Mitchell has also suggested that MPFC supports the ability to change perspective.
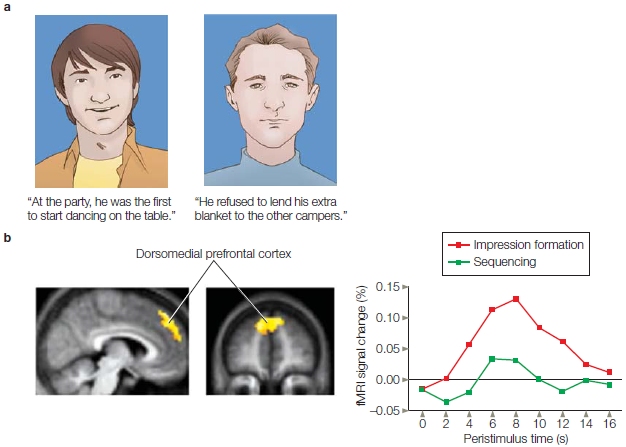
FIGURE 13.16 A study of personality inference.
(a) Participants were presented with a series of pictures that paired faces with statements about personality. They were instructed either to make an inference about the person’s personality or to pay attention to the order in which the statements were presented. (b) Medial prefrontal cortex activity was associated with forming impressions of personality in comparison to remembering sequence order.
The right temporoparietal junction Another brain region that has been associated with making inferences about other people’s mental states is the temporoparietal junction in the right hemisphere (rTPJ). Rebecca Saxe at the Massachusetts Institute of Technology conducted a series of studies to examine the specificity of this region (Saxe et al., 2005; 2006; 2009). First she localized the TPJ by using similar logic developed in fMRI studies of face perception. Recall from Chapter 6 that investigators explore the response characteristics of the fusiform face area (FFA) by using a localizer task to identify the FFA on an individual basis. For example, a participant might view faces or places, and the difference between the two conditions is used to specify the location of that person’s FFA. After identifying the FFA, researchers can perform further manipulations to ask how activity in the FFA varies as a function of other experimental manipulations (see Chapter 12).
Saxe developed a similar method to identify which rTPJ region is engaged during theory-of-mind judgments (Saxe et al., 2006). The localizer task is based on the Sally–Anne False-Belief Task (Figure 13.17)—a task, as mentioned earlier, used in many developmental studies of theory of mind. In one version of this task, participants view a series of drawings that depict scenarios involving the characters Sally and Anne. The pictures begin with Sally placing a marble in a basket and then leaving the room. After Sally is gone, Anne moves the marble into a drawer. Sally then comes back into the room. The key question here is, where will Sally look for the marble? To answer the question correctly, participants have to ignore their own knowledge about the location of the marble and answer from Sally’s perspective. Sally is unaware of Anne’s devious behavior, so she expects the marble to be in the basket where she left it. Participants who are unable to recognize that Sally does not share their knowledge predict that she will look in the drawer. To solve the Sally–Anne task, participants must understand that Sally and Anne can have different beliefs about the world. In other words, they must understand that people have different perspectives.
Saxe’s localizer task also presents a series of falsebelief stories as well as control scenarios involving falsehoods that have nothing to do with the mental states of other people. When these conditions are compared, a region of the rTPJ is consistently more active in the theory-of-mind condition. For each study participant, researchers define the exact location of activity within the rTPJ. Activity in this region is then examined for differential activity in relation to a series of other tasks that measure person perception (Figure 13.18).

FIGURE 13.17 The Sally–Anne False-Belief Task for investigating theory of mind.
This task is used with children to determine whether they can interpret what Sally is thinking about the location of the marble. Because Sally does not see Anne move the marble from the basket to the drawer, Sally should look for the marble in the basket.
Activity in the rTPJ is associated with reasoning about other people’s mental states, but it does not respond to just any condition involving socially relevant information about other people. In one study, participants were presented with three kinds of information about a person: social background, mental states, and a life event. For example, participants might learn about a fictional person named Lisa. Lisa lives in New York City with her parents (social background) but wants to move to her own apartment (mental state), and she finds out that the apartment she wants is available (life event). The study found that the rTPJ was significantly activated when participants were presented with the information about a mental state compared to information about a social background or a life event (Saxe & Wexler, 2005).
As you know from Chapter 1, neuroscientists favor a network approach to the relation between brain regions and psychological function rather than a strict localization approach. A single brain region is unlikely to support a psychological process as complicated as thinking about another person’s mental states. Although the rTPJ is theorized to be specialized for reasoning about the mental states of other people, we have learned in this chapter that the MPFC is also involved in this process. What roles do the rTPJ and MPFC play in reasoning about mental states of others?
Currently, two different hypotheses have been suggested. One is that the rTPJ is specialized for reasoning about the mental states of other people, and the MPFC more broadly supports reasoning about other people, including—but not limited to—their mental states. A second hypothesis suggests that the MPFC supports reasoning about social tasks, and the rTPJ is important for redirecting attention in both social and nonsocial tasks. Let’s look at the evidence for the first hypothesis. Participants’ brain activity was examined in relation to processing information about a person’s physical appearance (“Alfredo was a heavyset man”), internal physiology (“Sheila was starving because she had skipped breakfast”), or mental states (“Nicky knew that his sister’s flight was delayed by 10 hours”). The study found that the MPFC was activated in relation to information about physical appearance and internal physiology. In contrast, the rTPJ was selectively activated for the information about mental states (see Figure 13.18; Saxe & Powell, 2006).
What about evidence for the second hypothesis? Chapter 7 described the attentional cuing procedure popularized by Michael Posner and his colleagues (Posner et al., 1980). In that procedure, participants are presented with cues that provide either valid or invalid information about where to direct their attention to successfully identify a target object (see Figure 7.15). Many of the studies, which have found activation of the rTPJ in relation to mental states, use false-belief tasks that require participants to direct their attention away from invalid information to answer questions about a person’s mental states. Consider again the structure of a typical false-belief task (see Figure 13.17). Participants are told a story in which Sally puts her marble into the basket. Then, after she leaves the room Anne moves the marble to the drawer. Sally returns, and participants must decide where she will look for the marble. We know that participants’ most current representation of the marble is in the drawer. Therefore, they have to redirect their attention to other information to correctly answer that Sally will think the marble is in the basket. Although this task is specifically about mental states rather than someone’s physical appearance or other socially relevant information, it is also unique in its requirement that participants redirect their attention.
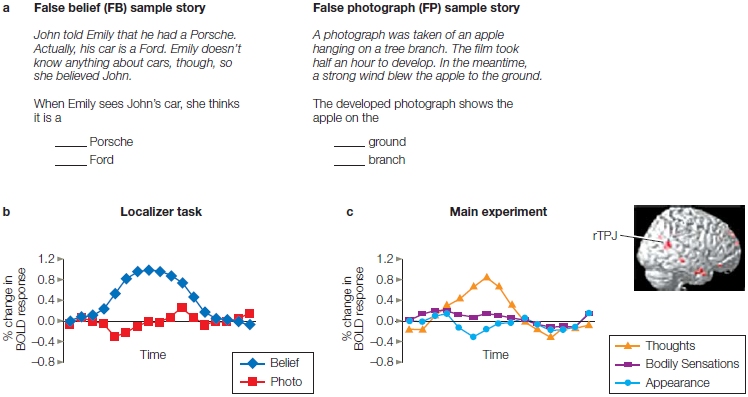
FIGURE 13.18 The localizer procedure for theory of mind and the right temporoparietal junction.
(a) Participants complete false-belief tasks that involve false beliefs about either people, as illustrated, for example, in the sample stories, or photos involving falsehoods that have nothing to do with the mental states of other people, such as viewing a photograph of an apple hanging from a branch. (b) Researchers identify a specific region in the brain that activates more strongly to the false beliefs of people when compared to false photographs. (c) They then examine this region of the brain for differential activity in relation to forming impressions about different aspects of people, such as their thoughts, body sensations, or physical appearance.
A later study found that the same region of rTPJ is significantly activated in relation to the false-belief localizer task used by Saxe and her colleagues and in relation to redirecting attention away from nonsocial cues that signaled invalid information in the attentional cuing procedure (J. P. Mitchell, 2008). This finding suggests that the same region of rTPJ supports the control of attention for social and nonsocial stimuli. But does it? Saxe and her colleagues took a second look, this time using a higher-resolution protocol. They found that the rTPJ actually has two distinct regions: one population of neurons engages for mentalizing, and the other engages for reorienting attention (Scholz et al., 2009).
Currently, there is no definitive answer about the differential roles of the rTPJ and MPFC in person perception. But research continues on this question and promises to deepen our understanding of how we accomplish the difficult task of understanding the minds of other people.
The superior temporal sulcus: Integrating nonverbal cues and mental states The studies described in the preceding discussion first provide information about someone’s mental states and then examine the brain systems that are recruited for reasoning about mental states versus reasoning about other kinds of information. In the real world, however, we are not given a paragraph about what someone is thinking, nor can we count on people telling us exactly what they are thinking. In fact, paying attention to nonverbal cues rather than verbal cues may be your best strategy when people try to hide their mental state from you. We know this is a good strategy, because patients with language comprehension deficits are better at detecting when someone is lying than are either patients without language deficits or control participants (Etcoff et al., 2000).
Nonverbal cues, such as body language, posture, facial expression, and eye gaze, play a powerful role in person perception. You have already learned about the neuroscience of facial perception, which involves regions such as the fusiform face area (Chapter 6) and the role of the amygdala in using the face to make social judgments (Chapter 10). Research has also shown that attention to the direction of eye gaze is an important source of nonverbal information about another person’s attentional state.
Within their first year of life, children develop joint attention, the ability to monitor another person’s attention. One of the most typical ways that children monitor where other people are directing their attention is by noting the direction of their eye gaze. Humans are the only primates that follow eye gaze rather than the direction of where the head is pointing. We humans can tell where the eye is gazing because of the large “whites of our eyes” that no other primate possesses (Kobayashi & Kohshima, 2001). Michael Tomasello and his colleagues (2007) suggest that eyes evolved a new social function in human evolution: supporting cooperative (mutualistic) social interactions. Eye gaze may also be helpful for understanding when people’s words may not match their mental states. For example, when your prospective date declines your invitation, does she make eye contact while turning you down? Or does she avoid your gaze so that you cannot see her true feelings? What neural systems support the ability to attend to another person’s eye gaze and use this information to reason about their mental state?
One of the earliest lines of research examining this question comes from single-cell recording studies in monkeys. David Perrett of the University of St. Andrews in Scotland discovered that cells in the superior temporal sulcus (STS) are helpful for identifying head position and gaze direction. The STS lies below the superior temporal gyrus and above the middle temporal gyrus. Amazingly, some cells responded to head position while others responded to gaze direction. Although head position and direction of eye gaze are often consistent, the ability to distinguish head position from eye gaze opens the door for using these cues to make inferences about mental states. Individuals who turn their head in the same direction as their gaze may be thinking something very different from individuals who keep their head facing forward but direct their gaze in a different direction.
Converging evidence showing that the STS is important for interpreting eye gaze in relation to mental states comes from human neuroimaging studies. Kevin Pelphrey and his colleagues at Duke University examined whether activity in the STS depended on the mental states indicated by shifts of eye gaze in another person. Participants watched an animated woman, who directed her attention either toward or away from a checkerboard that appeared and flickered in her left or right visual field (Figure 13.19). Randomly, the figure took either 1 or 3 seconds to shift her gaze. If the STS is involved solely in tracking shifts in eye gaze, then it would be activated to the same degree in relation to any shift in eye gaze. If, however, the STS is involved in integrating shifts in eye gaze with mental states, then activation of the STS should be related to where the character directs her attention, because eye gaze shifted toward the checkerboard and eye gaze shifted away from the checkerboard would indicate two different mental states.
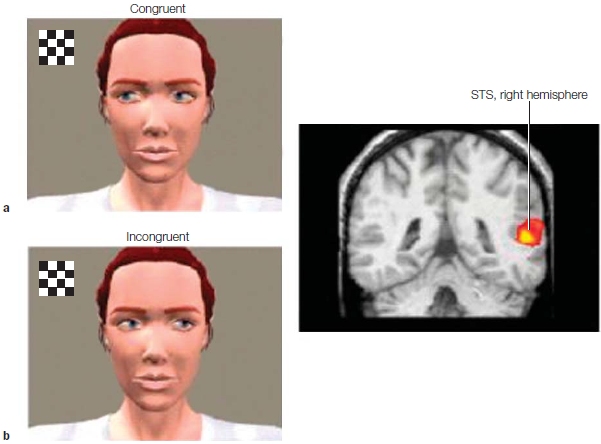
FIGURE 13.19 Participants viewed a virtual-reality character whose eye gaze moved either (a) in a congruent manner toward a flashing checkerboard or (b) in an incongruent manner away from a flashing checkerboard. The superior temporal sulcus tracked the intention behind shifts in eye gaze rather than all shifts in eye gaze.
Consistent with the latter prediction, activity in a posterior region of the STS varied in relation to shifts in eye gaze direction (Pelphrey et al., 2003). Gaze shifts to empty space evoked longer activation of the STS compared to when the gaze shifted to the checkerboard. The context of the gaze had an effect. The researchers conjectured that when the figure unexpectedly did not look at the target, observers were flummoxed and had to reformulate their expectation. This process takes longer, so STS activity was prolonged. The researchers found unexpectedly that STS activation was also related to the timing of the gaze. If the gaze shift occurred at 1 s after the checkerboard appeared, the context effect was seen; but if it took 3 s for the figure’s gaze to shift, the effect was not seen. They proposed that when the time between the presentation of the checkerboard and the gaze shift was too long, the gaze shift was more ambiguous. The observer did not necessarily link it to the appearance of the checkerboard, and no expectations were violated when the gaze direction varied.
In a related study, a similar region in the STS was more strongly activated when a virtual-reality character made eye contact with the participant versus when the character averted his gaze from the participant (Pelphrey et al., 2004). Thus the STS appears to signal the focus of attention of another individual as well as provide important social signals: That individual may be trying to direct our attention away from a novel object or maybe wishing to engage in a social interaction. Interestingly, these studies also demonstrate that the activity in a visual processing region is sensitive to the context of the observed action.
Autism as a Window on the Role of Mental State Attribution
The study of autism provides a fascinating window into the important role of theory-of-mind abilities in navigating our social worlds. If theory-of-mind impairments are a central feature of autism, then we should see differences in many of the neural regions involved in person perception between autistic people and controls. Is this the case? Anatomical studies suggest that a host of brain abnormalities are associated with autism. For example, Eric Courchesne and his colleagues at the University of San Diego have observed that infants with autism tend to have small head circumferences at birth, followed by an abrupt inflation of the head circumference in the first year of life (Courchesne & Pierce, 2005a). Brain abnormalities persist over the course of development, and studies suggest that autism is associated with reduced volume in a range of brain areas, including the frontal lobes, STS, amygdala, cerebellum, and hippocampus.
Changes in anatomy are accompanied by changes in connectivity. The researchers observed hyperconnectivity within the frontal lobe regions and decreased long-range connectivity and reciprocal interactions with other cortical regions (Courchesne & Pierce, 2005b). Aside from these anatomical changes, autism has been associated with abnormal function in a number of regions associated with person perception, including the MPFC, amygdala, FFA (discussed in Chapter 6), STS, anterior insula, and TPJ. It has become apparent that no single brain region, or even a single system, is responsible for the behaviors of autistic individuals. Although different brain regions support our ability to make sense of other people’s minds and visible cues, the study of autism suggests that they function as a network.
False-belief tasks are particularly challenging for children with autism. Even when they are well past the age when most children are able to solve these problems, autistic individuals perform these tasks as if the characters have access to all of the information in the story. For example, although they understand that Sally initially put the marble in the basket, they also act as if Sally knows that Anne moved the marble to the drawer. Therefore, they report that Sally will look for the marble in the drawer.
Michael Lombardo and his colleagues at Cambridge looked for the specific neural systems responsible for the impairments in representing mental state information in autism. They examined whether deficits are observed in processing information about both the self and the other, and they tried to find out how, or if, the atypical functioning of these neural systems relates to variation in social impairment (Lombardo et al., 2011). They designed a mentalizing task—a task that elicited robust activation of all the regions within the standard circuit known to be active when a nonautistic individual thinks about the thoughts of both the self and others: the MPFC, the PCC, and the bilateral TPJ. In answering the question about which neural system was responsible, they found that the rTPJ functioned atypically in autism. In nonautistic individuals, the rTPJ was selectively more responsive to thinking about thoughts than physical judgments (both in the self and other conditions). But in autistic individuals, the rTPJ was less responsive, and specialization was completely absent. This lack of selectivity correlated with the degree of social impairment. Put another way, the less selective the rTPJ response, the more impaired that individual was in representing the mental states of others (i.e., mindblindness).
False-belief tasks often give participants information about other people’s mental states; but as mentioned earlier, in real life we are often left to infer these states from nonverbal cues such as facial expression and eye gaze. People with autism, however, don’t pay attention to eye gaze as much as nonautistic individuals do (Spezio et al., 2007). Why is that? Some researchers have suggested that people with autism may avoid the eye gaze of others because they find eye contact unpleasant (Dalton et al., 2005). A recent study from Finland (Kylliäinen et al., 2012) combined EEG with skin conductive responses to explore whether frontal EEG asymmetry, as a measure of approach–avoidance brain activity, could clarify whether another person’s direct gaze is arousing or aversive to individuals with autism. These researchers found that a direct gaze with either normally open eyes or wide-open eyes (Figure 13.20) evoked neither avoidance-related brain responses nor approach responses in children with autism spectrum disorder (ASD). They did note, however, that autonomic arousal to faces increased as a function of the amount of sclera (white of the eye) visible in the direct gaze. This differed from the response of normally developing children for whom the normally open eyes evoked an approach response and wide-open eyes an avoidance response, but whose intensity of arousal was constant. This reaction is not so surprising when you look at the expressions (Figure 13.20) and recall that in facial expressions of fear, more of the sclera is visible (Chapter 10).
These findings do not support the suggestion that direct gaze is an aversive stimulus for children with autism (Kylliäinen et al., 2012). In fact, they support an alternate hypothesis proposed by Ami Klin and his colleagues at Yale University (2002b). Individuals with autism may fail to recognize the importance of eye gaze as a cue for understanding their social worlds. When watching the movie Who’s Afraid of Virginia Woolf? (Figure 13.21), nonautistic individuals spent much of their viewing time paying attention to the characters’ faces and eyes to gain understanding of their intentions and feelings. In contrast, autistic individuals fixated on mouths, bodies, and objects. Therefore, perhaps individuals with autism do not automatically distinguish eye gaze as an especially meaningful cue for perceiving other people.
Additional support for this explanation comes from several studies showing that autistic individuals exhibit significantly less activation in the STS when performing theory-of-mind tasks (see Frith, 2003, for a review). Instead, they exhibit activation in this region for a broader range of conditions. What happens when autistic individuals do the checkerboard task described in Figure 13.19? Not surprisingly, they show increased STS activation to any shift in eye gaze rather than specifically in response to eye gaze to unexpected locations.
The failure to pay attention to eye gaze can also be partially accounted for by the smaller amygdala size that is characteristic of autism. Recall from Chapter 10 that amygdala size correlates with attention to the eyes of other people. The smaller a person’s amygdala is, the less likely that individual is to attend to the eyes of another person (Nacewicz et al., 2006).
FIGURE 13.20 Three eye conditions: eyes closed, eyes opened normally, and widely opened eyes.

FIGURE 13.21 Study of eye gaze in healthy and autistic participants.
(a) The eye gaze of healthy participants is compared to that of participants with autism while they are watching characters in a film. (b) Healthy participants tend to focus on the eyes of characters in a film. In comparison, participants with autism do not show selective attention to the eyes in comparison to more noninformative aspects of the face.
Together, these studies suggest that in the brains of individuals with autism, the neural regions associated with person perception are not activated in the same way as in the brains of individuals without autism. It appears, then, that sometimes autism is associated with reduced function or volume in select brain regions, and sometimes it is associated with more inclusive activation that is not sensitive to subtle social cues (such as eye gaze specifically oriented toward an object of interest). Recently, upon considering these various findings, researchers have determined that ASD should be addressed by a systemslevel approach. With the realization that the brain is made up of multiple, distinct, and interacting networks, the complex symptomatology of ASD may become more understandable.
It has been suggested that, on a systems level, autism may affect the default brain network. As mentioned earlier in the chapter, the MPFC is part of a brain network that has a higher level of metabolism at rest—and this activity may reflect self-referential and social processing. The relation between autism and abnormalities in MPFC function may also extend to this region’s baseline mode. When healthy participants engage in thinking that takes their attention away from this self-referential processing, they experience deactivation in the MPFC. Participants with autism do not experience significantly less activity in their MPFC when performing non-self-referential tasks (D. P. Kennedy et al., 2006). That is, no change in activation takes place between “resting” and doing an active task. Is this because the default network is always on, or because it is always off? These researchers point out that PET studies are consistent with the always-off conclusion. Interestingly, participants with ASD report very different types of thoughts when their mind is at rest: Two out of three reported seeing only images but no internal speech, feelings, or bodily sensations. None of them had ever even thought about their inner experience. The third appeared to have no inner thoughts at all, but merely described what his current actions were (Hurlburt et al., 1994). Kennedy and his colleagues speculated that an absence of this resting activity in autism may be directly related to their differences in internal thought.
These researchers have since found that in some cases, the extent of these resting abnormalities correlates with the severity of autistic social impairments. They propose that one cause of autism may be unusually low metabolic rates in medial frontal cortex (D. P. Kennedy & Courchesne, 2008; Kennedy et al., 2006). If this proposal were true, we could infer that the social deficits seen in autistic individuals are partially due to their brains not being constantly prepared for the type of social thought that marks normal cognition. Some evidence does support this notion. When given explicit instructions to use a social process (e.g., pay attention to the faces), specific brain regions were activated in high-functioning autistic individuals. Unlike control participants, people with ASD did not exhibit activation in the same regions when given vague instructions in a social task (e.g., pay attention). Thus, people with ASD may fail to engage instinctively in social processing, but they can when explicitly instructed to do so. Possibly, they do not experience the constant impulse to view most events through a social lens (D. P. Kennedy & Courchesne, 2008; A. T. Wang et al., 2007).
Jason Mitchell (2011) has suggested that if autistic individuals truly are unencumbered by intensive social processing, then they are freer to attend to objects and other nonsocial aspects of the environment. Indeed, that is exactly where autistic individuals excel. Many people with ASD are unusually adept in visuospatial and other nonsocial domains, such as exceptional musical or drawing talent, puzzle-solving aptitude, or the capacity to perform complex mathematical or calendrical calculations mentally. Although about 10 % of autistic individuals demonstrate one such skill at the savant level, most have at least one enhanced nonsocial ability (Happé, 1999; Mottron & Belleville, 1993; Rimland & Fein, 1988). Freed from the constant demands of social cognition, their minds are able to engage intensely in nonsocial processing.
You may be wondering if anyone has made proposals about deficits in the mirror neuron systems of autistic individuals. Indeed they have. Deficits in mirror neuron systems come to mind when considering the difficulties autistic individuals exhibit with mimicry and imitation. Developmental psychologists have come to realize that imitative behavior is crucial for the development of social cognitive skills (Meltzoff & Prinz, 2002), and it is well recognized that humans automatically tend to imitate each other when interacting socially. The more they imitate, the more empathic they are. Some studies have shown that automatic mimicking is difficult for autistic children. For instance, they do not exhibit the degree of yawn contagion that normal children do (Senju et al., 2007), and although they can voluntarily mimic pictures of faces, they do not show automatic mimicry (D. N. McIntosh et al., 2006). For autistic individuals, some types of imitation are more difficult, such as imitating nonmeaningful or novel actions (for a review, see Williams et al., 2004), and some are easier, such as when the goal is clear or the person being imitated is familiar (Oberman et al., 2008). It seems, then, that sometimes children with ASD understand the goal of observed motor acts, a function of mirror neurons, and sometimes they don’t. This behavior suggests that several factors play a role in imitation and that if a mirror neuron system is involved, its role is not fully understood.
Luigi Cattaneo at the University of Parma suggested that in autistic individuals, the primary deficit in the mirror neuron system lies in how it links the initial motor acts into action chains, rather than in how responsive the mirror neurons are to the observation of other people’s actions. The idea is that mirror neurons respond to the initial motor action (such as reaching for food) by firing a specific action chain (in this case, reach, grasp, place in mouth) based on the initial motor movement. Thus, the observer of the action has an internal copy of the action before it occurs, allowing her to gain an understanding of the other person’s intentions. Cattaneo suspected something was awry in this system. To test this hypothesis, he designed a clever experiment using electromyography to record the activity of the mylohyoid muscle involved in mouth opening (Cattaneo et al., 2007). Children with ASD and typically developing children were asked either to reach, grasp a piece of food, and eat it or to reach, grasp a piece of paper, and place it in a container. In a second condition, the children observed an experimenter performing these actions (Figure 13.22). The two actions were subdivided into three movement phases: reaching, grasping, and bringing the object to the mouth or to the container. Cattaneo reasoned that if an action chain had been activated by the initial reaching movement, then the mouth muscle would be activated as soon as a person started for the food; but if not, then the muscle would be activated only as the food approached the mouth.
FIGURE 13.22 In one task, either the participant or the experimenter reaches for a piece of food, grasps it, and puts it in his mouth. In a second condition, a piece of paper is reached for, grasped, and put in a container on his shoulder.

FIGURE 13.23 The time course of MH muscle activity.
Reach action begins at time 0. (a) In typically developing children, the activity of MH muscle differs depending on the action. During execution of the bringing-to-the-mouth (red), the EMG indicated that the MH muscle’s activity increased several hundred ms before the hand actually grasped the food. When the activity is a placing action (blue) with no eating involved, the muscle remained inactive. (b) In children with ASD, there is no activation of the MH muscle during execution of either reaching or grasping. Similar results are seen during the observation of the bringing-to-the-mouth action (red) and the placing action (blue) in (c) normally developing children. (d) In children with autism, however, observing a hand grasping food and bringing it to the mouth does not illicit any MH action.
In typically developing children, mylohyoid (MH) activation was present early in the reaching and grasping phases of the grasping-for-eating action (Figure 13.23a) and when observing a grasping-for-eating action (Figure 13.23c). This early activation of the muscle involved in the final stage of the action indicates that understanding of the final goal of the action takes place early on. Not so for children with ASD, however. The MH was activated only during the last movement phase of bringing-to-the-mouth action (Figure 13.23b), and no MH activation occurred during observation of the action (Figure 13.23d). This evidence suggests that individual motor acts are not integrated into an action chain in children with ASD, resulting in their lacking full comprehension of the intention of others.
Intention can be broken down into what a person is doing and why he is doing it. These authors point out that the what of a motor act can be understood in two ways. One way is through a direct matching mechanism (i.e., mirror neurons). The what could also be predicted, however, by the semantic cues of the object itself. Just knowing what an object is can cue a person to what motor action will follow. So even if a person’s mirror neuron system were impaired, she could still predict the what goal of a motor act through external cues. In other words, sometimes the what process doesn’t actually depend on the person’s mental state, because recognizing the object is all the information that is needed to predict the goal.
FIGURE 13.24 The what and the why of an action inferred by motor and object cues.
(Experiment 1) Examples of hand grips that are either congruent with the function of the object (“why-use” trials) or the position typically used to move that object (“why-place” trials). (Experiment 2) Here, in both situations, hand grips are congruent with the use of the object, but only one is congruent with the inferred action cutting, and not with placing the object in the box. Autistic children infer intention through object cues.
How about the why goal of the motor act, especially when it is not related to an object? For example, when parents of autistic children extend their arms to hug their child, the child fails to extend his arms in return and does not understand why his parents are making the gesture. To analyze this kind of behavior, Sonia Boria and her colleagues at the University of Parma (2009) looked at whether autistic children understood both the what and the why of an action. Their experiment consisted of two parts. In the first part, children with ASD and typically developing children were presented with pictures showing hand–object interactions. In half of the why trials the children observed, the hand grip shown was congruent with the function of the object (“why-use” trials); in the other half, the grip corresponded to the position typically used to move that object (“why-place” trials; Figure 13.24). Then the children were asked what the individual was doing and why she was doing it. Both sets of children could accurately report the what, or goal, of the motor acts (i.e., she is grabbing the object). The children with ASD, however, made several errors in the why is she grabbing the object task, and all of these errors occurred in the “why-place” trials. In part two of the experiment, the children saw pictures of a hand grip that was compatible with the object’s use. The object was placed in a context suggesting either that it was going to be used (congruent with the grasp) or that it was about to be placed into a container (incongruent with the grasp). Here both sets of children performed equally, correctly reporting the agent’s intention. These researchers concluded that understanding the intentions of others can occur in two ways: by relying on motor information derived from the hand–object interaction, and by using semantic information, derived from the object’s standard use or the context in which it is being used. Children with ASD have no deficit in the second type of understanding, but they have difficulties in understanding the intentions of others when they have to rely exclusively on motor cues. In other words, they understand the intentions from external cues, not internal ones, thus, providing additional support for the notion that autism involves a deficit in the mechanics of the mirror neuron system.
This evidence in turn suggests that the mirror neuron system is highly interconnected. These studies and many others suggest that the imitation deficits and some of the other cognitive differences seen in autism may be a result of underconnectivity in the mirror neuron system and the involvement of alternative communication pathways (Kana et al., 2011).
The complicated business of understanding the thoughts, goals, intentions, desires, and beliefs of other people is made manifest when studying the deficits seen in ASD. The autistic individual’s difficulty in understanding other people is reflected in abnormal brain development and function affecting all of the major neural regions important for person perception and self-referential processing.
TAKE-HOME MESSAGES
Social Knowledge
In 1985, Simon Yates and Joe Simpson were the first mountaineers ever to reach the summit of Siula Grande, a remote peak in the Peruvian Andes. In his book, Touching the Void, Simpson explained the climb was made with no support or backup team. It would be remembered as much for these accomplishments as it was for the moral dilemma faced by the climbers. Early in the descent, Joe fell and broke his leg. How could he climb down the mountain now? Simpson later commented in an interview (Lloyd-Pierce, 1997) that when he told Simon Yates he had broken his leg,
What he should really have said was, “I’ll go off and get some help,” which would have been a euphemism for, “You’ve had it.” Instead, he chose to try and save my life by lowering me thousands of feet down the mountain on a rope, at great risk to himself. It was an incredible feat of mountaineering and we descended about 3,000 feet in this way.
The two men developed a system in which Simon would brace himself with his climbing axes and then lower Joe down using a 300-meter rope. After being lowered as far as the rope would permit, often out of Simon’s view, Joe used his climbing axes to brace himself on the mountain and then tugged on the rope. Simon would then make his way down to meet Joe and repeat the process. Late in the day, their slow progress became even slower and more treacherous when a storm hit, causing the icy mountain temperatures to drop even further. With only one more stretch to go before they could rest for the night in a sheltered spot, disaster struck a second time.
In the dark, Simon inadvertently lowered Joe down over an ice overhang. Instead of feeling a tug on the rope to signal him to start descending, Simon felt all of Joe’s weight tugging at him on the rope. Simon knew what this meant: Joe was dangling in the air. Unfortunately, Joe’s hands were so frostbitten that he was unable to tie the knots required to climb back up the rope. They were in this position for about an hour. Joe tried to yell to Simon, but he could not be heard over the storm. Simpson said:
I was dragging him down with me. In order to stop himself plummeting over the edge, the only thing he could do was cut the rope and let me go—to prevent us both being dragged to our deaths. He obviously knew that this could kill me, but he had no choice.
Simon grew colder. His hands were numb, and he no longer had his strength and grip and could not pull Joe back. Yates recalled:
I was being pulled towards the edge of the cliff, too. Cutting the rope was the only choice I had, even though it was obvious that it was likely to kill Joe. There wasn’t much time to think; it was just something which had to be done quickly or I’d have been dragged to my death.
He cut the rope.
The biggest taboo in the mountaineering community is to cut the rope attaching you to your partner. Ironically, Simon’s decision to violate the moral code of mountaineering may have been the only reason they both survived. The result, however, does not stop others from moralizing. Simon notes:
Sometimes someone who thinks what I did was unacceptable will come up and verbally assault me. The rope between two climbers is symbolic of trust and to cut it is viewed as a selfish act. What’s important is that Joe didn’t think that, and the first thing he did when he crawled back into camp was to thank me for trying to get him down.
Although Joe wrote that Simon did what he would have done in the same situation, Yates was ostracized by much of the mountaineering community.
To save his own life, Simon broke the moral code of the mountaineering community. Do you think he was justified in further endangering someone else’s life to save his own? Simon and Joe’s story is certainly an extreme case, but it illustrates the reality that social behavior is shaped by multiple influences. To negotiate our social worlds successfully, we must not only understand the rules for appropriate behavior, but make choices consistent with those rules. In this section, we consider questions about social knowledge and its use in decision making. How do we know which aspects of knowledge to apply to a particular situation? If our own interests conflict with societal norms, deciding how to proceed can be difficult. What can the brain systems used to make these sorts of decisions tell us about this psychological process?
Representations of Social Knowledge
One of the most complicated aspects of social behavior is the lack of straightforward rules. The very same behavior that is appropriate in one context may be wildly inappropriate in another. For example, hugging a close friend is an act of affection, but hugging a stranger may be considered intrusive. And should you hug someone you are getting to know better but do not yet consider a close friend? Or how about that guy you have a crush on? When is it appropriate to greet a person with a hug? Social cognitive neuroscientists are just beginning to research the neural systems that help us make these decisions. Current research findings suggest that the frontal lobes are important for taking into account the particular situation in order to apply the appropriate rules.
Orbitofrontal cortex Patients with orbitofrontal cortex (OFC) damage have the most difficulty when they need to draw on their social knowledge to make sense of social interactions. In one fascinating line of work, Valerie Stone and her colleagues developed a social faux pas task that measures a person’s ability to reason about the world. The task presents participants with a series of scenarios in which one of the characters commits a social faux pas by accidentally saying something impolite. One scenario tells the story of Jeannette and Anne. Anne receives a vase as a wedding gift from Jeannette. A year later, Anne has forgotten that the vase was from Jeannette. Jeannette accidentally breaks the vase while at Anne’s house. Anne tells Jeannette not to worry because it was a wedding gift that she never liked anyway. The researchers then measure social reasoning by asking participants to identify whether someone in this scenario made a social mistake, and if so, why. Stone and her colleagues gave this test to patients with orbitofrontal damage, patients with lateral prefrontal cortex damage, and healthy control participants (Stone et al., 1998).
In comparison to all other participants, patients with orbitofrontal damage did not perform as well on the test, thus demonstrating a decreased ability to apply their social knowledge to the scenarios (Table 13.1). Patients with orbitofrontal damage understood that a character like Jeannette would feel bad about breaking the vase, but they did not understand that Anne’s comment about not liking the vase actually was intended to reassure Jeannette. Instead, they often believed that Anne had intended to hurt Jeannette’s feelings. The patients with orbitofrontal damage were not as able to take the context into account when reasoning about the social mistakes. These results suggest that orbitofrontal damage impairs the ability to use social knowledge to reason about social interactions.
| table 13.1 Detection of Errors on Faux Pas Task | |
| Group Tested | Detected Faux Pas (n = 10 problems) |
| DFC patients | |
| L.S. | 10 |
| R.T. | 10 |
| O.A. | 10 |
| W.E. | 10 |
| Mean | 10 |
| OFC patients | |
| D.H. | 9 |
| M.R. | 6 |
| R.V. | 7 |
| R.M. | 8 |
| R.B. | 10 |
| Mean | 8 |
| Anterior temporal control | |
| B.G. | 10 |
| Normal controls | |
| Mean | 10 |
|
Source. From Stone et al., 1998. |
|
A series of studies conducted by Jennifer Beer provides some important clues that orbitofrontal cortex supports appropriate social behavior (Beer et al., 2003, 2006). In her study reported earlier in the chapter, patients with orbitofrontal damage, patients with lateral prefrontal damage, and healthy controls took part in a structured conversation with a stranger. Compared to the other participants, patients with orbitofrontal damage were likely to introduce impolite conversation topics. Before beginning the social interaction task, however, all the participants reported that it was inappropriate to discuss emotional and personal information with strangers. The patients with orbitofrontal damage were unaware that their actual social behavior violated these social rules for conversations with a stranger.
This lack of awareness may be especially problematic because it makes it difficult for patients with orbitofrontal damage to feel embarrassment that might motivate them to behave differently in the future. In another study (Beer et al., 2003), patients with orbitofrontal damage and healthy control participants took part in a teasing task that required them to make up nicknames for an experimenter they did not know well. Healthy control participants were careful to come up with flattering nicknames and to apologize for having to tease someone they did not know well. In contrast, patients with orbitofrontal damage offered unflattering nicknames and were likely to announce them in a singsong voice more often used for teasing someone you know well. The orbitofrontal patients were not embarrassed by their inappropriate teasing; instead, they reported feeling especially proud of their social behavior.
Without awareness of their social mistakes, patients with orbitofrontal damage never generate the emotional feedback they need to change their future behavior. When we do something that makes us feel embarrassed, we don’t like that feeling and are strongly motivated to avoid feeling that way again. When we do something that makes us feel proud, however, we are likely to repeat the action in order to continue the good feeling. These findings suggest that even though patients with orbitofrontal damage report an understanding of social rules, they do not apply this knowledge to their own social interactions (Figure 13.25). They are also unlikely to spontaneously recognize that their behavior is inappropriate, because they lack self-insight and do not generate the social emotions needed to correct their social mistakes in future social interactions.
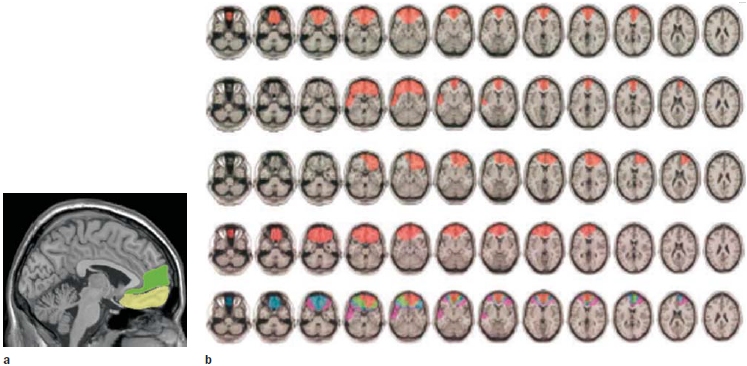
FIGURE 13.25 Patients with orbitofrontal damage may lack insight into their behavior at a particular moment while maintaining accurate summaries of their traits. (a) The orbitofrontal cortex (yellow) lies just beneath the medial prefrontal cortex region (green) associated with the summaries of personality traits. (b) Typical orbitofrontal damage. Damage is indicated in red. Each row represents ascending brain slices beginning on the left, with the most superior slice to the far right, of a single patient. The bottom row is a composite of the findings from all the patients, indicating the extent of overlap in the location of lesions. Red indicates 75–100% overlap, green 50–75%, blue 25–50%, and pink 0–25%.
Adult patients who have sustained orbitofrontal damage and behave inappropriately can retain intact social knowledge about what is proper—that is, social rules—but they appear to have trouble learning new social knowledge. This view is supported by case studies of orbitofrontal damage sustained in childhood. These patients also have inappropriate social behavior; but, in contrast to patients who receive this damage in adulthood, they do not understand social rules because they had not learned them before being injured (S. W. Anderson et al., 1999). This finding suggests that the orbitofrontal cortex is important for learning social knowledge as well as applying it to specific social interactions.
Using Social Knowledge to Make Decisions
The research described in the preceding discussion suggests that the orbitofrontal cortex is important for both learning social knowledge and using it in relevant situations. Even if we know the rules for a given social situation, we still have to decide what to do to ensure that we abide by the rules. Consider the following scenario. When you go to a friend’s house for a party, you know that there are certain rules for being a polite guest. These rules may help you avoid inappropriate behavior, but they do not always point to one specific behavioral choice. For example, you can do a number of things and still be polite. Do you hug someone you are introduced to, or just shake their hand? Do you get something to eat now, or wait until later? Do you mention that you are a vegetarian, or just eat what you can without mentioning it? How do we make decisions about our social behavior? What are the brain mechanisms that support decision making using social knowledge?
Patients with ventromedial prefrontal cortex damage are notoriously poor at making social decisions. (Here the ventromedial prefrontal cortex includes the medial OFC.) Early research attempting to identify and understand the function of the brain regions involved with social decision making gave gambling tasks to VMPFC patients. These patients had a difficult time making decisions when the outcome was uncertain. Leslie Fellows and Martha Farah (2007) wondered, however, if this difficulty was specific to decisions involving uncertainty, or if it reflected a general difficulty in assessing the relative value of options. In the experiment discussed earlier, where the task was a simple preference judgment between two options of colors, actors, or food, we learned that the VMPFC damage impairs value-based decision making even when no uncertainty exists.
In Chapter 10, we learned that people with OFC damage are unable to respond to changing patterns of reward and punishment. That is, they can learn that a stimuli is rewarding (its value), but when it becomes punishing (the value changes), they still choose it. Thus reversal learning does not take place, and individuals with OFC damage don’t learn from a negative experience. To learn from experience, we must be able to change behavior as a result of unexpected negative feedback. Thus, in a social situation, sometimes hugging someone is appropriate and you get a hug back—positive feedback that your behavior was okay. Sometimes, however, the hug is not appropriate and the person stands frozen in your embrace. If your behavior unexpectedly receives the cold shoulder, you feel embarrassed, and you are guided by that negative feedback to change your behavior. When we consider that the VMPFC is involved in coding stimulus value, it seems odd that patients with VMPFC lesions can selectively learn a stimulus value initially, but not when the stimulus value is reversed. Geoffrey Schoenbaum and his colleagues found in rats that although the OFC may be critical in reversal learning, it is not because it flexibly represents positive and negative value. They found that the better the reversal learning, the less flexible the OFC value coding was. It appeared to them that the OFC does not code stimulus value, but signals the amygdala when the value expectation is violated (Schoenbaum et al., 2007).
Following this idea, Elizabeth Wheeler and Lesley Fellows (2008) investigated whether positive and negative feedback of stimulus value expectation influences behavior through separate and distinct neural mechanisms. The study participants were patients with damage to the ventromedial frontal lobe (VMF, a term the researchers used to refer to the region encompassing both medial OFC and adjacent ventral medial PFC), healthy controls, and patients with dorsolateral frontal (DLF) damage. The researchers asked the participants to do a probabilistic learning task with positive and negative feedback while undergoing fMRI. They found that VMF damage selectively disrupted the ability to learn from negative feedback, but not from positive feedback. The controls and patients with DLF damage performed equally and were able to learn from both positive and negative feedback: This evidence suggests two distinct neural mechanisms.

|
FIGURE 13.26 Cortical atrophy in frontotemporal lobar degeneration patients with social disorder (shown in blue) overlaps with brain regions that are seen to activate in fMRI studies of healthy adults undertaking judgments of negative social scenarios (shown in orange). |
These researchers point out that these findings are consistent with much of the literature that implicates the VMF in reversal learning, extinction, fear conditioning, regret, and envy. The results, however, are hard to reconcile with the previous study by Fellows discussed earlier (and findings in neuroeconomics that we discuss in the next section), suggesting that this region represents relative reward value and preferences. Perhaps, as the researchers propose, the VMF may carry representations of the expected (relative) reward value not to guide choice per se, but to serve as a benchmark to compare outcomes against. When the outcomes are negative and unexpectedly fail to match expectations, the VMF enables avoidance learning. Perhaps, as suggested by Geoffrey Schoenbaum and his colleagues (2007), this process takes place not directly, but indirectly by signaling to the amygdala and other regions to form new associative representations that may flexibly change their behavior. This proposal would suggest that in patients where the VMF is not functioning, no benchmark is provided, no outcomes are being compared, no negative feedback is generated, and no reversal learning can take place. A bad social experience has no effect. The positive feedback system is intact, however, and learning can take place through positive feedback.
Can we apply this finding to social judgments? For instance, when you expect a hug back and don’t get one, is your OFC activated? Penn State researchers specifically addressed the role of VMPFC in the interpretation of negatively valenced feedback during social decision making (Grossman et al., 2010). They matched healthy controls with patients who had VMPFC degeneration due to frontotemporal lobar degeneration (FTLD). These patients make socially inappropriate comments, engage in socially unacceptable behavior, and often show little insight into the effects of these behaviors despite their social (and sometimes legal) consequences. The participants first judged 20 social situations (e.g., cutting into the ticket line at a movie theater) or minor infractions of the law (rolling through a red light at 2 a.m.) on a scale of 1 to 5 for social acceptability. These scenarios were then given contingencies that were either negatively biased (e.g., rolling through a red light at 2 a.m. when a police car is at the intersection) or positively biased (e.g., rolling through a red light at 2 a.m. when rushing a sick child to the emergency room). This time, participants were asked to judge according to two randomly presented instructions: “Should everyone do this all of the time?” (rule-based condition) or “Is this generally okay?” (similarity-based condition). This manipulation was intended to ferret out differences that could be due to insensitivity to perceived legal and social rules. No differences were noted in the performance of the FTLD patients.
Although both the FTLD patients and the healthy adults rated the positively biased scenarios as equally acceptable, they rated the negatively biased scenarios differently. The FTLD patients judged negative scenarios to be more acceptable than the healthy adults judged them to be. When healthy adults judged these negative social scenarios, significantly greater activation occurred in their VMPFC than when they judged the positive social scenarios—the very region of cortical atrophy in FTLD patients (Figure 13.26). These studies support the hypothesis that VMPFC plays a crucial role in evaluating the negative consequences of social decision making.
As suggested in the previous section, the orbitofrontal cortex plays a strong role in applying social knowledge to our decisions in social settings. This region likely helps us choose the correct behaviors by supporting reversal learning through the evaluation of the negative consequences of social decisions. As the case of patient M.R. from the chapter opener suggests, the orbitofrontal cortex is helpful for recognizing when a hug is appropriate and when it is not.
Neuroeconomics
A recent perspective on the problem of how we make decisions using social knowledge comes from a new field called neuroeconomics. Neuroeconomics integrates psychology, neuroscience, economics, and computational models to yield an understanding of how people make value-based decisions (Rangel et al., 2008). Economic models of decision making assume that people should make rational decisions—those that maximize their rewards and minimize their losses. Specifically, rational decision making focuses on the choice that will reap the largest monetary outcomes. As we all know, however, people often don’t make rational decisions, economic or otherwise. Recognizing that people do not always make decisions based on the greatest financial outcomes, these models have more recently begun to incorporate the role of emotional reactions that often arise in relation to concerns that are not financial. Some neuroeconomists propose that emotions may sometimes help people make optimal decisions by taking into account a wider range of consequences. These researchers are trying to create decision-making models that include cognitive and emotional variables driven by valuation of gains, losses, risks, and uncertainties.
Suppose you are given $50 and a chance to gamble with it. If you had either a guarantee of keeping $20 or a chance to gamble it all, which would you choose? What if you had a guarantee of losing $30 or a chance to gamble it all? Would you make a different choice then? Most people prefer to gamble when faced with a guaranteed loss, even when the monetary consequences of the guaranteed options are the same, as they are in the two bets outlined here (Figure 13.27). A guaranteed loss elicits a negative emotional response and makes people focus on any option that will help them avoid the guaranteed loss. Acting on emotion is detrimental, however, because participants are not making decisions based on the actual monetary consequences. Benedetto De Martino and his colleagues at University College London (De Martino et al., 2006) conducted an fMRI study to understand the neural systems that underlie emotion-driven and rational decision making in this task. They found that participants who were misled by the loss frame tended to show activation in the amygdala. Orbitofrontal cortex activation was correlated with rational decision making. Specifically, participants who made decisions based on monetary principles had significantly more orbitofrontal cortex activation than did participants who based their decisions on emotion.
In the preceding example, emotion shaped participants’ decision making in a detrimental manner. What about when we make financial decisions in the context of an interaction with another person? Some research suggests that emotions may lead to decision making that is financially irrational (because money will be lost) but beneficial for defending social reputation. One study examined decision making using the Ultimatum game (Sanfey et al., 2003). In the Ultimatum game, one player (P1) must split a sum of money with another player (P2). P1 offers a portion of the sum to P2 and P2 must decide to accept or reject the offer. The offers may be fair (e.g., very close to 50 % for each person) or unfair (e.g., 80 % for P1 and 20 % for P2). If P2 rejects the offer, however, neither player gets any money.
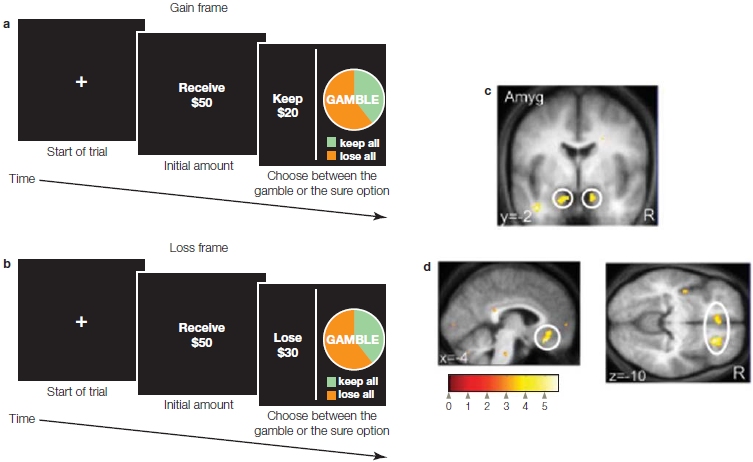
FIGURE 13.27 A gambling task in which participants can choose to gamble in the context of a guaranteed gain (a) or a guaranteed loss (b). In both guaranteed cases, the outcome is the same; it is merely couched in different terms. (c) Participants who react to the condition of gain or loss rather than actual money amounts activate their amygdala when placing a bet. (d) Participants who bet on the basis of money amounts and are not swayed by a guaranteed gain versus loss activate their medial and lateral orbitofrontal cortex.
In this study, the consideration of unfair offers was associated with dorsolateral prefrontal cortex and insula activity. Insula activity has often been associated with negative emotions such as disgust, anger, pain, and distress, suggesting that the participants experienced these emotions while considering the offer. What’s more, increased insula activity during consideration of an unfair offer predicted a likelihood that the offer would be rejected. From a rational economic perspective, participants should not let their negative emotional reaction lead them to reject the unfair offer. Even if it is unfair, they can gain some money instead of no money. From a broader perspective, however, the negative emotional reaction leads participants to reject unfair offers that might otherwise compromise their social standing. If you continually accept less than your share, word may get around and people may begin to view you as deserving of less than an equal share. By rejecting the offer, you also punish the other player, who then also receives nothing. You may thus gain social standing by punishing unfair players.
In the Ultimatum game, P2 can only react to P1’s offer. How does emotion help or hurt decision making when P2 has a more active role and has to speculate on the actions of P1? An fMRI study used the prisoner’s dilemma game to test this question (Rilling et al., 2002). In the prisoner’s dilemma game, participants again make decisions about how to divide a sum of money. Participants’ winnings are determined by various combinations of their own decision to cooperate or betray their partners, and their partners’ decisions to cooperate or betray them (e.g., combinations of whether each partner makes fair or unfair offers). The choice to cooperate is a double-edged sword; participants win the most if both players choose to cooperate, but lose the most if one player decides to cooperate and the other player decides to betray.
In this study, cooperation was related to areas associated with reward states, such as the nucleus accumbens, orbitofrontal cortex, anterior cingulate, and caudate nucleus. The authors suggest that this activation reflects a positive emotional experience that reinforces prosocial decision making.
It could be that being prosocial is its own reward, however. More recent investigations of the neural systems that underlie human prosociality consistently suggest that people experience prosocial acts as intrinsically rewarding. Help a stranger jump his car battery, and you get a little reward yourself and feel good. A rich and growing body of neuroscience research has reliably demonstrated that reward and subjective value rely on activity in mesolimbic dopaminergic targets—including the nucleus accumbens (NAcc) and OFC (Padoa-Schioppa & Assad, 2006; Rangel et al., 2008; Rolls, 2004; Tom et al., 2007). In humans and other animals, activity in these regions strongly correlates with the subjective value of a wide variety of reward types. These include primary rewards, such as food and juice, and secondary outcomes, such as monetary gains (Berns et al., 2001; Kable & Glimcher, 2007; Padoa-Schioppa & Assad, 2006; Schultz, 2002; Tom et al., 2007). (As described in Chapter 12, however, violations of expected value may be at the core of the brain activity, rather than value per se.) Surprisingly, even in the absence of direct, first-person rewards, these same regions are also activated by prosocial outcomes. For example, the NAcc responds robustly when a person is rewarded with money as well as when that person simply watches someone else win a cash reward he has gained fairly (Mobbs et al., 2009). This evidence suggests that perceivers experience positive outcomes for another person to be rewarding in their own right. Now you know why people like watching game shows. Along the same lines, similar patterns of neural response have been observed when one person agrees with others, suggesting that individuals experience interpersonal consensus as intrinsically rewarding (Klucharev et al., 2009). In both cases, these activations were observed even though participants received no immediate reward other than the prosocial outcomes associated with positive social events.
Moral Decisions
Neuroeconomics focuses on financial decisions, but the relative contributions of emotion and cognition have also been theorized to support other kinds of social decision making. How do we resolve moral dilemmas like the one that Simon Yates faced on the Siula Grande climb? What can the brain tell us about this process? Are we relying on emotion or on cognitive computations? For discussion of the implications of the relationships among brain function, moral judgment, and criminal behavior, see “The Cognitive Neuroscientist’s Toolkit: Neuroethics.”
Simon’s dilemma is a real-life example of the classic trolley dilemma in philosophy. In this problem, a conductor loses control of his trolley car (Figure 13.28). As a witness to this event, you can see that, if nothing is done, five people are likely to be killed because they are directly in the path of the speeding trolley. You can throw a switch and divert the trolley onto another track. This option, however, comes at the cost of ensuring the death of a single construction worker who is on the alternate track. Do you throw the switch or not? Now consider the footbridge dilemma. This time you are standing next to a large stranger on a footbridge that crosses over the tracks. You see an out-of-control trolley car speeding toward five people. This time, the only way to stop the trolley car is to push the person next to you off the footbridge onto the tracks to impede the movement of the trolley car. Do you push the stranger onto the tracks in order to save the other five people?
FIGURE 13.28 The trolley and footbridge problems.
Would you be willing to sacrifice one life to save five lives? Would your decision be different if you had to (a) pull a switch to direct a trolley toward one person or (b) physically push a person off a footbridge into the path of a trolley car? Research suggests that the strong emotional response to actually pushing someone would make you decide differently in these two scenarios.
Most people agree that is acceptable to throw the switch in the trolley dilemma, but they find it immoral to push the stranger in the footbridge dilemma. In both cases, one person’s life is sacrificed to save five others, so why do we make such different choices? Simon’s dilemma on Siula Grande draws on aspects of both the trolley car and the footbridge dilemmas. We know already that Simon could not simply walk away from Joe while he was alive. Thus he was willing to put his life at great risk to try to save Joe’s. When Joe’s life was again threatened, Simon made the opposite decision to save his own life and cut the rope, even though he could be sending Joe to his death. Do you think Simon would have cut the rope if he had been looking right at Joe? What would you have done if you were in Simon’s position on Siula Grande?
Joshua Greene and his colleagues at Princeton University (2004) argue that we make different choices in the trolley and footbridge dilemmas because the level of personal involvement in causing the single death differentially engages emotional decision making. If you throw a switch, you still maintain some distance from the death of the construction worker. When you actually push the stranger, you perceive yourself as more directly causing the death. Greene and his colleagues conducted a series of fMRI studies that contrasted moral dilemmas involving high levels of personal engagement with dilemmas involving low levels of personal engagement (Greene et al., 2001, 2004). As predicted, personal dilemmas and impersonal dilemmas were associated with distinct patterns of activation. Across the studies, impersonal decisions were associated with greater activation in the right lateral prefrontal cortex and bilateral parietal lobe, areas associated with working memory (Chapter 9). In contrast, when participants chose options that required more personal effort, regions such as the medial frontal cortex, the posterior cingulate gyrus, and the amygdala were significantly activated. These regions have been associated with emotional and social cognitive processes. Together, these studies suggest that the differences in our moral decisions are related to the extent that we permit emotions to influence our decisions about what is morally acceptable.
TAKE-HOME MESSAGES
THE COGNITIVE NEUROSCIENTIST’S TOOLKIT
Neuroethics: An Emerging Field
On July 10, 2003, William Safire of the New York Times coined the term neuroethics to refer to “the field of philosophy that discusses the rights and wrongs of the treatment of, or enhancement of, the human brain.” In the past few years, the term has come to encompass how society will “deal with social issues of disease, normality, morality, lifestyle and the philosophy of living” as informed by our understanding of the underlying brain mechanisms (Gazzaniga, 2005).
Antisocial personality disorder (APD) is a mental illness characterized by utter disregard for social rules and the rights of others. It is almost always accompanied by violence, aggression, deceitfulness, impulsivity, and lack of remorse. Genetic research on twins who were reared apart reveals some genetic influences on APD and aggressive behavior (Rowe, 2001). Genetic influences, however, are not always sufficient to produce the behaviors associated with APD. Such behaviors are most often expressed when environmental influences are also present.
Evidence for this observation comes from research on adopted children. In a study of Swedish male adoptees, researchers determined that, when both genetic factors for violence and environmental factors encouraging violence were present, 40% of adoptees had engaged in criminal behavior. When genetic factors were present in the absence of environmental factors encouraging violence, only 12% of adoptees had committed illegal acts. The percentages dropped to 7% of adoptees when genetic factors were absent and only environmental factors remained, and to just 3% when neither environmental nor genetic factors were present.
Based on this evidence, Adriane Raine of the University of Pennsylvania (2002) proposed a biosocial model for the development of violent behavior (Figure 1). The model outlines how genetic and environmental dispositions for violence, as well as genetic and environmental protective factors against violence, can influence the likelihood of violent behavior. In effect, this model demonstrates the subtle interplay between nature and nurture. Raine and his colleagues employed a variety of imaging techniques to assess this model. By using positron emission tomography, they found that individuals with violent and antisocial histories had reduced glucose metabolism in the orbitofrontal cortex. In terms of structural abnormalities, people with APD have reduced volume of prefrontal gray matter when compared to both a normal and a substance dependent control group (Figure 2). These findings suggest that a dysfunctional orbitofrontal cortex—resulting from environmental factors, genetic factors, and the interaction of environmental and genetic factors—is associated with abnormal social behavior and violence.

FIGURE 1 According to Adriane Raine’s biosocial model of violence, both genetic and environmental factors are necessary for the behavioral result of violence.
The advances of neuroscience raise important ethical questions. APD is present in overwhelming proportions in the prison community (65%–80%). If such behavior has a neural correlate, then is the person at fault for committing the crime? Could that person have done otherwise? Or did his brain make him commit the crime, thus absolving him of responsibility for the crime? This viewpoint ironically assumes a dualist stance, suggesting that the person and brain are separate—not something a cognitive neuroscientist usually accepts.
FIGURE 2 In patients with antisocial personality disorder, the volume of cortex (gray matter) in the prefrontal region of the brain is significantly reduced from both a normal and a substance dependent control group.
One of the authors of this book (M.S.G.) argues in his book, The Ethical Brain (2005), that the questions the law asks are not the same as those that neuroscience answers. We do not know that a specific amount of loss of prefrontal gray matter will cause antisocial behavior, nor do we know exactly how much neuronal loss or dysfunction correlates with the inability to choose to do the right thing. The people studying these issues in cognitive neuroscience fervently hope to better understand these correlations in the future.
This is just one example of the intersection of neuroscience and ethics. Many other examples are beginning to emerge (Figure 3), and we will consider some of them in the next chapter. In the years to come, it will be important to engage in a societal discussion of these issues.
FIGURE 3 Issues Facing Neuroethics.
Current topics of neuroethical debate include free will, the neural basis of criminal behavior, neuroenhancement, the reliability of memory, the possibility of a neural correlate of the soul, when consciousness begins, and when consciousness ends.
Summary
In the more than 100 years separating the cases of Phineas Gage and patient M.R., researchers have learned very little about the relation between brain function and social cognition. With the development of new research tools and new theories, however, the fields of social cognitive neuroscience and neuroeconomics are beginning to develop. Exciting insights into how the brain supports our ability to know ourselves, to know other people, and to make decisions about our social worlds have already resulted, though we still have a long way to go.
We know from behavioral research that self-perception is unique in many regards, even at the neural level. We store incredibly elaborate information about ourselves, and the medial prefrontal cortex supports the particularly deep processes by which we encode this information. The increased baseline metabolism in this region may indicate that we chronically engage in self-referential thought, and many other processes represent momentary diversions of our cognitive resources from self-referential thought. Although the orbitofrontal cortex helps us consider contextual information so that we remain relatively accurate in our self-perceptions, the anterior cingulate may help us view ourselves through rose-colored glasses by marking positive information about the self.
When we try to understand other people, we are faced with the difficult task of trying to reason about their mental states, which are not directly accessible to us. This process heavily relies on our ability to use nonverbal cues such as facial expression and eye gaze to gather information about possible mental states. Then we have to represent this abstract information and use it to form an impression of what the person might be thinking. A number of structures support our ability to make inferences about other people’s minds: the medial prefrontal cortex, right temporoparietal junction, superior temporal sulcus, fusiform face area, and amygdala. The widespread impairment of these regions in autism, a developmental disorder marked by deficits in person perception, reinforce the theory that these regions work together to support theory-of-mind abilities.
Although we often contrast self-perception and the perception of other people, the processes are not always completely distinct. The intrinsic relation between these two types of perception is illustrated by their neural commonalities. The medial prefrontal cortex may support the perception of both self and others when we draw on properties of self-perception to make sense of other people. In addition, mirror neurons in regions such as the insula, anterior cingulate, and somatosensory cortex appear to support our own emotional experiences as well as our ability to empathize with the same emotional states in other people.
Along with understanding ourselves and other people, we need to understand the rules for social interactions and how to make decisions to satisfy the multitude of rules that govern a particular social interaction. The process of making social decisions engages a large network of neural structures, including the orbitofrontal cortex, the dorsolateral prefrontal cortex, the amygdala, the anterior cingulate, the medial prefrontal cortex, the caudate, and the insula. Although this research is just emerging, the patterns of activations appear to reflect a tension between the use of emotional and cognitive processes for making social decisions.
Some of the same brain regions are activated in relation to the three main processes of social cognition: selfperception, person perception, and social knowledge. It may be tempting to describe these regions as the “social brain.” It is important to keep in mind, however, that almost every brain function has been adapted for social functions, even if they are not uniquely social. Although social interaction may influence how we select motor movements or where we direct our attention, motor movement and vision are also useful for finding food and other nonsocial functions. Disorders like autism suggest, however, that abnormal function in certain brain regions most powerfully affects social function. The interdisciplinary perspectives of social cognitive neuroscience and neuroeconomics promise to give us a deeper understanding of the processes that are most fundamental to social behavior.
Key Terms
autism (p. 561)
default network (p. 568)
empathic accuracy (p. 573)
empathy (p. 576)
False-Belief Task (p. 582)
imitative behavior (p. 589)
joint attention (p. 585)
neuroeconomics (p. 596)
orbitofrontal cortex (OFC) (p. 593)
reversal learning (p. 575)
self-reference effect (p. 563)
simulation theory (p. 575)
social cognitive neuroscience (p. 560)
theory of mind (p. 573)
theory theory (p. 575)
Thought Questions
Suggested Reading
Adolphs, R. (2003). Cognitive neuroscience of human social behaviour. Nature Reviews Neuroscience, 3, 165–178.
Baron-Cohen, S., & Belmonte, M. K. (2005). Autism: A window onto the development of the social and the analytic brain. Annual Review of Neuroscience, 28, 109–126.
Gazzaniga, M. S. (2005). The ethical brain. New York: Dana Press.
Gazzaniga, M. S. (2008). Human. New York: Harper & Row (Ecco).
Lieberman, M. D. (2007). Social cognitive neuroscience: A review of core processes. Annual Review of Psychology, 58, 259–289.
Macmillan, M. (2002). An odd kind of fame: Stories of Phineas Gage (reprint ed.). Cambridge, MA: MIT Press.