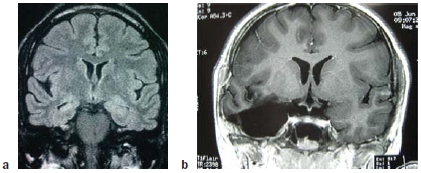
FIGURE 9.1 A temporal lobectomy.
(a) Coronal MRI image prior to surgery. (b) MRI image following removal of right amygdala, hippocampus, and anterior temporal lobe.

|
When I was younger, I could remember anything, whether it had happened or not. ~ Mark Twain |
Chapter 9
Memory
OUTLINE
The Anatomy of Memory
Memory Deficits: Amnesia
Mechanisms of Memory
The Medial Temporal Lobe Memory System
Imaging Human Memory
Memory Consolidation
Cellular Basis of Learning and Memory
FROM THE TIME HE WAS A CHILD, H.M. suffered from progressively worsening epilepsy. Over the years, his physicians had tried to control his seizures with the available drugs, but they were largely ineffective. While in his 20s, H.M.’s seizures became so bad that he was having 10 minor seizures a day and a major seizure every few days. In 1953, at age 27 (Figure 9.1), he was no longer able to work.
At that time, neurologists knew that many seizures originated in the medial portions of the temporal lobe, and their electrical impulses could spread across the brain, producing violent seizures and loss of consciousness. It was also becoming increasingly clear that surgically removing the seizure focus, the brain region where seizure activity originated, could help patients with epilepsy. William Beecher Scoville, a neurosurgeon at Hartford Hospital in Connecticut, offered H.M. an experimental surgical therapy: bilateral resection of his medial temporal lobes, or what the surgeon called a temporal lobectomy. Like W.J. in Chapter 4, H.M. was desperate. He agreed to the surgery. H.M.’s temporal lobes, including his amygdalae, entorhinal cortex, and hippocampi, were removed.
Although the surgery succeeded in treating his epilepsy, H.M.’s physicians, family, and friends soon realized that he was now experiencing new problems. H.M. had profound amnesia, a disorder of memory. He did not have the kind of amnesia that we usually see depicted in television shows or movies, in which the character loses all personal memories. H.M. knew who he was, remembered his personal history, facts he had learned in school, language, how to do things, social events, people, almost everything—that is, up until a couple of years before his surgery. For those previous couple of years, he drew a blank. More troubling was that when a nurse whom he had just spoken to left the room and returned after a short delay, he could not remember ever having seen or spoken with her before. He could follow a conversation and remember a string of numbers for a while, but he could not repeat them an hour later. So while his short-term memory was intact, H.M. could not form new long-term memories.
No surgeon had ever removed both of a patient’s temporal lobes before, so no one knew that it would lead to severe amnesia. Since then, great care is taken to avoid removing both medial temporal lobes, or even one medial temporal lobe if the other is compromised in any way from prior damage or disease. This adapted form of the surgery, known as unilateral temporal lobectomy (Figure 9.1), is still used successfully today for certain patients suffering from epilepsy.

|
FIGURE 9.1 A temporal lobectomy. |
While some of our knowledge about the world comes hard wired from the baby factory, much of it comes from experience. Learning and remembering information about the world around us enables us to make predictions about the future from our past experiences. In order for those past experiences to be useful, certain kinds of information have to be stashed away in memory: what happened, where and when, who was involved, and the value of experience. Being able to recall these facts allows us to guide our actions when confronted in the future with the same or similar situation (Nadel and Hardt, 2011). The cognitive abilities that allow us to store this type of information through learning and memory make us adaptable and provide a survival advantage by enabling us to avoid situations that we found dangerous in the past and to seek those that were previously beneficial.
Despite the vast stores of information contained in our brains, we continuously acquire new information. Learning is the process of acquiring that new information, and the outcome of learning is memory. That is, a memory is created when something is learned, and this learning may occur either by a single exposure or by repetition of information, experiences, or actions. We retain some forms of information only briefly, while some memories may last a lifetime. You may not remember what you had for dinner last Thursday, but you may remember the chocolate cake with the scuba divers on it you had for your birthday in second grade. Not only that, but you may also remember many of the guests who attended and the games you all played. This latter characteristic of memory led the University of Toronto’s Endel Tulving to describe some forms of memory as “mental time travel.” By this, Tulving meant that the act of remembering something that happened to us previously is to reexperience the context of the past experience in the present.
Not all memories are processed in the same manner. Researchers now generally believe that humans and animals have several types of memory, which may be mediated by different neural mechanisms. All forms of memory involve cellular and circuitry changes in the nervous system. Exactly what these changes are, and in which neural circuits and systems they are manifest, remain important questions for cognitive neuroscience. Let’s begin with an overview of memory and the basic steps of memory processing.
Models of memory include distinctions among very short-lived memories like sensory memory, which has a lifetime measured in milliseconds to seconds; shortto medium-lived memories like short-term memory and working memory, which persist for seconds to minutes; and memories that may persist for decades, which we call long-term memory. The various types of short-term and long-term memory are summarized in Table 9.1 and in Figure 9.2. We take a detailed look at these types of memory later in the chapter.
| table 9.1 Type of Memory | ||||
| Type of Memory | Characteristic of Memory | |||
| Time Course | Capacity | Conscious Awareness? | Mechanism of Loss | |
| Sensory | Milliseconds to seconds | High | No | Primarily decay |
| Short-Term and Working | Seconds to minutes | Limited (7 ± 2 items) | Yes | Primarily decay |
| Long-Term Nondeclarative | Days to years | High | No | Primarily interference |
| Long-Term Declarative | Days to years | High | Yes | Primarily interference |
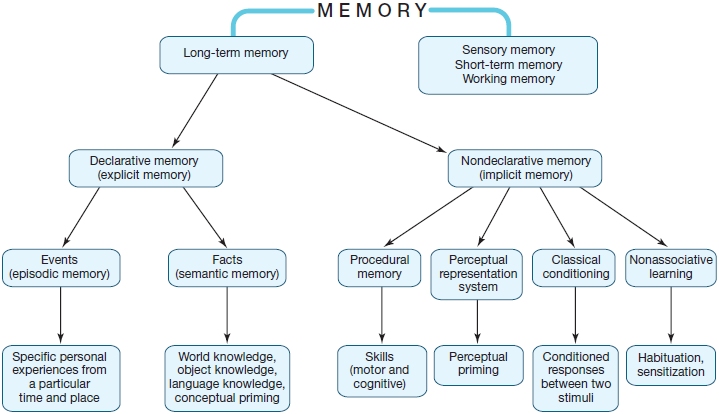
FIGURE 9.2 The hypothesized structure of human memory, diagramming the relationships among different forms of memory.
Researchers divide learning and memory into three major processing stages:
In this chapter, we explore what is known about the neuroscience of learning and memory, starting with a tour of the brain regions involved in memory encoding, storage, and retrieval. We also look at what we have learned about memory and learning from patients with amnesia. Then we look at how memory has been categorized and discuss the current thinking about what memory systems exist and how they work. At the end of the chapter, we discuss the cellular mechanisms that are thought to mediate memory formation.
The Anatomy of Memory
The brain has the ability to change through experience—in other words, to learn. At the neural level, this means that changes occur in the synaptic connections between neurons. It also implies that learning can occur in multiple regions of the brain. Learning can be accomplished in a number of ways, and it appears that different parts of the brain are specialized for different types of learning. For instance, in the last chapter we discussed the role of the basal ganglia in reinforcement learning and the involvement of the cerebellum in trial-and-error learning based on prediction error signals. The amygdala is involved with fear learning, which we will read more about in the next chapter.
As can be seen in the Anatomical Orientation box, many regions of the brain are also involved in one or more aspects of memory. What has come to be called the medial temporal lobe memory system, first described after H.M.’s surgery, is made up of the hippocampus, an infolding of the medial temporal cortex that is shaped like a sea horse (Hippocampus is the genus name for the marine fish known as a sea horse), and the various structures interconnected with the hippocampus. These include the surrounding entorhinal cortex, perirhinal cortex and parahippocampal cortex within the temporal lobe, and subcortical structures including the mammillary bodies and anterior thalamic nuclei. The hippocampus is reciprocally connected with wide regions of the cortex via the entorhinal cortex and the output projection pathway of the fimbria and fornix to the subcortical portions of the system, which themselves project to the prefrontal cortex. Although the amygdala, also located in the temporal lobe, is primarily involved in affective processing, which can have an influence on learning and memory as we will see later in the chapter, it is not involved with memory in general.
ANATOMICAL ORIENTATION
The anatomy of memory
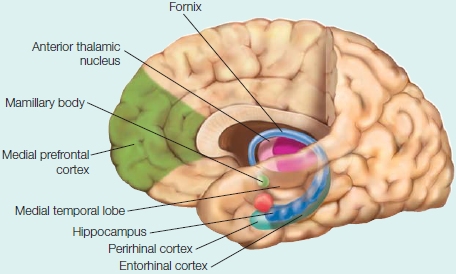
The components of the medial temporal lobe memory system are shown. Other regions of the brain, such as the prefrontal cortex, are involved in storage and retrieval of memories.
TAKE-HOME MESSAGES
Memory Deficits: Amnesia
Memory deficits and loss can result from brain damage caused by surgery, disease, or physical or psychological trauma, and are known collectively as amnesia. Amnesia is a form of memory impairment that affects all of the senses. Typically, amnesiacs display deficits in specific types of memory or in aspects of memory processing. Each type of functional deficit is associated with a lesion in a different brain region. For instance, left hemisphere damage can result in selective impairment in verbal memory, whereas right hemisphere damage may result in nonverbal memory impairment.
The loss of memory for events that occur after a lesion is known as anterograde amnesia. It results from the inability to learn new things. A loss of memory for events and knowledge that occurred before a lesion is called retrograde amnesia. Sometimes retrograde amnesia is temporally limited, extending back only a few minutes or hours. In other severe cases, it is extensive, sometimes encompassing almost the entire previous life span. Retrograde amnesia tends to be greatest for the most recent events. This effect, known as a temporal gradient or Ribot’s Law, was first postulated by Théodule Ribot, a 19th-century French psychologist. Amnesia can differentially affect short-term memory, working memory, or long-term memory abilities.
Because the extent and locations of lesions are known after surgery, a lot of the information about the organization of human memory was first derived from patients left accidentally amnesic after surgical treatments. We return now to the story of H.M., one of a series of patients who had surgery in the late 1940s and early 1950s to treat neurological and psychiatric disease. Elsewhere in the chapter, we will look at other patients with amnesia resulting from other types of lesions.
Brain Surgery and Memory Loss
In a 1954 report on the bilateral removal of the medial temporal lobe in H.M. and several schizophrenic patients, Scoville wrote:
Bilateral resection of the uncus [anterior aspect of the hippocampal gyrus], and amygdalum alone, or in conjunction with the entire pyriform amygdaloid hippocampal complex, has resulted in no marked physiologic or behavioral changes with the one exception of a very grave, recent memory loss, so severe as to prevent the patient from remembering the locations of the rooms in which he lives, the names of his close associates, or even the way to the toilet. (Scoville, 1954)
To better understand the deficits of his post-surgical patients with medial temporal lobe resections, Scoville teamed up with psychologist Brenda Milner (Chapter 1). Through neuropsychological examinations, Milner found that the extent of the memory deficit depended on how much of the medial temporal lobe had been removed. The more posterior along the medial temporal lobe the resection was made, the worse the amnesia was (Scoville & Milner, 1957). Strikingly, however, only bilateral resection of the hippocampus resulted in severe amnesia. By comparison, in one patient whose entire right medial temporal lobe (hippocampus and hippocampal gyrus) was removed, no residual memory deficit was reported by Scoville and Milner (although today’s more sensitive tests would reveal some memory deficits).
The most interesting and famous of these patients was H.M.—Henry Molaison, whose name was revealed after his death in 2008 at the age of 82. Over the years, he unstintingly allowed himself to be tested by over 100 researchers. His case holds a prominent position in the history of memory research for several reasons. One was that although he had a memory deficit, he had no other cognitive deficits. His problem was purely a memory problem: He was of normal intelligence, had normal perceptions, except for some olfactory deficits due to surgery, and had no psychological or mental illness. Also, because his memory loss was the result of surgery, the exact regions of the brain that were affected were thought to be known (Scoville & Milner, 1957; Milner et al., 1968). As we will see later in the chapter, this last point was not quite true.
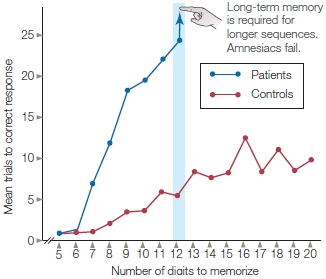
FIGURE 9.3 Digit span for amnesic patients and control participants.
A sequence of five digits was read to the participants, who were then asked to repeat the digits to the experimenter. If the digits were repeated correctly, one more digit was added to the next sequence presented. If the digits in a sequence were reported incorrectly, that sequence was repeated until the participant reported it correctly.
After the surgery, H.M. knew the autobiographical details of his life and all the other things he had learned in his life up to the 2 years immediately before his surgery. For those 2 years before surgery, however, he could not remember anything. He also showed selective memory loss for events as far back as a decade before the surgery. H.M. had normal short-term memory (sensory registers and working memory) and procedural memory (like riding a bicycle). Like many other amnesics (Figure 9.3), H.M. had normal digit span abilities (how many numbers a person can hold in memory over a short period of time) and did well at holding strings of digits in working memory. Unlike normal participants, however, he did poorly on digit span tests that required the acquisition of new long-term memories. It appeared that the transfer of information from short-term storage to long-term memory was disrupted. H.M. had anterograde amnesia, and could form no new long-term memories. Interestingly, even though he could not consciously remember new experiences, his behavior would be affected by them. The researchers were surprised when they discovered that H.M. could learn some things: tasks that involved motor skills, perceptual skills, or procedures became easier over time, though he could not remember practicing the new skill or being asked to learn it. There was a dissociation between remembering the experience of learning and the actual learned information.
Recent Studies on Memory Loss
Studies of H.M. changed how people thought about the brain’s memory processes. Previously, it was thought that memory could not be separated from perceptual and intellectual functions. These latter functions, however, remained intact in H.M., implying that memory was to some degree distinct from these processes. From H.M., researchers also learned that the medial temporal lobes are necessary for forming long-term memory and for transferring information about events and facts from short-term memory into long-term memory. Studies of H.M. also suggest that the medial temporal lobes are not necessary for the formation and retrieval of short-term memories or for learning new long-term memory that involves learning procedures or motor skills. Thus, the medial temporal lobe memory system is involved in certain memory functions, but not others, and is not critical for general intelligence, cognitive control, language, perception, or motor functions.
Studies in H.M. and other patients with amnesia have also shown that they can learn some forms of new information in addition to procedures, motor skills, and perceptual skills. They can also learn new concepts and world knowledge (semantic memory). But the amnesic patients, nonetheless, do not remember the episodes during which they learned or observed the information previously. The growing evidence from cases of amnesia suggests that long-term memories for events, facts, and procedures can be partially dissociated from one another, as expressed in their differential sensitivity to brain damage. Throughout this chapter, we explore additional studies that used patients with amnesia as participants.
TAKE-HOME MESSAGES
Mechanisms of Memory
Although patients with memory deficits have revealed many key aspects of human memory, models of memory continue to evolve, and different models emphasize different factors in the organization of learning and memory. Many different memory models have been proposed, including, for example, those based on how long memories persist, the type of information that is retained, whether memories are conscious or unconscious, and the time it takes to acquire them (see Figure 9.2 for a summary of the essential relations among different forms of long-term and short-term memory). In the next few sections, we discuss different forms of memory, and describe some of the evidence supporting theoretical distinctions among them.
Short-Term Forms of Memory
As mentioned earlier, short-term memory is memory that persists for milliseconds, seconds, or minutes. Short-term memories include the transient retention of sensory information in sensory structures (sensory memory), short-term stores for information about the world (short-term memory), and working memory. We discuss these three forms of memory in turn.
Sensory Memory Imagine that you are watching the final game of the World Cup. The score is tied and there are only seconds to go when your mother enters the room. She begins a soliloquy, but you’re not really paying attention. Suddenly you detect an increase in the volume of her voice and hear the words, “You haven’t heard a word I said!” Wisely, your response is not to admit it. Instead, and in the nick of time to avoid repercussions, you metaphorically reach back and retrieve the most recent sentence accurately enough to say, “Sure I did; you said that the neighbor’s goat is in our yard again eating the lettuce, and you want me to get it out.”
Almost everyone you ask about this phenomenon knows what you mean. The auditory verbal information just presented to you seems to persist as a sort of echo in your head, even when you are not really paying attention to it. If you try to retrieve it quickly enough, you find it is still there, and you can repeat it out loud to assuage your interrogator. We refer to this type of memory as sensory memory, which, for hearing, we call echoic memory. For vision, we say iconic memory.
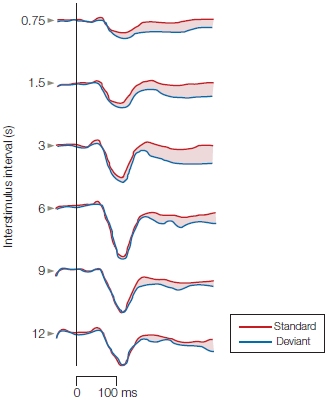
FIGURE 9.4 The mismatch field response.
The magnetic brain response known as the mismatch field (MMF) elicited by deviant tones (blue trace) in comparison to the magnetic responses elicited by standard tones (red traces). The amplitude of the MMF (indicated by the shaded difference between the blue and red traces) declines as the time between the preceding standard tone and the deviant tone increases to 12 s. This result can be interpreted as evidence for an automatic process in auditory sensory (echoic) memory that has a time course on the order of approximately 10 s.
The persistence of the auditory sensory memory trace in humans has been measured in different ways, including physiological recordings. An event-related potential (ERP) known as the electrical mismatch negativity (MMN), or its magnetic counterpart, the mismatch field (MMF), has proven highly informative about the duration of echoic memory. The MMN brain response is elicited by the presentation of a deviant stimulus, such as a high-frequency tone presented within a sequence of identical standard low tones. These mismatch responses are interpreted as representing sensory memory processes that hold recent auditory experience in echoic memory for comparison to new inputs: When the inputs differ, the MMN and MMF are generated. Hence, the amplitudes of these brain responses at different time intervals between the deviant and standard tones could be used to index how long the echoic memory trace persists.
Mikko Sams, Ritta Hari, and their colleagues (1993) at the Helsinki University of Technology in Finland did precisely that. They varied the interstimulus intervals between standard and deviant tones and found that the MMF could still be elicited by the deviant tone at interstimulus intervals of 9 to 10 s (Figure 9.4). After about 10 s, the amplitude of the MMF declined to the point where it could no longer be distinguished reliably from noise. Because the MMF is generated in the auditory cortex, these physiological studies also provide information about where sensory memories are stored: in sensory structures as a short-lived neural trace.
What about the time course of the neural trace for a visual sensory memory? Does it also last several seconds? No, and you know this is true because when you look at a painting and then turn away, the image does not persist very long. Most estimates of the time course of visual sensory memory suggest that the neural trace for a visual stimulus lasts only 300 to 500 ms. Both echoic and iconic sensory memory, however, have a relatively high capacity: These forms of memory can, in principle, retain a lot of information, but only for a very short period of time.
Short-Term Memory In contrast to sensory memory, short-term memory has a longer time course—seconds to minutes—and a more limited capacity. Early data on short-term memory led to the development of some influential models that proposed discrete stages of information processing during learning and memory. The modal model, developed by Richard Atkinson and Richard Shiffrin (1968), proposes that information is first stored in sensory memory (Figure 9.5). From there, items selected by attentional processes (see Chapter 7) can move into short-term storage. Once in short-term memory, if the item is rehearsed, it can be moved into long-term memory. The modal model suggests that, at each stage, information can be lost by decay (information degrades and is lost over time), interference (new information displaces old information), or a combination of the two. This model formalized the idea that discrete stages of memory exist and that they have different characteristics. In addition, this model has a strong serial structure: Information coming into sensory memory can be passed to short-term memory and only then into long-term memory.
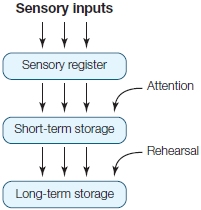
FIGURE 9.5 The Atkinson and Shiffrin modal model of memory.
Sensory information enters the information-processing system and is first stored in a sensory register. Items that are selected via attentional processes are then moved into short-term storage. With rehearsal, the item can move from short-term to long-term storage.
The ensuing decades have seen intense debate over this model from the standpoint of the psychology of memory as well as the neuroscience of memory. Data has been presented to support, challenge, and extend the model. A key question is whether memories have to be encoded in short-term memory before being stored in long-term memory. Another way to look at this question is to ask whether the brain systems that retain information over the short term are the same or different from those that store information over the long term. Atkinson and Shiffrin pondered this issue themselves, writing in 1971:
Our account of short-term and long-term storage does not require that the two stores necessarily be in different parts of the brain or involve different physiological structures. One might consider the short-term store simply as being a temporary activation of some portion of the long-term store. (p. 89)
HOW THE BRAIN WORKS
Short-Term Memory Capacity
Short-term memory is limited, but how limited? Precisely how much information a healthy person can retain in short-term memory varies among individuals. Experiments have demonstrated an interesting characteristic of human memory. In the 1950s, George Miller (G. Miller, 1956; see Figure 1.20 in Chapter 1) investigated how much information individuals can process. Although the initial work centered on perception, the research has been extended to memory for the retention of items.
Volunteers were presented with items to be remembered, in groups of varying size. The results were amazing: Regardless of the content of the items (e.g., digits, letters, or words), the number of items that were retained typically proved to be about seven. When more than seven items were presented, volunteers were less successful at recalling all of them. Miller referred to this characteristic feature of human memory as the span of immediate memory, or, in the terminology we have been using up to now, the span of short-term memory. When digits are used, this feature is referred to as digit span, and it is commonly measured in neuropsychological tests.
The memory limits discovered in these studies are defined by the number of items, not the content of each item, so they tell us about the way information is coded in short-term stores. This distinction has sometimes been cast as the difference between a bit of information and a chunk—a bit being the elementary piece of information, and a chunk being a unit composed of bits. The use of words allows individual letters to be chunked into one meaningful piece of information. The word cerebellum is either 10 letters or one word. If 10 letters have to be remembered, the short-term memory system is taxed; but if the letters can be chunked as one word (cerebellum), then about seven of these chunks (or words) can be remembered. The consequence of this chunking is that, during recall of the material, the chunked information can be essentially unpacked (unchunked) to yield more bits of information than normally could be retained. That is, if we can retain in our memory 7 words of 10 letters each, we can unpack them into 70 bits of information by using knowledge about word spelling. This evidence points to the ability of humans to recode information in manageable packets, packets that can be handled within the constraints of short-term memory.
Studies of patients with brain damage permit a test of the hierarchically structured modal model of memory. In 1969, neuropsychologists Tim Shallice and Elizabeth Warrington at University College London reported that a patient (K.F.) with damage to the left perisylvian cortex (the region around the Sylvian fissure) displayed reduced digit span ability (about 2 items, as opposed to 5 to 9 items for healthy persons). The test involves first reading lists of digits for the participants to remember and then, after a delay of only a few seconds, having participants repeat those digits. The lists can be from two to five or more digits long, and the maximum number that a person can recall and report is known as his digit span ability (see Figure 9.3).
Remarkably, however, in a long-term memory test of associate learning that pairs words, K.F. retained the ability to form certain types of new long-term memories that could last much longer than a few seconds. Therefore, it seemed that the patient displayed an interesting dissociation between short-term and long-term memory. If this interpretation of the finding is true, it has important implications for models of memory: Short-term memory might not be required in order to form long-term memory. This conclusion is in contrast to how the information flows in the modal model (Figure 9.5), which requires serial processing. One issue with this view is that the two tests presented to K.F. were different (digit span and word association), and it’s hard to pinpoint whether the dissociation is one of memory processes or actually due to the different tasks.
A more recent example of a similar patient comes from the work of Hans Markowitsch and colleagues (1999) at Bielefeld University in Germany. Their patient, E.E., had a tumor centered in the left angular gyrus. The tumor affected the inferior parietal cortex and posterior superior temporal cortex (Figure 9.6), regions similar to but slightly different from those affected in patient K.F. After undergoing surgery to remove the tumor, E.E. showed below-normal short-term memory ability but preserved long-term memory—a pattern similar to K.F.’s. E.E. showed normal speech production and comprehension, and normal reading comprehension. He had poor short-term memory for abstract verbal material, however, as well as deficits in transposing numbers from numerical to verbal, and vice versa, even though he could calculate normally. Interestingly, on tests of his visuospatial short-term memory and both verbal and nonverbal long-term memory, E.E. performed normally.
The pattern of behavior displayed by these patients demonstrates a deficit of short-term memory abilities but a preservation of long-term memory. This pattern suggests that short-term memory is not the gateway to long-term memory in the manner laid out in the modal model. Perhaps information from sensory memory registers can be encoded directly into long-term memory.
The data from patients like K.F. and E.E. demonstrate a dissociation between long-term memory ability and short-term retention of information. In contrast, patients like H.M. have preserved short-term memory but deficits in the ability to form new long-term memories. Together, these two different patterns of memory deficit present an apparent double dissociation for short- and long-term retention of information, specifically in relation to both the memory processes and the underlying neuroanatomy (i.e., left perisylvian cortex vs. the medial temporal lobes).
As described in Chapter 3, a double dissociation is the strongest pattern of effects that can be obtained in attempts to identify and distinguish two mental processes. Investigators disagree, however, on whether these interesting patient case studies demonstrate a true double dissociation. Some have argued that the evidence from these patient cases does not support a strong double dissociation of short- and long-term memory. Because the short-term memory tests are testing for the retention of overlearned materials such as digits and words, such tests may not be effective for learning about short-term memory. In fact, when novel materials are used to test short-term memory retention, patients with medial temporal lobe lesions sometimes fail.
Working Memory The concept of working memory was developed to extend the concept of short-term memory and to elaborate the kinds of mental processes that are involved when information is retained over a period of seconds to minutes. Working memory represents a limited-capacity store for retaining information over the short term (maintenance) and for performing mental operations on the contents of this store (manipulation). For example, we can remember a list of numbers, and we can also add (manipulate) them in our head by using working memory. The contents of working memory could originate from sensory inputs (as in the modal model), such as when someone asks you to multiply 55 times 3, or it could be retrieved from long-term memory, such as when you visit the carpet store and recall the dimensions of your living room and multiply them to figure out its square feet. In each case, working memory contains information that can be acted on and processed, not merely maintained by rehearsal, although such maintenance is one aspect of working memory.
Psychologists Alan Baddeley and Graham Hitch (1974) at the University of York argued that the idea of a unitary short-term memory was insufficient to explain the maintenance and processing of information over short periods. They proposed a three-part working memory system consisting of a central executive mechanism for controlling two subordinate systems involved in rehearsal of different types of information: phonological and visuospatial (Figure 9.7). The proposed central executive mechanism is a cognitive system, a command-and-control center that presides over and coordinates the interactions between two subordinate systems that are short-term memory stores (the phonological “loop” and the visuospatial “sketch pad”) and long-term memory.
|
FIGURE 9.6 MRI scans reconstructed to provide a three-dimensional rendering of patient E.E.’s left hemisphere. |

|
The phonological loop is a hypothesized mechanism for acoustically coding information in working memory (thus, it is modality specific). The evidence for modality specificity first came from studies that asked participants to recall strings of consonants. The letters were presented visually, but the pattern of recall errors indicated that perhaps the letters were not coded visually over the short term. The participants were apparently using an acoustic code, because during recall they were more likely to replace a presented letter with an erroneous letter having a similar sound (e.g., T for G) rather than one with a similar shape (e.g., Q for G). This was the first insight suggesting that an acoustic code might play a part in rehearsal.
In line with this idea is evidence that immediate recall of lists of words is poorer when many words on the list sound similar than when they sound dissimilar, even when the dissimilar words are semantically related. This finding indicates that an acoustic code rather than a semantic code is used in working memory, because words that sound similar interfere with one another, whereas words related by meaning do not. The phonological loop might have two parts: a short-lived acoustic store for sound inputs and an articulatory component that plays a part in the subvocal rehearsal of visually presented items to be remembered over the short term.
The visuospatial sketch pad is a short-term memory store that parallels the phonological loop and permits information storage in either purely visual or visuospatial codes. Evidence for this system came from studies of participants who were instructed to remember a list of words using either a verbal strategy such as rote rehearsal or a visuospatial strategy based on an imagery mnemonic. Under control conditions in which the memory rehearsal was the only task, participants were better on the memory test when they used the visuospatial strategy. The verbal strategy, however, proved better when the participants were required to concurrently track a moving stimulus by operating a stylus during the retention interval. In contrast, people are impaired on verbal memory tasks (but not nonverbal memory tasks) when they are required to repeat nonsense syllables during the retention interval, presumably because the phonological loop is disrupted. Dissociations like these cannot be explained by assuming that there is a unitary memory system.

FIGURE 9.7 Simplified representation of the working memory model proposed by Baddeley and Hitch.
This three-part working memory system has a central executive that controls two subordinate systems: the phonological loop, which encodes information phonologically (acoustically) in working memory; and the visuospatial sketch pad, which encodes information visually in working memory.
Deficits in short-term memory abilities, such as remembering items on a digit span test, can be correlated with damage to the subcomponents of the working memory system. Evidence about the distinct nature of these subsystems and their anatomical substrates in the human brain first came from studies of patients with specific brain lesions. In fact, each system can be damaged selectively by different brain lesions.
One expectation is that the phonological loop and the visuospatial sketch pad might correspond to working memory functions of the left and right hemispheres, respectively—an idea consistent with the general picture of hemispheric specialization (see Chapter 4). Indeed, patients with lesions of the left supramarginal gyrus (Brodmann area 40) have deficits in phonological working memory (Figure 9.8; see also Figure 9.6) resulting in reduced auditory–verbal memory spans: They cannot hold strings of words in working memory. The rehearsal process of the phonological loop involves a region in the left premotor region (area 44). Thus, a left-hemisphere network consisting of the lateral frontal and inferior parietal lobes is involved in phonological working memory. These deficits in working memory for auditory–verbal material (digits, letters, words) have not been found to be associated with deficits in speech perception or production. This distinction between aphasia—language deficits following brain damage (see Chapter 11)—and deficits in auditory–verbal short-term memory is important to keep in mind.
The visuospatial sketch pad is compromised by damage to the parieto-occipital region of either hemisphere, but damage to the right hemisphere produces more severe deficits in visuospatial short-term memory. Patients with lesions in the right parieto-occipital region have difficulty with nonverbal visuospatial working memory tasks like retaining and repeating the sequence of blocks touched by another person. For example, if an investigator touches blocks on a table in sequences that the patient must repeat, and gradually increases the number of blocks touched, patients with parieto-occipital lesions show below-normal performance, even when their vision is otherwise normal. Similar lesions in the left hemisphere can lead to impairments in short-term memory for visually presented linguistic material.
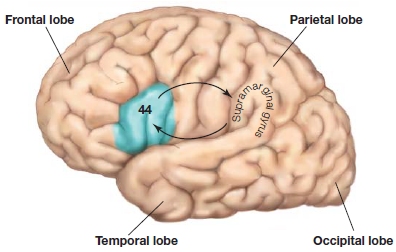
FIGURE 9.8 Lateral view of the left hemisphere, indicating that there is an information loop involved in phonological working memory flowing between BA44 and the supramarginal gyrus (BA40).
Early neuroimaging studies have helped to support this distinction. Using PET imaging in healthy volunteers, Edward Smith and his colleagues (1996) at Columbia University provided evidence for dissociations in the brain regions activated while performing spatial versus verbal working memory tasks. Participants were presented with either an array of locations marked on a computer screen or an array of letters, and were asked to remember the locations or the letters during a delay period of 3 s. Next, they presented a location marker for the spatial memory task or a letter at fixation for the verbal memory task and asked participants whether the location or letter had been in the original array. For verbal working memory tasks, they found activation (increasing blood flow coupled to increased neural activity) in left-hemisphere sites in inferolateral frontal cortex, but for the spatial working memory task, activation was primarily in right-hemisphere regions (inferior frontal, posterior parietal, and extrastriate cortex in the occipital lobe; Figure 9.9).
Several years later, Smith and colleagues compiled a meta-analysis of 60 PET and fMRI studies (Wager & Smith, 2003). Although their analysis confirmed that activation is found during working memory tasks with verbal stimuli in the left ventrolateral prefrontal cortex, the evidence for spatial working memory showed activation to be more bilateral in the brain. Why is there a behavior difference in visuospatial tasks with right-sided lesions, but activity with these tasks is also seen on the left side on fMRI? The left-hemisphere activity during spatial working memory may reflect, at least in some studies, a verbal recoding of the nonverbal stimuli. For example, when asked to remember the locations of a set of stimuli, we might think “upper left” and “lower right.” We will return to further discussion of working memory in Chapter 12.
Long-Term Forms of Memory
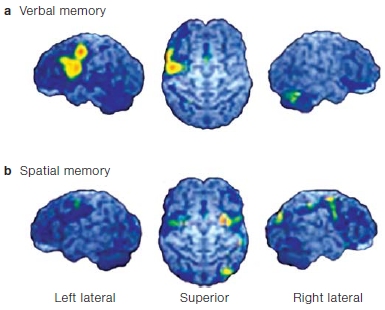
FIGURE 9.9 Changes in local cerebral blood flow, measured with positron emission tomography.
Verbal (a) and spatial (b) working memory tasks were tested in healthy volunteers. In each case, the views of the cortical surface show the left hemisphere (left); superior (dorsal) surface of both hemispheres, with the frontal lobe at the top (middle); and right hemisphere (right). See text for details.
Information retained for a significant time (days, months or years) is referred to as long-term memory. Theorists have tended to split long-term memory into two major divisions, taking into account the observable fact that not all stored knowledge is the same. The key distinction is between declarative and nondeclarative memories.
Declarative Memory Declarative memory is defined as memory for events and for facts, both personal and general, that we have conscious access to and that can be verbally reported. This form of memory is sometimes referred to as explicit memory. Declarative memory is the type of memory that is impaired in H.M. and, thus, it is dependent on the medial temporal lobe. In the 1970s, psychologist Endel Tulving introduced the idea that declarative memory can be further broken down into episodic memory and semantic memory. Episodic memories are memories of personal experiences that we recall about our own lives and what, where, when, and with whom they happened. They are our own personal, autobiographical memories. They differ from personal knowledge (Figure 9.10). For instance, you have personal knowledge of what day you were born, but you do not remember the experience. Episodic memories always include the self as the agent or recipient of some action. For example, the memory of falling off your new red bicycle (what) on Christmas day (when), badly skinning your elbow on the asphalt driveway (where), and your mother (who) running over to comfort you is an episodic memory. Episodic memory is the result of rapid associative learning in that the what, where, when, and who of a single episode, its context, become associated and bound together and can be retrieved from memory after a single episode. More recently, evidence has been unearthed that not all memory of experiences is conscious. We will discuss this research later in the chapter, when we examine relational memory.

FIGURE 9.10 Tulving and his cat.
According to Tulving, animals like his cat have no episodic memory, although they have knowledge of many things. Tulving argues that they therefore do not remember their experiences the same way we do; they can merely know about such experiences.
Semantic memory, in contrast, is objective knowledge that is factual in nature but does not include the context in which it was learned. For instance, you may know that corn is grown in Iowa, but you most likely don’t remember when or where you learned that fact. A fact can be learned after a single episode, but it may take many exposures. Semantic memory reflects knowing facts and concepts such as how to tell time, who the lead guitarist is for the Rolling Stones, and what quantum mechanics is all about. The take-home message is that world knowledge is fundamentally different from our recollection of events in our own lives.
Interestingly, in human development, episodic and sematic memory appear at different ages. Babies who are 2 years old have been able to demonstrate recall of things they had witnessed at age 13 months (Bauer & Wewerka, 1995). It isn’t until children are at least 18 months, however, that they actually seem to include themselves as part of the memory, although this ability tends to be more reliably present in 3- to 4-year-olds (Perner & Ruffman, 1995; M. Wheeler et al., 1997).
When Tulving introduced the idea of episodic versus semantic memory decades ago, the dominant thinking was that there was a unitary memory system. If Tulving is right, however, then perhaps different underlying brain systems support these two different flavors of declarative long-term memory.
Nondeclarative Memory Nondeclarative memory is so named because it cannot be “declared,” that is, verbally reported. It is also known as implicit memory, knowledge that we have no conscious access to. Several types of memory fall under this category: priming, simple learned behaviors that derive from conditioning, habituation, sensitization, and procedural memory, such as learning a motor or cognitive skill. This form of memory is revealed when previous experiences facilitate performance on a task that does not require intentional recollection of the experiences. This type of memory was unimpaired in H.M. because nondeclarative memory is not dependent on the medial temporal lobe. It involves other brain structures, including the basal ganglia, the cerebellum, the amygdala, and the neocortex.
Procedural Memory Procedural memory is one form of nondeclarative memory that depends on extensive and repeated experience. Tasks that require us to use procedural memory include learning motor skills like how to ride a bike, type, or swim, and learning cognitive skills such as how to read. Studies of amnesia have revealed some fundamental distinctions between long-term memory for events in your life, such as seeing your first bike under the Christmas tree, and procedural memory, such as riding a bicycle.
One test of procedural learning is the serial reaction time task. In one experimental setup, participants sit at a console having four buttons. Placing the fingers of one hand over the buttons, participants would press buttons that correspond to locations of stimuli in front of them. Each button corresponds to one of four lights—the mapping between button and light can simply be their spatial relationships (i.e., the left light maps to the left button). The task would be to press the button with the finger that corresponds to the light that is illuminated (Figure 9.11a). The lights can be flashed in different sequences: A totally random sequence can be flashed; or a pseudorandom sequence might be presented, in which the participant thinks the lights are flashing randomly when in reality they are flashing in a complex, repetitive sequence.
Over time, normal participants respond faster to the repeating sequence than they do to a totally random sequence (Figure 9.11b). Thus, their improved performance indicates that they have learned the sequence. When asked whether the sequences were random, however, participants report that the sequences were completely random. They do not seem to know that any pattern existed, yet they learned the skill. Such behavior is typical of procedural learning, which requires no explicit knowledge about what was learned. This kind of evidence has been used to argue for the distinction between declarative and procedural knowledge, because participants appear to acquire one (procedural knowledge) in the absence of the other (declarative knowledge).
Some have challenged the idea that normal participants learn without having any explicit knowledge of what was learned. For example, sometimes the investigators ask normal volunteers about the sequences and find that they can in fact explicitly describe the learned material. Perhaps those who deny any such knowledge have less confidence in their knowledge and hence deny it. Given this possibility in normal participants, if we do not find evidence for explicit knowledge during skill acquisition, how can we be sure it is not there? Perhaps the person merely failed to demonstrate it.
An answer comes from procedural learning studies in persons with anterograde amnesia, like H.M.. These people cannot form new declarative (or at least episodic) memories. When tasks like the one in Figure 9.11a were presented to amnesic patients, it was found that those with dense anterograde amnesia (with loss of episodic learning) improved their performance for repeated sequences (compared to random ones) over a series of days; their improvement was shown as a speeding up of reaction time (as in Figure 9.11b). Even though they state they have never performed the task before, these amnesic participants have learned the procedure. Therefore, procedural learning can proceed independently of the brain systems required for episodic memory.
What brain systems support procedural memory? Learning motor skills may involve the basal ganglia. Patients with disorders of the basal ganglia or inputs to these subcortical structures show poor performance on a variety of procedural learning tasks. As we learned in the previous chapter, these individuals include patients with Parkinson’s disease, in which cell death in the substantia nigra disrupts dopaminergic projections into the basal ganglia, and patients with Huntington’s disease, who have degeneration of neurons in the basal ganglia. These patients, who are not amnesic per se, have impairments in acquisition and retention of motor skills as assessed by a variety of tests involving motor skill learning.
|
FIGURE 9.11 Procedural learning of sequences in the serial reaction-time task. |
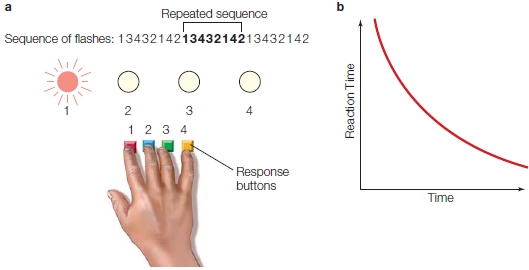
|
Priming Another form of nondeclarative memory is priming. Priming refers to a change in the response to a stimulus, or in the ability to identify a stimulus, following prior exposure to that stimulus. For instance, if you were to see a picture of bicycle handlebars from an odd angle, you would recognize them as part of a bike faster if you had just seen a typical picture of a bike. If you had not, you would find them more difficult to identify. Priming can be perceptual, conceptual, or semantic.
Perceptual priming acts within the perceptual representation system (PRS). In the PRS, the structure and form of objects and words can be primed by prior experience, and the effects persist for months. For example, participants can be presented with lists of words, and their memory of the lists can be evaluated using a word-fragment completion task. In such a task, during the later test phase, participants are shown only some letters from real words; for example, t_ou_h_s for “thoughts.” These fragments can be from either new words (not present in the original list) or old words (present in the original list). The participants are simply asked to complete the fragments. Participants are significantly better and faster at correctly completing fragments for words presented in the initial list—they show priming. The important idea is that participants benefit from having seen the words before, even if they are not told and do not realize that the words were in the previous list. This priming for fragment completion does not lessen over time, and it is specific for the sensory modality of the learning and test phases. To put this another way, if the word lists are presented auditorily and the word-fragment completion is done visually, then the priming is reduced, suggesting that priming reflects a PRS that subserves structural, visual, and auditory representations of word form. Lastly, perceptual priming can also be seen with non-word stimuli, such as pictures, shapes, and faces. In summary, the PRS mediates word and non-word forms of priming. Moreover, it is not based on conceptual systems, but rather is perceptual in nature. Interestingly, this type of priming is also found in amnesia patients like H.M. H.M. would show evidence of priming even when he could not remember ever having seen the word list or ever having done a fragment-complete task before. This behavior tells us that the PRS system does not rely on the medial temporal lobe, because both of H.M.’s were removed surgically. But this is merely a single dissociation. Is there any evidence that brain lesions can affect the PRS system while leaving long-term memory intact?
There is: John Gabrieli and his colleagues (1995) at Stanford University tested a patient, M.S., a man who had a right occipital lobe lesion. M.S. had experienced intractable epileptic seizures and at age 14 underwent surgery to treat them. The surgery removed most of Brodmann areas 17, 18, and 19 of his right occipital lobe, leaving him blind in the left visual field. He has above average intelligence and memory. Explicit tests of memory (recognition and cued recall) and implicit memory (perceptual priming) were administered to M.S., and his performance was compared to amnesiacs similar to H.M., who had anterograde amnesia for episodic memory. The test materials were words briefly presented visually and then read aloud by the subjects. During the implicit memory test, the words were presented and then masked with rows of X’s. The duration of presentation increased from 16 ms to a time when the participant could read the word. If less time was required to read the word after it had been seen previously, then there would be evidence for implicit perceptual priming. In a separate explicit recognition test, participants saw old and new words and had to judge whether they had seen them before.
The amnesic patients displayed the expected impairments of explicit word recognition, but they did not show impairment in the implicit perceptual priming test. In contrast, M.S. had normal performance on explicit recognition, but impairment in the implicit perceptual priming test. This deficit was not due to his partial blindness, because his explicit memory for word recognition and recall indicated that he perceived them normally by using the intact portions of his visual field. M.S. showed a pattern opposite to that typical of amnesiacs like H.M. These data show that perceptual priming can be damaged even when explicit memory is not impaired, thereby completing a double dissociation for declarative and nondeclarative memory systems. The anatomical data indicate that perceptual priming depends on the perceptual system, because M.S. had lesions to the visual cortex leading to deficits in perceptual priming.
Priming also occurs for conceptual features rather than perceptual features, though it doesn’t last nearly as long. Here, participants are quicker at answering general knowledge questions if the concept had been presented earlier. For example, if we had been talking about pasta and its different shapes, and then you were asked to name an Italian food, most likely you would say pasta, rather than pizza or veal parmigiana. Conceptual priming is also not affected by lesions to the medial temporal lobe, but rather by lesions to the lateral temporal and prefrontal regions.
Another form of priming is semantic priming, in which the prime and target are words that are different but related semantically. For instance, the prime may be the word hammer, but the target word is wrench. Semantic priming is brief, lasting only a few seconds.
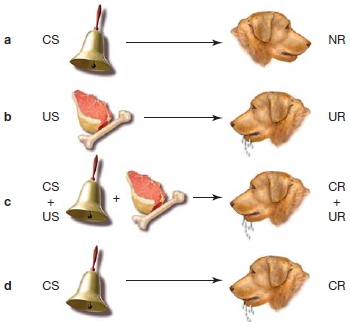
FIGURE 9.12 Classical (Pavlovian) conditioning.
When a stimulus is presented that has no meaning to an animal, such as the sound of a bell (CS), there is no response (NR) (a). In contrast, presentation of a meaningful stimulus like food (US) generates an unconditioned response (UR) (b). When the sound is paired with the food, however, the animal learns the association (c); and later the newly conditioned stimulus (CS) alone can elicit the response, which is now called a conditioned response (CR) (d).
Classical Conditioning and Nonassociative Learning Two other domains of nondeclarative memory include classical conditioning, a type of associative learning, and nonassociative learning. In classical conditioning, sometimes referred to as Pavlovian conditioning, a conditioned stimulus (CS; an otherwise neutral stimulus to the organism) is paired with an unconditioned stimulus (US; one that elicits an established response from the organism) and becomes associated with it. The conditioned stimulus will then evoke a conditioned response (CR) similar to that typically evoked by the unconditioned stimulus (the unconditioned response, UR). Russian Ivan Pavlov (1849–1936) received a Nobel Prize after first demonstrating this type of learning with his dogs, which started to salivate at the sound of a bell that Pavlov rang before he gave them food (Figure 9.12). Before conditioning, the bell was not associated with food and did not cause salivation. After conditioning, in which the bell and the food were paired, the bell (CS) caused salivation even in the absence of the food (US). We will discuss more about conditioning in Chapters 10 and 12. Classical conditioning comes in two flavors: delay and trace conditioning. In delay conditioning, the US begins while the CS is still present; but in trace conditioning, there is a time gap, and thus a memory trace is necessary for an association to be made between the CS and US. Studies with normal participants and those with amnesia resulting from hippocampal damage have found that damage to the hippocampus does not impair delay conditioning, but does impair trace conditioning (R. Clark & Squire, 1998). Thus, some types of associative learning depend on the hippocampus, and others do not.
Nonassociative learning, as its name implies, does not involve the association of two stimuli to elicit a behavioral change. Rather, it consists of forms of simple learning such as habituation, where the response to an unchanging stimulus decreases over time. For instance, the first time you use an electric toothbrush, your entire mouth tingles; but after a few uses, you no longer feel a response. Another type of nonassociative learning is sensitization, in which a response increases with repeated presentations of the stimulus. The classic example is rubbing your arm. At first it merely creates a feeling of warmth. If you continue, however, it starts to hurt. This is an adaptive response that warns you to stop the rubbing because it may cause injury. Nonassociative learning primarily involves sensory and sensory motor (reflex) pathways. We do not consider classical conditioning, nonassociative learning, or nonassociative memory further in this chapter. Instead, we focus on the neural substrates of declarative (episodic and semantic memory) and nondeclarative memory (procedural memory and the perceptual representation system).
TAKE-HOME MESSAGES
The Medial Temporal Lobe Memory System
So far, we have learned from H.M. that the brain’s ability to acquire new declarative memories (episodic and semantic memory) depends on the medial temporal lobe, whereas short-term and nondeclarative memories are supported more directly by brain mechanisms outside the medial temporal lobe system. We now explore how the medial temporal lobe affects long-term memory by looking first at patients with memory deficits, then lesion studies in animals, and finally imaging evidence from humans.
Evidence From Amnesia
As we have learned, the medial temporal lobe includes the amygdala, the hippocampus, and the surrounding parahippocampal, entorhinal, and perirhinal cortical areas. We also know that memory mechanisms have been divided into acquisition, consolidation, storage, and retrieval. Let’s look first at those functions lost in amnesic patients like H.M., and ask: What neural mechanisms and brain structures enable us to acquire new long-term memories?

FIGURE 9.13 Region of the medial temporal lobe believed to have been removed from H.M.
As reported by his surgeon, the areas of H.M.’s brain that were removed are shown in red. (The resection is shown here on the left side only, for comparison of the resected region with an intact brain, on the right side, at the same level. H.M.’s actual lesion was bilateral.) At the top is a ventral view of the brain, showing both hemispheres and the details of the right medial temporal area (at left). The four anterior-to-posterior levels (a–d) shown in this ventral view correspond to the four coronal sections below.
H.M.’s original surgical reports indicated that his hippocampi were completely removed bilaterally (Figure 9.13). Decades later, Suzanne Corkin of the Massachusetts Institute of Technology and journalist author Philip Hilts (1995) discovered through some detective work that the clips used in H.M.’s surgery were not ferromagnetic—which meant he could have an MRI. So in 1997, more than 40 years after his surgery, H.M.’s surgical lesion was investigated with modern neuroimaging techniques (Figure 9.14).
Data gathered by Corkin and her colleagues were analyzed by neuroanatomist David Amaral of the University of California, Davis (Corkin et al., 1997). This analysis revealed (Figure 9.15) that H.M.’s lesion was smaller than originally reported. Contrary to Scoville’s reports, approximately half of the posterior region of H.M.’s hippocampus was intact, and only 5 cm (not 8 cm) of the medial temporal lobe had been removed. Thus, the posterior parahippocampal gyrus was mostly spared; but the anterior portion, the perirhinal and entorhinal cortices, was removed. The remaining portions of H.M.’s hippocampi, however, were atrophied, probably due to the loss of inputs from the surrounding perihippocampal cortex that had been removed in the 1953 surgery. Thus, despite the original error in our knowledge about H.M.’s lesion, it may be that no functional hippocampal tissue remained. Consequently, H.M.’s lesions cannot help us determine the role of the hippocampus versus parahippocampal cortex in memory.
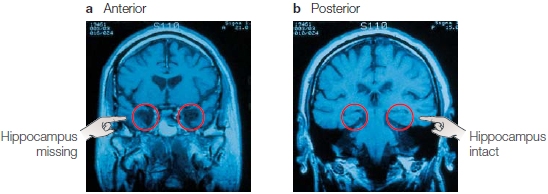
FIGURE 9.14 Coronal MRI scans of H.M.’s brain.
(a) In this anterior slice, the hand points to where the hippocampus has been removed bilaterally. (b) In this more posterior slice, however, the hand points to where the hippocampus is still intact in both hemispheres! This finding is in marked contrast to the belief that H.M. has no hippocampus—a view, based on the surgeon’s report, that the scientific community held for 40 years.
Consider another remarkable patient story, that of R.B. In 1978, R.B. lost his memory after an ischemic episode (reduction of blood to the brain) during heart bypass surgery. Changes in his memory performance were studied in detail by Stuart Zola, Larry Squire, and David Amaral at the University of California, San Diego. R.B. developed dense anterograde amnesia similar to H.M.’s: He could not form new long-term memories. He also had a mild temporal retrograde amnesia that went back about 1 or 2 years, so R.B.’s amnesia was slightly less severe than H.M.’s retrograde loss. After his death, an autopsy revealed that R.B.’s lesions were restricted to a particular region of his hippocampus only. Although on gross examination his hippocampus appeared to be intact (Figure 9.16a), histological analysis revealed that, within each hippocampus, he had sustained a specific lesion restricted to the CA1 pyramidal cells (Figure 9.16b). Compare his hippocampus (Figure 9.16c) with that of a normal person after death (Figure 9.16b).
These findings of specific hippocampal damage in patient R.B. support the idea that the hippocampus is crucial for the formation of new long-term memories. R.B.’s case also supports the distinction between areas that store long-term memories and the role of the hippocampus in forming new memories. Even though retrograde amnesia is associated with damage to the medial temporal lobe, it is temporally limited and does not affect long-term memories of events that happened more than a few years prior to the amnesia-inducing event. Subsequently, several patients with similar medial temporal lobe lesions also have been identified and studied, and they show highly similar patterns of memory loss.
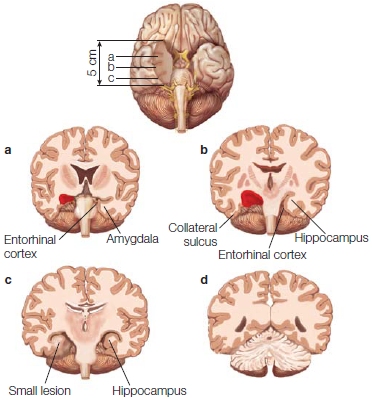
FIGURE 9.15 Region of the medial temporal lobe actually removed from H.M.
Modern reconstruction by Amaral and colleagues, showing that portions of H.M.’s posterior hippocampus were not removed during surgery. This tissue, however, shows signs of atrophy and may no longer be functioning normally. Red areas indicate where portions were removed. Compare with Figure 9.13.
Further evidence that the hippocampus is involved in long-term memory comes from patients with transient global amnesia (TGA). This syndrome is triggered by a number of causes, but most commonly by physical exertion in men and emotional stress in women over 50. In this situation, the normal blood flow is disrupted in the brain. In particular, the vertebral-basilar artery system, which supplies blood to the medial temporal lobe and the diencephalon has been implicated as a critical site. The result is a transient ischemia that later returns to normal. High-resolution imaging data now suggest that the lesions caused by such an event are located within the CA1 subfield of the hippocampus and that these neurons are selectively vulnerable to metabolic stress (see Bartsch & Deuschl, 2010). This disruption of blood flow results in a sudden transient anterograde amnesia, and retrograde amnesia spanning weeks, months, and sometimes even years. In a typical scenario, a person may wind up in the hospital but not be sure about where he is, or why, or how he got there. He knows his name, birth date, job, and perhaps address; but if he has moved recently, he will supply his past address and circumstances. He performs normally on most neuropsychological tests, except for those that call for memory. He has normal short-term memory, and thus, can repeat lists of words told to him. When asked to remember a list of words, however, he forgets it within a couple of minutes if he is prevented from rehearsing it. He continually asks who the physician is and why he is there. He does show an awareness that he should know the answer to some questions, which worries him. He manifests a loss of time sense, and so he responds incorrectly to questions asking how long he has been in the hospital. During the hours following the amnesia-inducing event, distant memories return, and his anterograde memory deficit is resolved. Within 24 to 48 hours, he is essentially back to normal, although mild deficits may persist for days or weeks.

FIGURE 9.16 Comparison of R.B.'s brain with that of a normal participant.
(a) This section is from R.B.’s brain following his death. In contrast to the MRI sections from H.M. in Figure 9.15, which show an absence of the anterior and middle portions of the hippocampus, R.B.’s medial temporal lobe appeared intact on gross examination. (b) Compare normal histology here with R.B.’s in (c). This histological section from the brain of a normal participant shows an intact CA1 region (labeled “CA1” and delimited as the region between the arrows). (c) Careful histological examination of R.B.’s temporal lobe revealed that cells in the CA1 region of the hippocampus were absent (see the region between the arrows). The absence of cells was the result of an ischemic episode following surgery. Cells of the CA1 region are particularly sensitive to transient ischemia (temporary loss of blood supply to a brain region).
As you may have noticed, the patients with transient global amnesia have symptoms similar to those of people with permanent damage to the medial temporal lobe, such as H.M. So far, we do not know whether these patients have normal implicit learning or memory, in part because their impairment does not last long enough for researchers to adequately index things like procedural learning. The answer to this question would improve our understanding of human memory and of a form of amnesia that any of us could experience later in life.
Converging evidence for the role of the hippocampus in forming long-term memory also comes from patients with amnesia caused by lesions in regions connected to, but outside of, the medial temporal lobes (e.g., damage to the diencephalon). Damage to these midline subcortical regions can be caused by stroke, tumors, trauma, and metabolic problems like those brought on by chronic alcoholism, such as Korsakoff’s syndrome. Because patients with Korsakoff’s syndrome initially have no damage to the medial temporal lobe, it is likely that connections between the anterior and dorso-medial diencephalon and medial temporal lobe are disrupted, giving rise to the deficit by compromising the circuitry that involves the hippocampus.
Further evidence comes from patients with Alzheimer’s disease (AD). This disease causes widespread neuronal deterioration, including severe disruptions in the parietal lobe structures of the retrosplenial cortex, posterior cingulate, precuneus, and angular gyrus. But neuroscientists now widely believe that the hippocampus also deteriorates more rapidly in patients with AD than in people undergoing the normal aging process. The amyloid plaques (clumps of insoluble protein between neurons) and neurofibrillary tangles (tangles of protein fibers within cortical neurons) that are characteristic of AD congregate in this medial temporal area (Figure 9.17). MRI measurements of brain volumes have shown that the size of the hippocampus changes with the progression of AD: People with thicker hippocampi develop dementia to a lesser extent (Jobst et al., 1994; Jack et al., 2002).
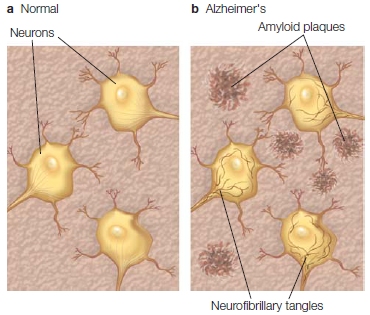
FIGURE 9.17 Comparison of cortex in Alzheimer’s patients and normal participants.
These diagrams depict a normal section of cortex with cortical neurons (a) and a section of cortex in an Alzheimer’s patient containing amyloid plaques between neurons and neurofibrillary tangles within neurons (b).
Morris Moscovitch and colleagues at the Rotman Research Institute and the University of Toronto, Canada, have demonstrated that the extent of atrophy in the medial temporal lobe in Alzheimer’s patients is most closely related to their deficits in episodic memory (Gilboa et al., 2005). In addition, there is a large loss of acetylcholine cells that connect to the hippocampus and prefrontal cortex in Alzheimer’s disease. This dysfunctional connectivity between the hippocampus and prefrontal cortex due to the loss of acetylcholine appears to play a role in the progressive loss of ability to form new episodic memories in Alzheimer’s patients.
Evidence From Animals With Medial Temporal Lobe Lesions
Studies in animals with lesions to the hippocampus and surrounding cortex have been invaluable to improving our understanding about the contributions of the medial temporal lobe to memory. This immense field of study includes investigations in invertebrates as well as studies in various mammalian species, including nonhuman primates. A comprehensive review of this field of work is beyond the scope of this textbook, but a few of the most important findings from animal studies are essential for understanding memory mechanisms. A key question in memory research has been how much does the hippocampus alone, as compared with surrounding structures in the medial temporal lobe, participate in the memory deficits of patients like H.M.? In other words, what structures of the medial temporal lobe system are involved in episodic memory? For example, does the amygdala influence memory deficits in amnesiacs (Figure 9.18)? Data from amnesic patients indicate that the amygdala is not part of the brain’s episodic memory system, although—as we will learn in Chapter 10—it has a role in emotion and emotional memories. Another question is, what kind of memory and learning is impaired with various temporal lobe lesions?
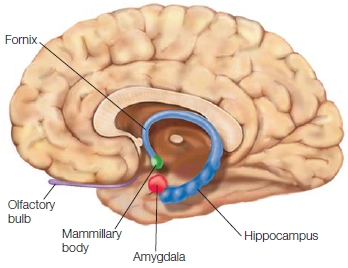
FIGURE 9.18 The amygdala.
The medial temporal lobe structures are shown in a medial view of the right hemisphere.
Nonhuman Primate Studies To test whether the amygdala is essential in memory formation, surgical lesions were created in the medial temporal lobe and amygdala of monkeys. In classic work on monkeys conducted by Mortimer Mishkin (1978) at the National Institute of Mental Health, either the hippocampus, the amygdala, or both were removed surgically. Mishkin found that the resulting amount of impairment varied according to what had been lesioned.
The brain-lesioned monkeys were tested with a popular behavioral task that Mishkin developed, known as the delayed nonmatch-to-sample task. A monkey is placed in a box with a retractable door in the front (Figure 9.19). When the door is closed so the monkey cannot see out, a food reward is placed under an object (Figure 9.19a). The door is opened, and the monkey is allowed to pick up the object to get the food (Figure 9.19b). The door is closed again, and the same object plus a new object are put in position (Figure 9.19c). The new object now covers the food reward, and after a delay that can be varied, the door is reopened and the monkey must pick up the new object to get the food reward. If the monkey picks up the old object, as in Figure 9.19d, there is no reward. With training, the monkey picks the new, or nonmatching, object; hence, learning and memory are measured by observing the monkey’s performance.
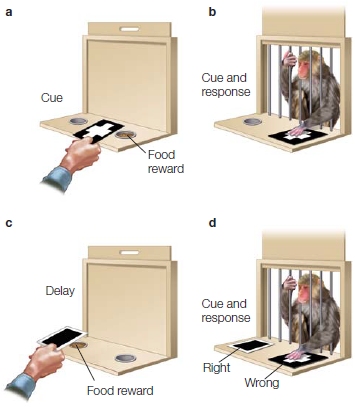
FIGURE 9.19 Delayed nonmatch-to-sample task.
(a) The correct response has a food reward located under it. (b) The monkey is shown the correct response, which will yield a reward for the monkey. (c) The door is closed, and the reward is placed under a second response option. (d) The monkey is then shown two options and must pick the correct response (the one that does not match the original sample item) to get the reward. Here the monkey is shown making an error.
In his early work, Mishkin found that, in the monkey, memory was impaired only if the lesion included both the hippocampus and the amygdala. This finding led to the (incorrect) idea that the amygdala is a key structure in memory. That idea, however, does not fit well with data from amnesiacs like R.B. (described earlier), who had anterograde amnesia caused by a lesion restricted to CA1 neurons of the hippocampus and no damage to the amygdala.
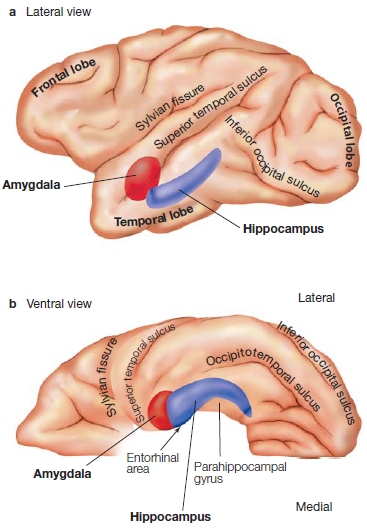
FIGURE 9.20 Gross anatomy of the medial temporal lobe.
(a) This lateral, see-through view of the left hemisphere shows the amygdala (red) and hippocampus (purple) within the temporal lobe. (b) This view from the ventral surface of the same hemisphere shows the amygdala and hippocampus, and indicates the locations of the parahippocampal gyrus and the entorhinal area (consisting of Brodmann areas 28 and typically also 34, which are located in the most anterior portion of the parahippocampal gyrus).
Stuart Zola, Larry Squire, and colleagues (Zola-Morgan et al., 1993) at the University of California, San Diego, investigated this dilemma. They created more selective lesions of the brains of monkeys by distinguishing between the amygdala and the hippocampus as well as the surrounding cortex near each structure. They surgically created lesions of the amygdala, the entorhinal cortex (Brodmann areas 28 and 34; Figure 9.20), or the surrounding neocortex of the parahippocampal gyrus and the perirhinal cortex (Brodmann areas 35 and 36). They wanted to extend Mishkin’s work, which always involved lesions of the neocortex surrounding the amygdala or hippocampus owing to the way the surgery was performed.
The results indicated that lesions of the hippocampus and amygdala produced the most severe memory deficits only when the cortex surrounding these regions was also lesioned. When lesions of the hippocampus and amygdala were made but the surrounding cortex was spared, the presence or absence of the amygdala lesion did not affect the monkey’s memory. The amygdala, then, could not be part of the system that supported the acquisition of long-term memory.
In subsequent investigations, Zola and his colleagues selectively created lesions of the surrounding cortex in the perirhinal, entorhinal, and parahippocampal regions. The parahippocampal and perirhinal areas receive information from the visual, auditory, and somatosensory association cortex and send these inputs to the hippocampus (Figure 9.21) and from there to other cortical regions. These selective lesions worsened memory performance in delayed nonmatch-to-sample tests (Figure 9.22). Follow-up work showed that lesions of only the parahippocampal and perirhinal cortices also produced significant memory deficits.
How do we reconcile these results with R.B.’s profound anterograde amnesia, caused by damage limited to the hippocampus and not involving the surrounding parahippocampal or perirhinal cortex? The answer is that the hippocampus cannot function properly if these vital connections are damaged. But more than this, we now know that these regions are involved in a great deal of processing themselves, and hence lesions restricted to the hippocampus do not produce as severe a form of amnesia as do lesions that include surrounding cortex.
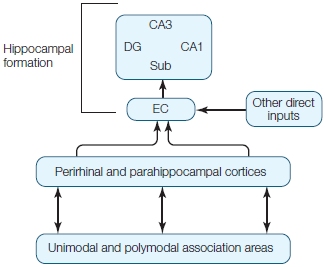
FIGURE 9.21 Flow of information between the neocortex and the hippocampal system.
CA = cornu ammonis neuronal fields (CA1–CA3); DG = dentate gyrus; EC = entorhinal cortex; Sub = subiculum.
In summary, the data from animals are highly consistent with evidence from amnesic patients such as R.B. and H.M. implicating both the hippocampal system in the medial temporal lobe and the associated cortex as critical for forming long-term memories. Lesions that damage the hippocampus directly, or damage the input– output relations of the hippocampus with the neocortex (Figure 9.23), produce severe memory impairments. The amygdala is not a crucial part of the system for episodic memory, but it is important for emotional memory (see Chapter 10).
Moreover, the animal data corroborates the data from amnesic patients with regard to the preservation of short-term memory processes after the medial temporal lobe has been damaged. That is, monkeys’ memory deficits in the delayed nonmatch-to-sample task became more pronounced as the interval between the sample and the test increased. The medial temporal lobe, then, is not essential for short-term or working memory processes. As we noted earlier, the medial temporal lobe is most likely not the locus of long-term storage, because retrograde amnesia is not total after damage to this area. Rather, the medial temporal lobe is a key component in organizing and consolidating long-term memory that is permanently stored in a distributed fashion in the neocortex.
Rodent Studies Another key question that animal researchers have addressed involves the kind of memory and learning that is impaired with lesions to the hippocampus. Early studies in rodents found that hippocampal lesions did not disrupt stimulus–response learning, yet the lesioned rats did exhibit a bewildering variety of abnormal behaviors. These observations led to the suggestion that the hippocampus was involved with the storage and retrieval of one specific type of memory: contextual memory (Hirsh, 1974).
For instance, when electrodes were implanted in the rat hippocampus, certain cells, later known as place cells, fired only when the rat was situated in a particular location and facing a particular direction (O’Keefe & Dostrovsky, 1971). A particular place cell may become silent when the animal moves to a different environment, but then assume a location-specific firing in that new area. As the animal moves about an environment, the activity of specific CA1 and CA3 hippocampal neurons correlates with specific locations. This study led to the idea that the hippocampus represented spatial contexts (O’Keefe & Nadel, 1978), the where in context memory. It was soon found to be involved in spatial navigational learning.
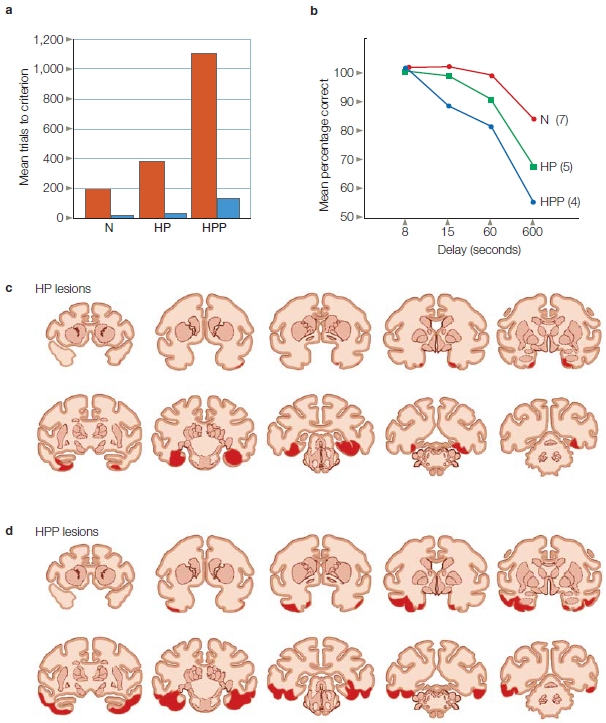
FIGURE 9.22 Selective lesions around the hippocampus worsen memory.
Performance on the delayed nonmatch-to-sample task on two different occasions for normal monkeys (N); monkeys with lesions of the hippocampal formation and the parahippocampal cortex (HP); and monkeys with lesions of the hippocampal formation, parahippocampal cortex, and perirhinal cortex (HPP). (a) Initial learning of the task with a delay of 8 s. Red bars = first test; blue bars = second test. (b) Performance across delays for the same groups. Lesions (red) in HP (c) and HPP monkeys (d) are shown in coronal sections.
In rats, spatial navigational learning is tested using the Morris water maze (R. Morris, 1981). This apparatus is a circular tank filled with opaque water. Above the water are different identifiable visual cues, such as windows and doors, and somewhere below the surface of the water is an invisible platform. Rats are dropped into the tank at different points on different trials. The time it takes for them to reach the platform becomes shorter over time, indicating that they have learned where the platform is in relation to the visual cues above the water. Rats with hippocampal lesions do not learn to associate the visual cues with the platform’s location when dropped from different spots, but swim randomly about on every trial looking for the platform (Schenk & Morris, 1985). If they are always dropped into the water from exactly the same spot, however, they do learn where the platform is located (Eichenbaum et al., 1990). Thus, with hippocampal lesions they can learn a repeated, practiced task (a stimulus–response task) but are unable to relate space information with different contextual information.
Context is not just about space. Some rat hippocampal neurons have been found to fire for specific odors and for specific combinations of odors and locations (Wood et al., 1999), some for visual or auditory stimuli or a combination of both (Sakurai, 1996), and some for many other nonspatial features, including behavior (see Eichenbaum et al., 1999). These findings have led to the suggestion that the function of the hippocampus may be to bind together different contextual information to form a complex contextual memory.

FIGURE 9.23 Anatomy of the hippocampal memory system in monkeys and rats.
Most areas of cortex send information to the hippocampus. Different neocortical zones (blue) project to one or more subdivisions of the parahippocampal region. These subdivisions are the perirhinal cortex (light purple), the parahippocampal cortex (dark purple), and the entorhinal cortex (pink). These latter areas are interconnected and project to different regions of the hippocampus (green), including the dentate gyrus, the CA3 and CA1 fields of the hippocampus, and the subiculum. As a result, various cortical inputs converge within the parahippocampal region. In addition, the parahippocampal region passes this information from the cortex to the hippocampus. Following processing in the hippocampus, information can be fed back via the parahippocampal region to the same areas of the cortex that the original inputs came from.
Although initial work in both animals (see Squire, 1992) and humans suggested that the hippocampus was not involved in the retrieval of long-term distant memories and had only a temporary involvement with forming and retrieving new contextual memories, more recent work has suggested otherwise. For instance, in spatial navigation tasks, both recent and remote memories are equally disrupted after hippocampal lesions (S. Martin et al., 2005).
The retrieval of contextual memory in rats is often studied using contextual fear learning, where rats are placed in a small chamber with specific visual features and a foot shock is delivered. The rats then show a variety of conditioned responses, such as freezing, when placed back into the same visually identifiable chamber. The retention of fear conditioning is evaluated by the amount of freezing the rats show. In one study, after experiencing a single shock episode, some rats underwent sham (control) surgery. Other rats had their hippocampus partially or fully destroyed either 1 week, 2 months, or 6 months later. None of the rats had been put back into the shock chamber in the interval between the shock and the surgery. Two weeks after surgery, all of these groups were tested for fear retention. The control rats froze when put back in the chamber, though the response lessened with longer retention intervals. The rats with a completely destroyed hippocampus did not freeze no matter what the interval, while the rats with partial damage showed some, but less freezing than controls, especially at longer intervals. The severity of retrograde amnesia for the contextual fear was related to the extent of hippocampal damage, but amnesia existed for even remote retrograde contextual memory (Lehmann et al., 2007).
Such studies suggest that the hippocampus has a more extensive role in long-term contextual (and episodic) memory retrieval than was originally postulated after early studies of H.M. There is yet another variable to be considered: memory detail and its accuracy. For example, mice are initially able to distinguish between a fear conditioning chamber and slightly different chambers: They freeze only in the specific chamber where they were first shocked. Over time, however, they no longer distinguish between the similar chambers, and their fear generalizes to the similar chambers (Wiltgen & Silva, 2007). Thus, contextual memories become less detailed and more general with time, allowing the animal to be more adaptable, such that the fear memory is activated in novel, but similar, contexts. It has been proposed that memory quality may be a critical factor that determines whether the hippocampus is essential for retrieval. The proposal is that it plays a permanent role in retrieving detailed contextual memory, but is not necessary for retrieval once detail is lost and memory has generalized. Thus, if testing conditions promote retention of detailed memories, such as spatial navigation in water mazes where the exact location of a platform is required, the hippocampus is needed in their retrieval for both short- and long-term memories. If the conditions result in memory generalization across time, such as in fear conditioning, they will lead to a temporal gradient of hippocampal involvement in memory retrieval, as was seen in the last experiment.
Interestingly, if the fear memory was reactivated 45 days after it was formed (when it no longer requires the hippocampus for expression) and then a hippocampal lesion was made, the rats no longer showed fear when placed back in the chamber (Debiec et al., 2002). It seems that retrieval and reactivation of a hippocampal-independent memory made that memory hippocampal-dependent again and susceptible to hippocampal damage. In the next sections, we see how some of these findings have been mirrored in humans.
TAKE-HOME MESSAGES
Imaging Human Memory
The work described so far has dealt primarily with evidence from humans and animals with brain damage. It suggests a degree of independence of procedural memory and perceptual priming (as well as conditioning and nonassociative learning) from the medial temporal lobe memory system. Let’s now integrate into the story some of the studies done over the past 15 years using functional brain-imaging methods (both magnetic resonance methods and electromagnetic recording methods). These methods have helped to clarify the role of various brain structures and systems in different memory processes.
As mentioned earlier, long-term memory is created when new information is encoded and consolidated; stored information can then be retrieved to create a conscious memory or to produce an action. Researchers have eagerly tested the role of the hippocampal system in creating long-term memories using functional brain-imaging methods in healthy human volunteers with intact memory ability.
A key question has been whether the hippocampus becomes active during the encoding of new information, during the retrieval of information, or both. In this section, we review evidence demonstrating that the hippocampus is involved in both, which is in agreement with the animal studies discussed earlier. We also see that different types of memory rely on different subregions of the medial temporal lobe during encoding and retrieval and that during retrieval, the medial temporal lobe memory systems reactivate cortical regions that were important during the original encoding of the information. We end this section with a discussion of the role of the frontal cortex in long-term memory encoding and retrieval.
Encoding and the Hippocampus
Functional MRI studies have shown that the human hippocampus is active when new information is encoded. This kind of work typically involves the subsequent-memory paradigm, where participants are presented with items that they are asked to remember. Their brain activity is measured with fMRI or ERPs while they are encoding the information. Later, their memory for the items is assessed. This can be done in different ways. One is to ask them whether they have seen the item previously when it is embedded in lists containing new items. Using event-related methods, it is then possible to sort and analyze the original data gathered during encoding as a function of whether the items were later correctly remembered or forgotten.
One study that demonstrates the involvement of the hippocampus in encoding (among other findings that we will return to) was done by Charan Ranganath and his colleagues (2003). They combined fMRI with the subsequent-memory paradigm, as shown in Figure 9.24. Healthy volunteers were presented with words on a screen at a rate of about one word every 2 seconds. The words (360 in total), printed in either red or green, were of either animate or inanimate items that were either large or small. Depending on the color, the participants were required to make either an animacy judgment or a size judgment for each item. While the participants viewed the words and made their decisions (the encoding phase of the experiment), they were scanned using fMRI. Later, outside of the scanner, they were tested on their memory for the items that they had been shown by being presented with the 360 “old” items that were mixed with 360 “new” items. Participants were asked to rate each item (which were shown in black) on a scale of 1 to 6 to indicate how confident they were that they had seen the word before. They also were asked to specify whether the item had been presented in green or red (a source memory judgment, where the source was the context in which the item had been previously viewed). Researchers had to sort and separately analyze the old items as a function of whether they were properly recollected, and whether the source was correctly identified. What brain regions were active at encoding for correctly recollected items (those that the participant had seen, reported having seen before, and correctly indicated the color of their presentation)? Correctly recollected words activated regions of both the hippocampus and the posterior parahippocampal cortex (Figure 9.25a) during encoding. This evidence that the medial temporal lobe, including the hippocampus proper, was activated during encoding fits well with evidence from studies in animals and patients with amnesia. Those studies suggested that the hippocampus is important for the formation of new long-term memories. Thus, one problem with hippocampal damage may be an inability to encode the information properly in the first place. Ranganath and colleagues also observed that regions of the frontal cortex were activated during encoding (Figure 9.25b), a topic that we will return to later in the chapter.
FIGURE 9.24 Testing for involvement of the hippocampus in information encoding.
(a) The sequence of events in one scanning run. At encoding, participants viewed a series of words and made either an animacy (animate versus not animate) or size (large versus small) judgment for each word, depending on the color of that word (e.g., green font meant, perform an animacy judgment, so for the word NICKEL in green ink, the correct response would be “inanimate”). Later, in a test at retrieval after the scan session, participants made two decisions about the items presented, which included the old items and new items never seen before. First, participants were asked to indicate whether and how well (how confidently) they recognized the items (e.g., on a scale of 1 to 6, from definitely new to definitely old). Second, for each word they had to make a source memory judgment (had it previously been presented in red or in green?). (b) Mean proportions of studied (“old”) and unstudied (“new”) items endorsed at each confidence level. Performance on the source memory judgment (red or green) is not shown.
Retrieval and the Hippocampus
The hippocampus is also involved in the retrieval of information from long-term memory. In one study, similar to that described in the preceding section, event-related fMRI methods were used to reveal that the hippocampus is activated when information is correctly recollected (Eldridge et al., 2000). Participants in this task memorized a list of words. No other task was involved at the encoding stage, and no memory strategy was suggested. Twenty minutes later, in a retrieval task, participants were presented with a new list consisting of previously studied (old) and unstudied (new) words and were asked, one by one, if they had seen the word before. If they answered yes, then they were asked to make a decision about whether they actually remembered (recollected) seeing it before (an episodic memory with a spatial and temporal context) or whether the item merely seemed familiar to them.
The interesting part of this study was that neuroimaging data were collected during the retrieval phase of the study, and brain responses measured with fMRI could be sorted according to whether the participants actually recollected the item, were only familiar with the item, were sure they had not seen the word before, or were mistaken about whether they had seen the word. The neuroimaging results were clear: During retrieval, the hippocampus was selectively active only for items that were actually correctly recollected (Figure 9.26), thus indicating an episodic memory. This finding strongly suggests that the hippocampus is involved in retrieval for episodic memories but not memories based on familiarity.
Recollection, Familiarity, and the Medial Temporal Lobe
What are the roles of different subdivisions of the medial temporal lobe in long-term memory? In 1999, John Aggleton and Malcolm Brown (1999) proposed that encoding processes that merely identify an item as being familiar, and encoding processes that correctly identify the item as having been seen before (recollection), each depend on different regions of the medial temporal lobes. Support for this idea soon followed. For example, in the study of retrieval described in the previous section and illustrated in Figure 9.26, the hippocampus was activated only for episodic recollections. It was not activated for memory that did not contain awareness of the prior event—that is, when the items recollected merely seemed familiar to the participants and were recognized by their familiarity alone. Such data raised the question of what brain regions are involved in episodic versus nonepisodic (familiarity-based) memory encoding and retrieval.
|
FIGURE 9.25 Correct recollections trigger activity in medial temporal lobe and frontal cortex. |
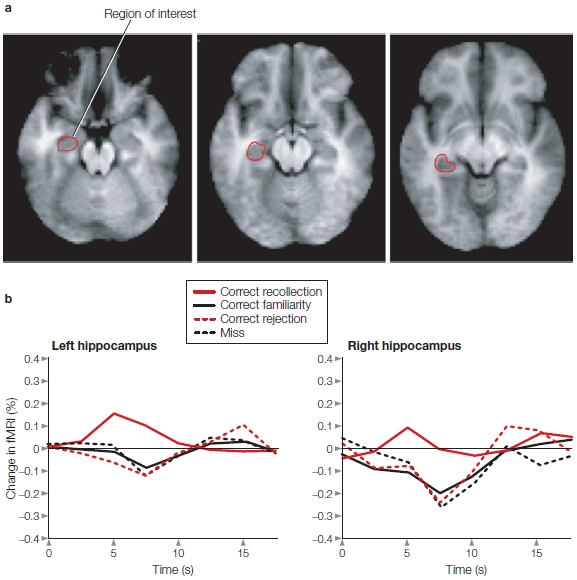
FIGURE 9.26 Retrieval in the hippocampus.
(a) Horizontal sections through the brain at the level of the inferior (left panel), middle (center panel), and superior (right panel) hippocampus. The red outline of the region of interest in the left hippocampus is based on anatomical landmarks. (b) Hemodynamic responses from event-related fMRI measures taken during the retrieval of previously studied words (see text for paradigm). The hippocampus was activated by correctly recollected words (solid red line) but not by words that the participants had previously seen but could not recollect, indicating that the words merely seemed familiar (solid black line). No hippocampal activity occurred for words that were correctly identified as new (not seen previously; dashed red line) nor for errors in which the participant did not remember the words (dashed black line) despite having seen them previously.
The process of encoding episodic memory involves encoding an event and binding it to a time and place, as we hinted at in the rodent studies. When you recall the first rock concert that you ever attended, you may recall who you saw, where you saw them, and with whom you went. This memory may be distinguished from memories of other rock concerts, other events held at the same place, and other places you have been with the same friend. How does our brain accomplish this?
Anatomy offers some clues: Different types of information from all over the cortex converge on the medial temporal lobe regions surrounding the hippocampus, but not all types pass through the same structures. Information about the features of items (“what” an item is) coming from unimodal sensory regions of neocortex passes through the anterior parts of the parahippocampal region known as perirhinal cortex (PRC). In contrast, information from polymodal neocortical areas about “where” something is located passes through the more posterior parts of the parahippocampal cortex. Both information types project into the entorhinal cortex but do not converge until they are within the hippocampus (Eichenbaum et al., 2007). A model known as the binding of items and contexts (BIC) model proposes that the perirhinal cortex represents information about specific items (e.g., who and what), the parahippocampal cortex represents information about the context in which these items were encountered (e.g., where and when), and processing in the hippocampus binds the representations of items with their context (Diana et al., 2007; Ranganath, 2010). As a result, the hippocampus is able to relate the two types of information about something that the individual encounters. This form of memory is referred to as relational memory. So, to recognize that something is familiar, perirhinal cortex is sufficient; but to remember the full episode and everything related to it, the hippocampus is necessary.
For support of this theory we return to the encoding study of Ranganath and his colleagues (2003). In that study, participants were required to make source memory judgments related to episodic memory (see Figures 9.25 and 9.26). Study participants also had to rate their confidence about whether they had seen the item before—a measure of familiarity. Figure 9.27 presents the neuroimaging results from this analysis of confidence ratings. Regions of the left anterior medial parahippocampal gyrus—in and around the perirhinal cortex—were activated during recognition based on familiarity, but the hippocampus itself was not activated. Combining these results with those in the previous paragraph, this work demonstrates a double dissociation in the medial temporal lobe for encoding different forms of memory: one medial temporal lobe mechanism for recognition based on the recollection of episodic (source) information involving the hippocampus and posterior parahippocampal cortex, and the other for supporting familiarity-based recognition memory in the perirhinal cortex.
A similar distinction has also been found between the recollection and familiarity components of retrieval of long-term memories. One study that nicely makes this point is the work of Daniela Montaldi and her colleagues at the University of Oxford (2006). They showed study participants pictures of scenes during an encoding session. Two days later, researchers tested the participants’ recognition with a mixed batch of new and old scenes while monitoring their brain activity with fMRI.
Montaldi and colleagues asked the participants to rate the pictures of scenes as new, slightly familiar, moderately familiar, very familiar, or recollected. Their results showed the same activity pattern as in the Ranganath encoding study. The hippocampus was activated only for pictures of scenes that the participants could recollect having seen before. Regions of the medial temporal lobe, like the perirhinal cortex, that are located outside the hippocampus showed activity patterns that correlated with the strength of familiarity with scenes other than recollected ones (Figure 9.28).
In sum, evidence from a number of studies indicates that the medial temporal lobe supports different forms of memory and that these different forms of memory (recollective experience versus familiarity) are supported by different subdivisions of this brain region. The hippocampus is involved in encoding and retrieval for episodic memories that are recollected, whereas areas outside the hippocampus, especially the perirhinal cortex, support recognition based on familiarity. These findings also suggest that the nature of the representations should be considered in distinguishing between memory systems (Nadel & Hardt, 2011).
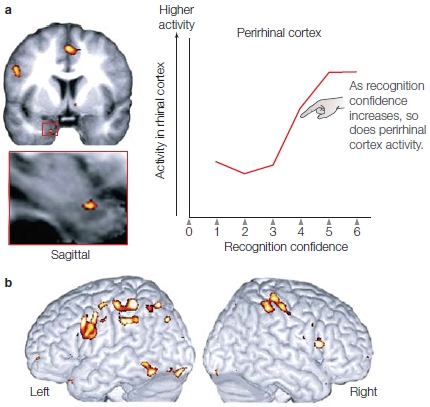
|
FIGURE 9.27 Familiarity-based recognition memory. Brain activity during encoding correlates with the confidence of recognizing that an item has been seen before. (a) Coronal section through the brain at the level of the anterior medial temporal lobe. Functional MRI activations that correlated with confidence ratings can be seen in the entorhinal cortex (red box and in an expanded view below the coronal section). The graph shows that as recognition confidence increases, activity in the perirhinal cortex also increases. (b) Images of the left and right hemispheres show additional regions of cortical activation. |
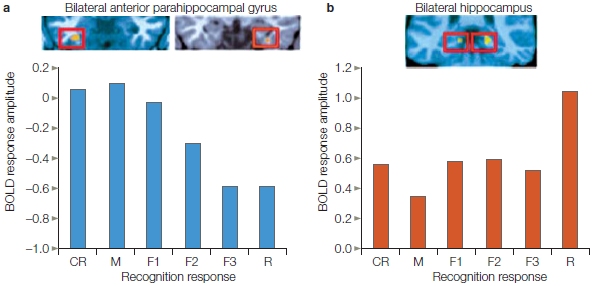
FIGURE 9.28 Recollection and familiarity during retrieval.
Participants studied scenes and were scanned during recognition testing. The partial images of the brain are coronal sections through the hippocampus. (a) Activation in bilateral anterior parahippocampal regions decreased with increasing confidence. (b) In contrast, activations in bilateral hippocampal regions increased for recollected items only, as compared with nonrecollected items. CR = correct rejection (an item that was correctly identified as new); M = miss (an item that was seen before but the participant reported as not having seen previously); F1 = weak familiarity, F2 = moderate familiarity, F3 = strong familiarity; R = recollected.
Relational Memory What we have been referring to as episodic information that leads to recollective experiences is relational memory, so called because we can indicate that something has been encountered previously. Moreover, we can retrieve the relational context (the sources) in which it was previously encountered and know that it is different from the present encounter. For instance, if you live in Los Angeles, you may see Tom Hanks drive past in a Porsche and know that you’ve seen him before—not in a Porsche, but in a movie. Neal Cohen and his colleagues (Ryan et al., 2000) at the University of Illinois have investigated relational memory using measures of eye fixation as study participants watched complex scenes where the object and spatial relationships were experimentally manipulated. They found that healthy participants were sensitive to changing relationships in the scenes, even when they were unaware of them, as demonstrated by their altered patterns of eye movements (Figure 9.29). In contrast, patients with amnesia as a result of hippocampal damage were insensitive to the changes (Figure 9.29b). These researchers have argued, therefore, that medial temporal amnesia is a disorder of relational memory and is distinct from episodic memory, which requires conscious awareness. Cohen and colleagues amassed additional evidence to support their argument in a study on amnesic patients with damage limited to the hippocampus (Konkel, 2007). The researchers evaluated memory performance for three different types of relational tasks: spatial, associative, and sequential. They also compared single-item recollection by the amnesiacs to that by normal participants and patients with more extensive medial temporal lobe damage. Those with hippocampal-only damage were impaired on all of the relational tasks, but not on the single-item recollection task. Patients with more extensive medial temporal lobe damage were impaired on both types of tests. Multiple neuroimaging studies show increased hippocampal activation when the relationship between items is being evaluated; in contrast, when an item is being individually encoded, activity is not observed in the hippocampus but is seen in other medial temporal lobe cortical regions, especially in the perirhinal cortex (Davachi & Wagner, 2002; Davachi et al., 2003).
Retrieval and Reactivation in Long-Term Memory Where in the brain is the what and where information stored? The projections of “what” and “where” information from the neocortex into the hippocampus described in the previous section are matched by a similar outflow from the hippocampus that travels back to the entorhinal cortex, then to the perirhinal and parahippocampal cortex, and then to the neocortical areas that provided the inputs to the neocortex in the first place. You may already have guessed the role of this feedback system in memory storage and retrieval, and some findings from neuroimaging studies during retrieval may back up your guess.
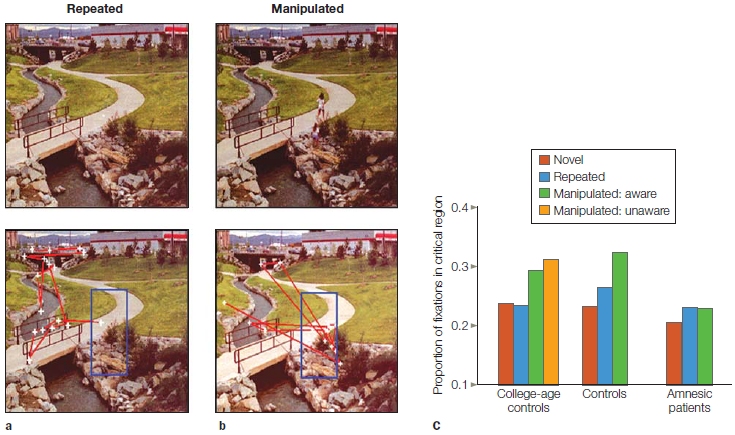
FIGURE 9.29 Scenes with changing relational information used to test for relational memory.
(a,b) Eye movements were recorded from healthy participants as they viewed scenes at two time points. Eye movements (red lines) and fixations (white crosses) are shown superimposed on the same scene (bottom panels) under two conditions. (a) The scene the participants viewed did not change (top vs. bottom panels). (b) The scene changed. At first viewing, it contained two people in a critical region (top panel), while in the second it did not (bottom panel). The critical region where the people were located is outlined by the blue rectangle (the box was not on the screen; it was placed in the figure to indicate the region of interest in this test). (a) When nothing changed in the critical area, the critical area did not attract eye fixations (bottom panel). (b) When the scene was viewed as a manipulated scene (the people present during the first viewing were removed in the second viewing), many eye fixations focused on the critical region that had contained the people. Some participants were aware of the change, while others were not. (c) Quantification of proportions of fixations in the critical area of a and b for healthy young controls, age-, education and intelligence-matched controls, and six patients with amnesia. Both control groups showed more fixations in the critical region when the scene changed, as in b, than when it did not change, as in a. The amnesic patients failed to show this effect of relational memory.
Mark Wheeler and his colleagues at Washington University in St. Louis (2000) investigated brain regions involved in the retrieval of different types of information. They asked participants to learn a set of sounds (auditory stimuli) or pictures (visual stimuli) during a 2-day encoding period. Each sound or picture was paired with a written label describing the item (e.g., the word BELL, followed by the sound of a bell). On the third day the participants were given perceptual and memory tests while in an fMRI scanner. In the perceptual test, stimuli (label plus sound or picture) were presented and brain activity was measured to identify brain regions involved in the perceptual processing of items. In the memory retrieval test, only the word label was presented and the participant pressed a button to indicate whether the item was associated with a sound or a picture.
Wheeler and coworkers found that during retrieval of pictures, regions of neocortex that had been activated during perception of the pictures were reactivated. Similarly, during retrieval of sounds, different areas of the neocortex that had been activated during the perception of sounds were reactivated. In each case, during memory retrieval the modality-specific regions of activity in the neocortex were subsets of the areas activated by presentation of the perceptual information alone, when no memory task was required (Figure 9.30). The activated areas of sensory-specific neocortex were not lower-level sensory cortical regions; they were later stages of visual and auditory association cortex, where incoming signals would have been perceptually well processed (e.g., to the point where identity was coded).
These results suggest that the specific relational information for items stored in long-term memory may be coded during retrieval by reactivation of the original neocortical areas that provide input to the hippocampus during the original encoding. In subsequent work, Wheeler and colleagues (M. Wheeler et al., 2006) showed that visual processing regions in inferotemporal cortex were involved in the preparation to retrieve visual information, whereas the more dorsal parietal and superior occipital activity was related to the process of searching memory for the relevant item. These findings help refine the role of different brain regions in reactivation during long-term memory retrieval.
HOW THE BRAIN WORKS
False Memories and the Medial Temporal Lobes
When our memory fails, we usually forget events that happened in the past. Sometimes, however, something more surprising occurs: We remember events that never happened. Whereas forgetting has been a topic of research for more than a century, memory researchers did not have a good method to investigate false memories in the laboratory until Henry Roediger and Kathleen McDermott at Washington University rediscovered an old technique in 1995. In this technique participants are presented with a list of words (e.g., thread, pin, eye, sewing, sharp, point, haystack, pain, injection, etc.) in which all the words are highly associated to a word that is not presented (in this case, needle; did you have to go back and recheck the list?). When participants are asked subsequently to recall or recognize the words in the list, they show a strong tendency to falsely remember the associated word that was not presented. The memory illusion is so powerful that participants are willing to claim that they vividly remember seeing the nonpresented critical word in the study list.
When participants are interrogated carefully about the conscious experience associated with remembering items from the list (true items) and the critical nonpresented words (false items), however, they tend to rate true items higher than false items in terms of sensory details (Mather et al., 1997; K. Norman & Schacter, 1997). This finding introduced a conundrum in false-memory research: How can human participants believe in their illusory recollections, and at the same time be able to differentiate them from genuine recollections in terms of sensory detail?
Roberto Cabeza at Duke University and collaborators (2001) provided a possible answer to this conundrum. In their study, participants watched a videotape segment in which two speakers alternatively presented lists of associated words. The participants then were required to perform an old/new recognition test that included true items, closely related false items, and unrelated new words (new items) while their brains were scanned with functional MRI. Changes in blood flow in the brain that indicated changing patterns of neural activity were measured separately for each kind of item. Memory performance showed the same pattern as in previous studies: Participants were able to reject new items but showed a strong tendency to falsely recognize closely related false items.
The researchers found a dissociation between two medial temporal lobe regions (Figure 1). In the hippocampus bilaterally, false items elicited more neural activity than did new items, and as much activity as true items. But in the left parahippocampal gyrus, a region surrounding the hippocampus, closely related false items elicited about the same amount of activity as new items and significantly less activity than true items. In other words, the hippocampus responded similarly to true and false items, and the parahippocampal gyrus responded more strongly to true than to false items. Because true and closely related false items were similar in their semantic content but differed in sensory content, these results suggest that the hippocampus is involved in the retrieval of semantic information, whereas the parahippocampal gyrus is involved in the retrieval of sensory information.
This dissociation provides a possible solution for the aforementioned conundrum: The memory system in the medial temporal lobes can generate two different types of messages when information is presented. Whereas anterior hippocampal activity suggests that closely related false items are like true items, posterior parahippocampal activity suggests that they are like new items. These two messages are not contradictory. Closely related false items are like true items in terms of their semantic properties, but they are like new items in terms of their sensory properties.
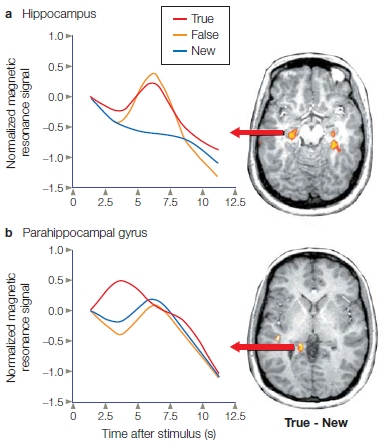
FIGURE 1 Significant increases in blood flow in regions of the medial temporal lobes (right side of figure), and their corresponding hemodynamic response functions (left side of figure).
(a) Bilateral hippocampal regions were more activated for true and closely related false items than for new items. There was no difference between activations for true and false items. (b) A left posterior parahippocampal region was more activated for true items than for closely related false and new items. There was no difference between activations for false and new items. The hemodynamic response functions at left were taken from the regions of interest defined by the statistical contrast of true activations minus new activations, which is shown at right in the brain sections.
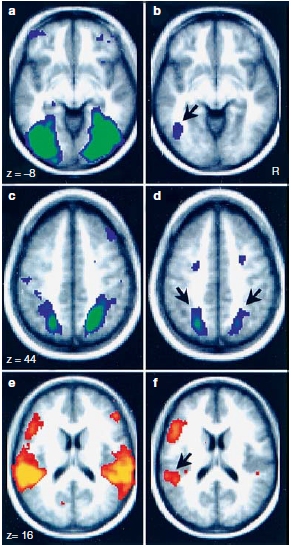
FIGURE 9.30 Reactivation of modality-specific cortex during long-term memory retrieval.
Areas activated by viewing pictures (a, c) and hearing sounds (e). Areas activated during the retrieval of pictures (b, d) or sounds (f) from memory. Arrows indicate regions of overlap between memory and perceptual activations. The right hemisphere of the brain is on the right of each image.
Encoding, Retrieval, and the Frontal Cortex
Neuroimaging research and studies of amnesic patients have consistently found that the frontal cortex is involved in both short-term and long-term memory processes. Its role in the encoding and retrieval of long-term memory, however, has been a key point of debate. A meta-analysis of the literature (Nyberg et al., 1996) found that the left frontal cortex is often involved in encoding of episodic information, whereas the right frontal cortex is often found to be activated in episodic retrieval (Figure 9.31). These findings led Roberto Cabeza and colleagues to develop a model proposing that contributions to episodic encoding (left frontal) and retrieval (right frontal) were lateralized within frontal cortex. Figure 9.31 also shows, however, that both semantic encoding and retrieval involve the left frontal cortex, including Broca’s area (Brodmann area 44 extending into area 46) and the ventral lateral region (Brodmann areas 44 and 45). This lateralization to the left hemisphere for semantic information remains regardless of whether the memories being retrieved are of objects or of words.
Others, including William Kelley at Dartmouth College and his colleagues, have argued that lateralization of frontal cortex activity during long-term memory retrieval is related more to the nature of the material to be processed than to a distinction between encoding and retrieval (Buckner et al., 1999). They believe that the left hemisphere is more involved in processes coded by linguistic representations, whereas the right frontal cortex is more involved in object and spatial memory information (Figure 9.32). Much work remains to be done to establish the roles of the frontal cortex in memory processing. For now, various competing models remain viable explanations of the patterns of deficits seen in amnesia and the activations in functional imaging that have been reported.
Retrieval and the Parietal Cortex
FIGURE 9.31 Summary of regions in the prefrontal cortex that show activation for episodic encoding and semantic retrieval or episodic retrieval.
The data are from many studies, reported in Nyberg, Cabeza, and Tulving (1996, 1998) and Tulving et al. (1994).
Over the past half century, memory researchers have largely ignored the parietal lobe, partially because parietal lobe lesions are not generally associated with memory loss. There is, however, a notable exception: Retrosplenial lesions can produce both retrograde and anterograde amnesia. A finding dubbed the old/new effect, which was first identified in ERP studies, stimulated memory research that focused on the parietal cortex. The findings showed that the parietal cortex displays different responses when an individual correctly recognizes a previously encountered item (termed a hit) as compared to correctly identifying that a new item was not previously encountered (a correct rejection). Event-related fMRI studies have revealed greater activation during hits than during correct rejections in posterior parietal cortex (PPC), including inferior and superior parietal lobules as well as medial structures that extend from precuneus into posterior cingulate cortex (PCC) and retrosplenial cortex (RSC).
|
FIGURE 9.32 Material-specific frontal cortex activation during memory encoding. |
|
The past few years have witnessed an explosion of functional neuroimaging studies, which have revealed that successful memory retrieval, especially for contextual information, is consistently associated with activity in lateral PPC, including the RSC. During encoding, however, these areas are usually less active than baseline levels (Figure 9.33; Daselaar et al., 2009), unless the items are encoded in a self-relevant manner (Leshikar & Duarte, 2012), or are likely to evoke self-referential (V. C. Martin et al., 2011) or emotional processing (Ritchey et al., 2011). This encoding preference for self-referential items suggests that the RSC is more attuned to internal information sources. Interestingly, these same parietal regions are active during conscious rest when a highly interconnected network of cortical association areas is activated; this is the so-called default mode network which we discuss in Chapter 13. The salient point for this discussion is that the default network is active whenever an individual’s mind turns to thinking about self-related past and future scenarios.
The anatomical connections of the parietal cortex are also suggestive of its involvement in memory. The lateral parietal, retrosplenial, and posterior cingulate cortices are connected to the medial temporal lobe, both directly and indirectly. Notably, the retrosplenial cortex is extensively interconnected with the parahippocampal cortex (PHC), and both interface with similar regions in the posterior hippocampus, subiculum, mammillary bodies, and anterior thalamus as well as the default network. Meanwhile, the perirhinal cortex displays a completely different connectivity pattern, not with the posterior hippocampus but with the anterior hippocampus, amygdala, ventral temporopolar cortex (VTPC), and lateral orbitofrontal cortex (Suzuki & Amaral, 1994).

FIGURE 9.33 Encoding and retrieval flip in ventral parietal cortex.
While encoding different types of stimuli (faces or words), fMRI revealed lower activity (red bars) in both left and right ventral parietal cortex for a stimulus that was successfully encoded (i.e., later remembered, referred to as Encoding Hits) than for one that was not encoded (i.e., later forgotten; Encoding Misses). During retrieval, the opposite was found. Activity was greater (blue bars) for remembered items (called Retrieval Hits) than for items remembered incorrectly (called Retrieval Misses).
Building on this anatomy and on the binding of items and contexts model, Charan Ranganath and Maureen Ritchey (2012) have proposed a memory model made up of two systems: the anterior temporal (AT) system, which includes the perirhinal cortex and its above-mentioned connections; and the posterior medial (PM) system, which is composed of the core components of the PHC and RSC, the mammillary bodies, anterior thalamic nuclei, subiculum, and default mode network (Figure 9.34). Ranganath and Richey propose that these two systems support different forms of memory-guided behavior. Thus, they are involved not only in memory, as in traditional medial temporal lobe models, but also in other aspects of cognition (Figure 9.35). The PRC in the anterior system supports memory for items, and it is involved in familiarity-based recognition, associating features of objects, and making fine-grained perceptual or semantic discriminations. Ranganath and Richey suggest that the overall cognitive job of the anterior system (in collaboration with the amygdala, VTPC, and lateral orbital frontal cortex) may be to assess the significance of entities. The PHC and RSC, which are not traditionally included in medial temporal lobe systems, support recollection-based memories, such as memory for scenes, spatial layouts, and contexts. These researchers also propose that this system, together with the other posterior medial system structures, may construct mental representations of the relationships between entities, actions, and outcomes. Some support for this theory comes from neurological patients. Recall that along with hippocampal damage, Alzheimer’s disease, with its episodic memory impairment, is associated with severe disruptions in the retrosplenial cortex, posterior cingulate, precuneus, and angular gyrus, which together are the proposed posterior medial system. In contrast, patients with semantic dementia, which is characterized by a loss of knowledge about objects, have extensive damage to the anterior temporal lobes.
In closing, while the parietal cortex is well known for its role in attention (see Chapter 7), it also appears to have a greater role in memory than had been considered previously. What is that role? Although the answers are not known, several hypotheses have been suggested. The working memory maintenance hypothesis (Wagner et al., 2005) says that activation of the parietal cortex is related to the maintenance of information in working memory. The multimodal integration hypothesis (Vilberg et al., 2008; Shimamura, 2011) suggests that parietal activations indicate integration of multiple types of information. The bottom-up attention hypothesis (Cabeza, 2008) proposes that the activity reflects the capture of bottom-up attention by information entering working memory either from the senses or from long-term memory.
FIGURE 9.34 Anatomy of the perirhinal, parahippocampal, and retrosplenial cortices.
(a) The perirhinal cortex (PRC), parahippocampal cortex (PHC), and retrosplenial cortex (RSC) regions are shown. (b) Functional connectivity profiles of the PRC (top) and PHC (bottom) showing regions that were significantly correlated with the PRC and PHC during resting-state scans. Resting state fMRI scans evaluate covariations in spontaneous fluctuations in the BOLD signal across the brain while the participant performs no task, and are taken as evidence of intrinsic functional connectivity between brain regions that covary. PRC was found to be functionally connected to ventral temporopolar cortex (VTPC) where higher-order visual areas are located. In contrast, PHC is functionally connected to the dorsal temporopolar cortex (DTPC), the retrosplenial cortex (RSC), the posterior cingulate cortex (PCC), the precuneus (PREC), the medial prefrontal cortex (MPFC), and the angular gyrus (ANG).
Finally, keep in mind that the studies presented so far implicate specific brain regions in distinct forms of memory impairment. For individuals to learn and retain new information about their autobiographical history (episodic memory), they must have an intact medial temporal lobe (primarily hippocampus) and related structures, such as the midline diencephalon and the retrosplenial cortex in the parietal lobe. Damage to these areas impedes the formation of new declarative memories (anterograde amnesia) and leads to difficulties in remembering events in the years immediately before the injury (time-limited retrograde amnesia). It leaves intact, however, most previous episodic and semantic memories acquired during life. Therefore, these structures are not likely to be the storage sites of information in long-term memory, but they appear to be essential for consolidating new information in long-term stores. In contrast, damage to regions of the temporal lobe outside the hippocampus can produce dense retrograde amnesia, an apparent loss of episodic memories, even though the ability to acquire new memories may be intact.

FIGURE 9.35 Model of two neocortical systems for memory-guided behavior.
The components of the anterior temporal (AT) system are shown in red. The posterior medial (PM) system is shown in blue. Regions with strong anatomical connections are indicated with arrows.
TAKE-HOME MESSAGES
Memory Consolidation
Consolidation is an old concept, first proposed by Marcus Fabius Quintilianus, a first-century Roman teacher of rhetoric, who stated:
[It] is a curious fact, of which the reason is not obvious, that the interval of a single night will greatly increase the strength of the memory.... Whatever the cause, things which could not be recalled on the spot are easily coordinated the next day, and time itself, which is generally accounted one of the causes of forgetfulness, actually serves to strengthen the memory. (as quoted in Walker, 2009)
The Hippocampus and Consolidation
Consolidation is the process that stabilizes a memory over time after it is first acquired. In most current models, consolidation consists of an initial rapid consolidation process, followed by a slower permanent consolidation process. One line of evidence for temporal consolidation comes from patients who have undergone electroconvulsive therapy (ECT) to treat psychological disorders. In ECT, an electrical current is passed through the brain by electrodes placed on the scalp—a useful treatment for conditions such as severe depression. This procedure can result in a retrograde amnesia that is more likely to affect items that were learned close to the time of the treatment (Figure 9.36). A similar phenomenon is observed with severe head trauma that results in a closed head injury. Retrograde amnesia is more likely for recent events, and even as the amnesia fades over time, the most recent events are affected for the longest time—sometimes permanently. The items that are lost appear to be those that have undergone initial rapid consolidation but have not yet completed the slower permanent consolidation process.
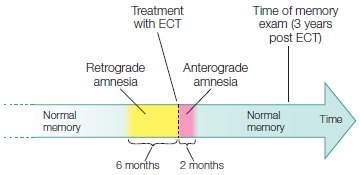
FIGURE 9.36 Effects of electroconvulsive therapy (ECT) on memory performance.
After electroconvulsive therapy, patients show a temporally graded retrograde memory loss. This tells us that memory apparently changes for a long time after initial learning. Some material is forgotten, and the material that remains becomes more resistant to disruption.
The medial temporal lobes, particularly the hippocampi, are essential for the rapid consolidation and initial storage of information for episodic and semantic memories. The mechanisms of the slow consolidation process, however, remain more controversial. There are two main theories. The standard consolidation theory, proposed by Larry Squire and his colleagues, considers the neocortex to be crucial for the storage of fully consolidated long-term memories, whereas the hippocampus plays only a temporary role. In this view, the representations of an event that are distributed throughout the cortex come together in the medial temporal lobe where the hippocampus binds them. Then, through some sort of interaction between the medial temporal lobe and the neocortex, the bound information is slowly transferred and replaced by a permanent memory trace in the neocortex. Consolidation occurs after repeated reactivation of the memory creates direct connections within the cortex between the various representations. This process takes place when an individual is either conscious or asleep, and it eventually makes the memory independent of the hippocampus. This model proposes the same process for both episodic and semantic memories. Although it can explain why there is a temporal gradient to retrograde amnesia (some memories just hadn’t completed the consolidation process before damage occurred), it doesn’t explain why some people who have amnesia due to hippocampal damage have good long-term memory and others have severe loss.
An alternative model, the multiple trace theory, proposed by Lynn Nadel in Arizona and Morris Moscovitch in Toronto, suggests that only the long-term stores for semantic information rely on the neocortex while some aspects of episodic memory, consolidated or not, continue to rely on the hippocampus. In this formulation, a new memory trace, composed of a combination of attributes, is set down in the hippocampus every time a memory is retrieved: The more times a memory is retrieved, the more traces are set down. Remote events that have been retrieved more often have more hippocampal traces and become resistant to hippocampal damage. The traces are not exactly alike, but may differ in attributes. Slowly, the common elements of the traces are extracted into “gist” information and then stored as semantic memory elsewhere in the cortex. This theory suggests that episodic memories degrade over time and are slowly converted to semantic memory. It predicts that partial hippocampal damage would partially affect episodic memory, but complete damage would completely destroy it. Although a more detailed discussion of these models is beyond the scope of this chapter, both models agree on one point: Memory consolidation via the hippocampus is rapid.
The Lateral Anterior Temporal Lobe and Consolidation
The temporal neocortex outside the medial temporal lobe is important for the permanent consolidation of semantic information. Lesions that damage the lateral cortex of the anterior temporal lobe near the anterior pole, such as those associated with semantic dementia and herpes simplex encephalitis, can lead to severe retrograde amnesia, which may extend back many decades or may encompass the patient’s entire life. In severe cases of semantic dementia, perirhinal atrophy is also observed (Davies et al., 2004). Some patients with anterior temporal lobe damage and the consequent dense retrograde amnesia can still form new long-term episodic memories. This condition is known as isolated retrograde amnesia. For instance, patients with semantic dementia have progressive loss of previously established semantic knowledge (non-context-specific fact, word, and object knowledge), yet their episodic memory is intact and they are still able to learn new episodic information (Hodges et al., 1992). Thus, these portions of the temporal lobe are not essential for acquiring new episodic information. What role do they play?
HOW THE BRAIN WORKS
Stress and Memory
Stress, both physical and psychological, triggers the release of cortisol. This hormone is produced by the adrenal cortex in the adrenal glands, which are located above the kidneys. In small quantities, cortisol can aid learning and increase attentiveness. Chronic high stress, however, has detrimental effects on cognitive functions, including memory. The receptors in the brain that are activated by cortisol are called glucocorticoid receptors and are found at concentrated levels in the hippocampus (especially in the dentate gyrus and CA1 region; see Figure 9.16b).
The CA1 region of the hippocampus is the origin of connections from the hippocampus to the neocortex that are important in consolidation of episodic memory. The functions of this circuitry can be disrupted by high levels of cortisol, perhaps by impairment of long-term potentiation (LTP; see the last section of this chapter for a discussion of LTP). Researchers have discovered that episodic memory (but not procedural memory) is impaired by high levels of cortisol. Clemens Kirschbaum at the Technical University of Dresden in Germany and his colleagues (1996) showed that a single dose of hydrocortisone (10 mg) had a detrimental effect on verbal episodic memory. Participants were given a list of words to study, after which they received either a dose of hydrocortisone or a placebo. In a cued recall test given an hour after administration of the dosages, participants who received the hydrocortisone recalled significantly fewer words than did the control participants who received a placebo.
Clinical evidence from all disorders characterized by high levels of cortisol—including Cushing’s syndrome, major depression, and asthma treated with the glucocorticoid prednisone—show impaired memory function (Payne & Nadel, 2004). Furthermore, Sonia Lupien of McGill University, and her colleagues (2005) found that elderly individuals, who have experienced chronic stress and have prolonged high levels of cortisol, have a 14% reduction in hippocampal volume as compared to age-matched individuals without chronic stress and with normal levels of cortisol, indicating a long-term deleterious effect of cortisol on the hippocampus. These individuals also show marked impairment of episodic memory.
Interestingly, cortisol levels normally rise gradually during the night as we sleep, from low levels at the beginning of sleep to the highest levels before awaking. In concert with this knowledge is the finding that dreams rich in episodic material are concentrated at the beginning of sleep, and episodic memory consolidation is most likely to occur early in the sleep process. Jessica Payne and Lynn Nadel (2004) at the University of Arizona propose that “variations in cortisol... determine the functional status of hippocampal/neocortical circuits, thereby influencing the memory consolidation processes that transpire during sleep” (p. 671). Sleep research is a hot topic in neuroscience (see How the Brain Works: Sleep and Memory Consolidation), and new studies should elucidate whether Payne and Nadel’s appealing theory is correct.
One possibility is that these lateral and anterior regions of the temporal lobe are sites where long-term semantic memories are stored. Another view is that these regions may be important for the retrieval of information from long-term stores. This latter hypothesis is supported by neuroimaging studies suggesting that memories are stored as distributed representations throughout the neocortex, involving the regions that originally encoded the perceptual information along with the regions representing information that was associated with this incoming information. As noted already in this chapter, the medial temporal lobe may coordinate the consolidation of this information over time.
TAKE-HOME MESSAGES
Cellular Basis of Learning and Memory
Most models of the cellular bases of memory hold that memory is the result of changes in the strength of synaptic interactions among neurons in neural networks. How would synaptic strength be altered to enable learning and memory? Hebb (1949) proposed one possibility: Hebb’s law states that, if a synapse is active when a postsynaptic neuron is active, the synapse will be strengthened; this phenomenon is known as Hebbian learning.
HOW THE BRAIN WORKS
Sleep and Memory Consolidation
Recent evidence suggests that sleep plays a crucial role in memory consolidation after learning. Matt Wilson and his colleagues at the Massachusetts Institute of Technology have studied the relationship between sleep and memory in the rat. They used multi-electrode methods to record from ensembles of neurons in the rat hippocampus that fire when an animal is in a specific place in its environment (in relation to a landmark cue). These cells are called place cells. In the initial study, Matt Wilson and Bruce McNaughton (1994) of the University of Arizona found that the place cells that fired together during the learning of spatial behavioral tasks were more likely to fire together during post-learning sleep than they had been before the task was learned, indicating that the neurons might be “replaying” the learned tasks during sleep.
Further studies of a similar nature have shown that hippocampal cells tended to replay not only with spatial coordination but also in the same temporal sequence of neuronal firing in which they were learned. Activities (and resulting neuronal firing patterns) that took place over minutes were replayed during sleep in a sequential pattern corresponding to that of the awake activity. These studies implicate the hippocampus in the consolidation of memory via “replaying” of the neuronal firing of spatial and temporal patterns that were first activated during awake learning. Replay of this type is not limited to sleep.
Foster and Wilson (2006) recently reported that sequential replay also takes place in the rat hippocampus when the animal is awake, in the period just after the rat experiences a pattern of spatial activity. Interestingly, replay during waking has the unusual property of taking place in the reverse temporal order of the original experience (e.g., running in a maze). One hypothesis is that this sort of waking replay of neural activity represents a basic mechanism for learning and memory.
Thus two mechanisms are involved in replaying an activity: the reverse waking replay of neural activity, and the sleep-related replay, in which activity is replayed in the same temporal order as it was experienced. Something about the sleep-related forward replay is apparently related to memory consolidation. But the reverse waking replay must be doing something different. Foster and Wilson propose that it reflects a mechanism that permits recently experienced events to be compared to their “memory trace” and may, potentially, reinforce learning.
Long-Term Potentiation and the Hippocampus
Due to the role of the hippocampal formation in memory, it has long been hypothesized that neurons in the hippocampus must be plastic—meaning able to change their synaptic interactions. Although it is now clear that storage itself is not in the hippocampus, this fact does not invalidate the hippocampal models that we will examine, because the same cellular mechanisms can operate in various cortical and subcortical areas.
First, let’s review the major excitatory neural components of the hippocampus (Figure 9.37): Neocortical association areas project to the entorhinal cortex via the parahippocampal cortex or perirhinal cortex. The entorhinal cortex projects via the perforant pathway onto the granule cells of the dentate gyrus with excitatory inputs. Distinctive unmyelinated axons, known as mossy fibers because of their appearance, connect the granule cells of the dentate gyrus to the dendritic spines of the hippocampal CA3 pyramidal cells. The Schaffer collaterals connect the CA3 pyramidal cells to the CA1 pyramidal cells. This system is used to examine synaptic plasticity as the mechanism of learning at the cellular level.
In studies by Bliss and Lømo (1973), stimulation of axons of the perforant pathway of the rabbit resulted in a long-term increase in the magnitude of excitatory postsynaptic potentials (EPSPs). That is, stimulation led to greater synaptic strength in the perforant pathway so that when stimulated again later, larger postsynaptic responses resulted in the granule cells of the dentate gyrus. This phenomenon, named long-term potentiation (LTP) (potentiate means “to strengthen or make more potent”), later was also found to occur in the other two excitatory projection pathways of the hippocampus. The changes could last for hours in isolated slices of hippocampal tissue placed in dishes, where recording was easier. LTP can even last days or weeks in living animals. It has since been found that the LTP in the three pathways varies and also takes place in other brain regions, including the amygdala, basal ganglia, cerebellum, and cortex (all involved with learning).
|
FIGURE 9.37 Synaptic organization of the rat hippocampus. |
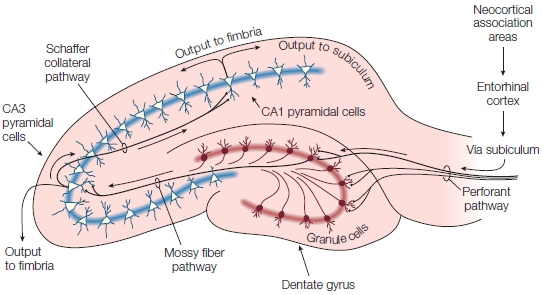
|
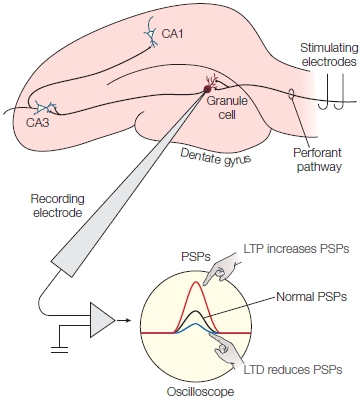
FIGURE 9.38 Stimulus and recording setup for the study of long-term potentiation (LTP) in perforant pathways.
The pattern of responses (in millivolts) before and after the induction of LTP is shown as the red curve. The pattern of responses in long-term depression (LTD) is shown as the blue curve. PSPs = postsynaptic potentials.
Hebb’s law was confirmed physiologically by the discovery of LTP. LTP can be recorded by placing stimulating electrodes on the perforant pathway and a recording electrode in a granule cell of the dentate gyrus (Figure 9.38). A single pulse is presented, and the resulting EPSP is measured. The size of this first recording is the strength of the connection before the LTP is induced. Then the perforant pathway is stimulated with a burst of pulses; early studies used approximately 100 pulses/s, but more recent studies have used as few as 5 pulses/s. After LTP is induced, a single pulse is sent again, and the magnitude of the EPSP in the postsynaptic cell is measured. The magnitude of the EPSP increases after LTP is induced, signaling the greater strength of the synaptic effect (Figure 9.38, red curve). A fascinating finding is that, when the pulses are presented slowly (as low-frequency pulses), the opposite effect—long-term depression (LTD)—develops (Figure 9.38, blue curve).
Hebbian Learning Associative LTP is an extension of Hebb’s law. It asserts that, if a neuron is simultaneously activated by a pathway with a weak input and another pathway with a strong input, both pathways show LTP and the weak synapse becomes stronger. This association has been tested directly by manipulating LTP in the CA1 neurons of the hippocampus. When two weak inputs (W1 and W2) and one strong input (S1) are given to the same cell, and when W1 and S1 are active together, W1 is strengthened but W2 is not. Subsequently, if W2 and S1 are active together, W1 is not affected by the LTP induced from W2 and S1. From this finding, three rules for associative LTP have been drawn:
For LTP to be produced, in addition to receiving excitatory inputs, the postsynaptic cells must be depolarized; in fact, LTP is reduced by inhibitory inputs to postsynaptic cells. This is what happens when habituation occurs. Moreover, when postsynaptic cells are hyperpolarized, LTP is prevented. Conversely, when postsynaptic inhibition is prevented, LTP is facilitated. If an input that is normally not strong enough to induce LTP is paired with a depolarizing current to the postsynaptic cell, LTP can be induced. Thus, through associative LTP, weak pathways become strengthened and specifically associated with other pathways. This process supports learning in the way that Hebb proposed.
The NMDA Receptor The molecular mechanism that mediates LTP is fascinating. It is dependent on the neurotransmitter glutamate, the major excitatory transmitter in the hippocampus. Glutamate binds to two types of glutamate receptors. Normal synaptic transmissions are mediated by the AMPA (a-amino-3-hydroxyl-5- methyl-4-isoxazole propionate) receptor. LTP is initially mediated by the NMDA (N-methyl-D-aspartate) receptors (Figure 9.39), which are located on the dendritic spines of postsynaptic neurons. When the NMDA receptors of CA1 neurons are blocked with the chemical AP5 (2-amino-5-phosphonopentanoate), then LTP induction is prevented. Once LTP is established in these cells, however, AP5 treatment has no effect. Therefore, NMDA receptors are central to producing LTP, but not maintaining it. Maintenance of LTP probably depends on the AMPA receptors, although the mechanisms are not fully understood.
NMDA receptors are also blocked by magnesium ions (Mg2+), which prevent other ions from entering the postsynaptic cell. The Mg2+ ions can be ejected from the NMDA receptors only when the cell is depolarized. Thus, the ion channel opens only when two conditions are met: (1) when the neurotransmitter glutamate binds to the receptors, and (2) when the membrane is depolarized. These two conditions are another way of saying that the NMDA receptors are transmitter- and voltage-dependent (also called gated; Figure 9.40).
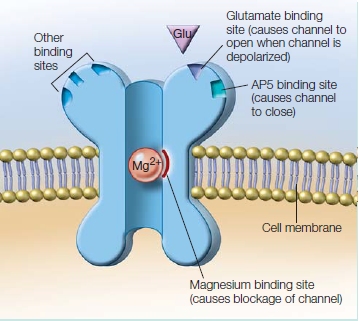
FIGURE 9.39 The NMDA receptor.
As this simplified cross-sectional schematic shows, the NMDA receptor is naturally blocked by Mg2+ ions. Unblocking (channel opening) occurs when the proteins that form the channel shift following the binding of glutamate to the glutamate binding site.
The open ion channel allows Ca2+ ions to enter the postsynaptic cell. The effect of Ca2+ influx via the NMDA receptor is critical in the formation of LTP. The Ca2+ acts as an intracellular messenger conveying the signal, which changes enzyme activities that influence synaptic strength. Despite rapid advances in understanding the mechanisms of LTP at physiological and biochemical levels, the molecular mechanisms of synaptic strengthening in LTP are still the subject of extensive debate.
The synaptic changes that create a stronger synapse after LTP induction likely include presynaptic and postsynaptic mechanisms. One hypothesis is that LTP raises the sensitivity of postsynaptic AMPA glutamate receptors and prompts more glutamate to be released presynaptically. Or perhaps changes in the physical characteristics of the dendritic spines transmit EPSPs more effectively to the dendrites. Finally, via a message from the postsynaptic cell to the presynaptic cell, the efficiency of presynaptic neurotransmitter release is increased.
Long-Term Potentiation and Memory Performance
With a candidate cellular mechanism for long-term plastic changes in synaptic strength identified, it should be possible to produce deficits in learning and memory, which can be demonstrated behaviorally, by eliminating LTP. Chemically blocking LTP in the hippocampus of normal mice impairs their ability to demonstrate normal place learning; thus, blocking LTP prevents normal spatial memory. In a similar way, genetic manipulations that block the cascade of molecular triggers for LTP also impair spatial learning. These experiments provide strong evidence that blocking NMDA receptors and preventing LTP impairs spatial learning.
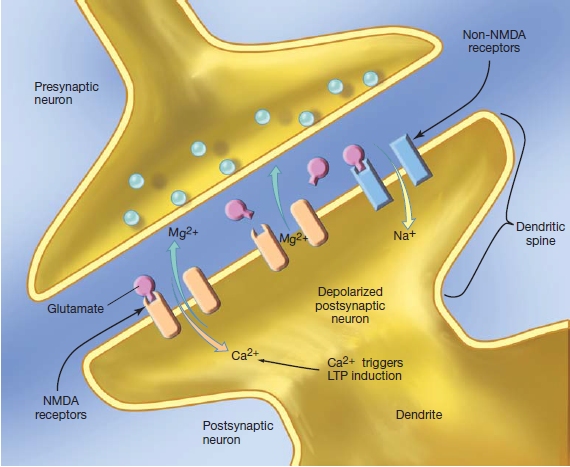
FIGURE 9.40 The role of Mg2+ and Ca2+ in the functioning of the NMDA receptor. See text for details.
NMDA receptors in the CA1 region of the hippocampus are necessary for most forms of synaptic plasticity, and their activation is required for spatial and contextual learning. Once learning has occurred, however, new memories can be formed without their activation. This surprising finding came from two classic water-maze studies (Bannerman et al., 1995; Saucier & Cain, 1995). Both experiments found that pharmacological NMDA receptor blockers did not stop rodents that had been pretrained to navigate in one maze from learning how to navigate in a second water maze; the animals were able to develop a new spatial map even when LTP was prevented. The conclusion is that NMDA receptors may be needed to learn a spatial strategy but not to encode a new map.
In another experiment, when mice were pretrained with a nonspatial task, spatial memory was not interrupted by the introduction of an NMDA antagonist. The conclusion is that the pretraining merely allowed the motor-related side effects of NMDA receptor blockage to be avoided. Although neither study has excluded the possibility that new spatial learning involves NMDA receptors, they do point to the possibility that at least two memory systems could use NMDA receptors. These systems participate in the water-maze task, but they might be consolidated by pretraining.
On the cellular and behavioral levels, the role of LTP in memory is still being unraveled. Whether the maintenance of LTP is located presynaptically or post-synaptically, and even whether LTP is necessary for spatial memory, is the subject of much debate. Daniel Zamanillo and his colleagues (1999) at the Max Planck Institute in Heidelberg, Germany, used gene knockout protocols to study mice that could not produce LTP in the synapses of neurons between the CA3 and CA1 regions of the hippocampus. Behaviorally, however, these mice could learn spatial tasks just as easily as normal control mice.
Bannerman and his colleagues (2012) found that genetically modified mice, which lacked NMDA receptor function in hippocampal CA1 and dentate gyrus granule cells, could not produce LTP in the neurons in these two regions. These mice performed as well as controls in a water-maze learning and memory task. Where the mice did show impairment was in the radial arm maze. The radial arm maze is a circular arena, rather like the hub of a wagon wheel, with six identical arms radiating from it. Like the water maze, there are physical identifiers in the larger environment in which the maze sits. Food rewards are put at the end of three of the corridors. In this type of maze, the NMDA knockout mice showed little improvement in identifying the corridors with the food. By contrast, controls were able to learn to pick the right corridor and rarely erred. Why the difference in learning success? These researchers suggested that the problem lies in picking between ambiguous local cues—that is, the six identical corridors—versus more distant predictive cues.
To test this idea, they used a modified water maze that added ambiguous local cues. A small local cue was put above the platform and the same cue, a faux cue, at the opposite end of the tank. The mice were dropped into the water at different positions in the maze. Although both the controls and the genetically modified mice could find the platform, when they were dropped near the faux cue, the knockout mice were more likely to swim to the faux cue. The control mice (using their spatial memory) were not influenced by the faux cue, and instead swam to the platform. It appears then, that the mice lacking the NMDA receptor function are able to form spatial memories, but they don’t use them when confronted with ambiguous local cues, suggesting that the NMDA receptor function is more subtle than previously thought (Maford, 2012). Martine Migaud and colleagues (1998) at the University of Edinburgh studied mice with enhanced LTP and found that they exhibited severe impairments in spatial learning.
Although much remains to be understood about the cellular and molecular basis of learning, two points of agreement are that (a) LTP does exist at the cellular level, and (b) NMDA receptors play a crucial role in LTP induction in many pathways of the brain. Because LTP is also in brain areas outside of the hippocampal system, the possibility that LTP forms the basis for long-term modification within synaptic networks remains promising.
TAKE-HOME MESSAGES
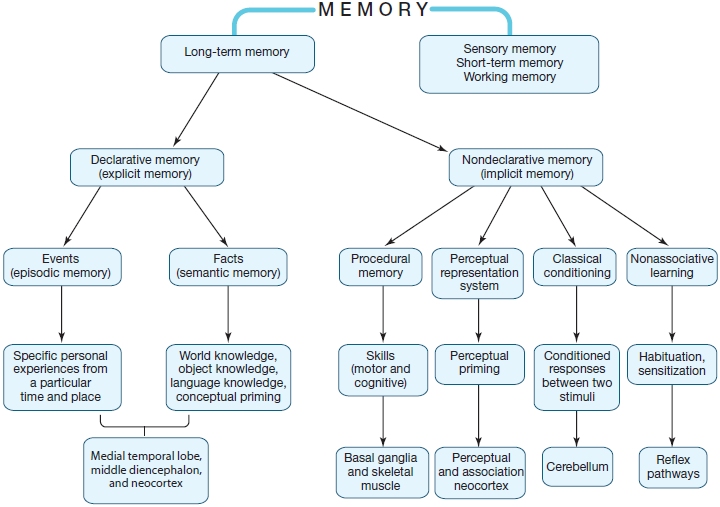
FIGURE 9.41 Generalized diagram of the relationships of long-term memory systems to the underlying brain systems.
This figure elaborates on Figure 9.2 to include candidate brain areas.
Summary
The ability to acquire new information and to retain it over time define learning and memory, respectively. Cognitive theory and neuroscientific evidence argue that memory is supported by multiple cognitive and neural systems. These systems support different aspects of memory, and their distinctions in quality can be readily identified. Sensory registration, perceptual representation, short-term and working memory, procedural memory, semantic memory, and episodic memory all represent systems or subsystems for learning and memory. The brain structures that support various memory processes differ, depending on the type of information to be retained and how it is encoded and retrieved.
The biological memory system includes (a) the medial temporal lobe, which forms and consolidates new episodic and perhaps semantic memories, and is involved in binding together the relationships among different types of information about an episode; (b) the parietal cortex, which is involved in the encoding and retrieving of episodic or contextual memory; (c) the prefrontal cortex, which is involved in encoding and retrieving information based perhaps on the nature of the material being processed; (d) the temporal cortex, which stores episodic and semantic knowledge; and (e) the association sensory cortices for the effects of perceptual priming. Other cortical and subcortical structures participate in the learning of skills and habits, especially those that require implicit motor learning. The data from studies in human amnesic patients, in animals, and in normal volunteers using electrophysiological and neuroimaging methods permit us to elaborate on the cognitive model first presented in Figure 9.2, by including our best current estimates of the neural systems that support the memory functions listed (Figure 9.41).
Not all areas of the brain have the same potential for storing information, and although widespread brain areas cooperate in learning and memory, the individual structures form systems that support and enable rather specific memory processes. At the cellular level, changes in the synaptic strengths between neurons in neural networks in the medial temporal lobe, neocortex, cerebellum, and elsewhere are the most likely mechanisms for learning and memory. Bit by bit, we are developing a very clear understanding of the molecular processes that support synaptic plasticity, and thus learning and memory, in the brain.
Key Terms
acquisition (p. 381)
amnesia (p. 382)
anterograde amnesia (p. 382)
consolidation (p. 381)
declarative memory (p. 390)
distributed representation (p. 415)
encoding (p. 381)
episodic memory (p. 390)
familiarity (p. 406)
Hebbian learning (p. 416)
learning (p. 380)
long-term memory (p. 380)
memory (p. 380)
nondeclarative memory (p. 390)
perceptual representation system (PRS) (p. 392)
priming (p. 392)
procedural memory (p. 390)
relational memory (p. 406)
retrieval (p. 381)
retrograde amnesia (p. 382)
Ribot’s Law (p. 382)
semantic memory (p. 390)
sensory memory (p. 380)
short-term memory (p. 380)
storage (p. 381)
temporal gradient (p. 382)
temporally limited amnesia (p. 382)
transient global amnesia (TGA) (p. 395)
working memory (p. 380)
Thought Questions
Suggested Reading
Aggleton, J. P., & Brown, M. W. (2006). Interleaving brain systems for episodic and recognition memory. Trends in Cognitive Sciences, 10, 455–463.
Clark, R. E., Manns, J. R., & Squire, L. R. (2002). Classical conditioning, awareness, and brain systems. Trends in Cognitive Sciences, 6, 524–531.
Collingridge, G. L., & Bliss, T. V. P. (1995). Memories of NMDA receptors and LTP. Trends in Neurosciences, 18, 54–56.
Corkin, S. (2002). What’s new with the amnesic patient H.M.? Nature Reviews Neuroscience, 3, 153–160.
Eichenbaum, H., Yonelinas, A. P., & Ranganath, C. (2007). The medial temporal lobe and recognition memory. Annual Review of Neuroscience, 30, 123–152.
McClelland, J. L. (2000). Connectionist models of memory. In E. Tulving & F. I. M. Craik (Eds.), The Oxford handbook of memory (pp. 583–596). New York: Oxford University Press.
Miller, G. (2003). The cognitive revolution: A historical perspective. Trends in Cognitive Sciences, 7, 141–144.
Nadel, L., & Hardt, O. (2011). Update on memory systems and processes. Neuropsychopharmacology, 36, 251–273.
Ranganath, C., & Blumenfeld, R. S. (2005). Doubts about double dissociations between short- and long-term memory. Trends in Cognitive Sciences, 9, 374–380.
Ranganath, C., & Richey, M. (2012). Two cortical systems for memory guided behaviour. Nature Reviews Neuroscience, 13, 713–726.
Squire, L. (2006). Lost forever or temporarily misplaced? The long debate about the nature of memory impairment. Learning and Memory, 13, 522–529.
Squire, L. (2008). The legacy of H.M. Neuron, 61, 6–9.