|
You have brains in your head.
You have feet in your shoes.
You can steer yourself any direction you choose.
~ Dr. Seuss
|
Chapter 8
Action
OUTLINE
The Anatomy and Control of Motor Structures
Computational Issues in Motor Control
Physiological Analysis of Motor Pathways
Goal Selection and Action Planning
The Brain–Machine Interface
Movement Initiation and the Basal Ganglia
Action Understanding and Mirror Neurons
Learning and Performing New Skills
IN JU LY 1982, emergency room physicians in the San Jose, California area were puzzled. Four patients, ranging in age from 26 to 42 years, had been seen recently at different hospitals, all presenting a similar picture. Although they were conscious, they were essentially immobile. None of them could speak, their facial expressions seemed frozen, and they showed extreme rigidity in their arms. It was as if they had each peered into Medusa’s eyes and been turned into stone statues. The symptoms and their rapid onset resembled no known disease. The physicians knew they had to act fast—but without a diagnosis, they could not prescribe a treatment. Interviews with the patients’ friends and family uncovered a few clues. Two of the patients were brothers, but they did not know the other two. All four patients, however, were heroin users.
Yet their symptoms were the opposite of what might be expected from taking a large dose of heroin, a powerful central nervous system (CNS) depressant. Instead of the typical muscular flaccidity, these patients were rigid. No one could recall seeing a case of heroin overdose that produced these effects, nor did the symptoms resemble those of other street narcotics. A new substance was at work here. A few friends, who had taken smaller doses, confirmed this suspicion. When injected, this heroin had unexpectedly produced a burning sensation at the site of injection, rapidly followed by a blurring of vision, a metallic taste in the mouth, and, most troubling, an almost immediate jerking of the limbs.
Computer tomography (CT) and magnetic resonance imaging (MRI) revealed no structural abnormalities in the brains of the rigid patients, or in the brains of those who had luckily used a smaller dose. A few days later, a neurologist at Stanford University, William Langston (1984), examined the patients. He was struck by how similar their symptoms were to those of a patient with advanced Parkinson’s disease. This condition is marked by muscular rigidity and disorders of posture and akinesia, the inability to produce volitional movement (Figure 8.1).
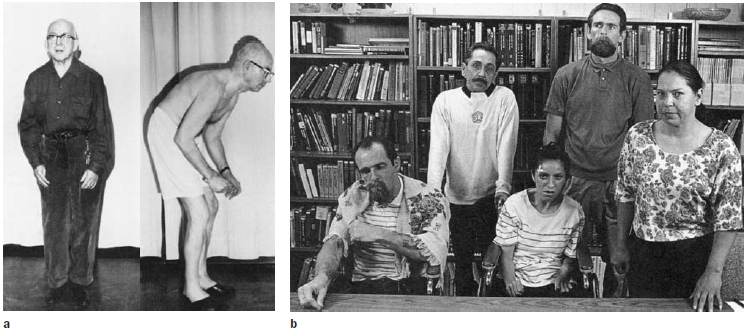
FIGURE 8.1 Parkinson’s disease disrupts posture as well as the production and flexibility of voluntary movement.
(a) This man has had Parkinson’s disease for many years and is no longer able to maintain an upright posture. (b) These people developed symptoms of Parkinson’s disease in their 20s and 30s, after ingesting the drug MPTP. Facial expression, including blinking, is frequently absent, giving people with PD the appearance of being frozen.
Everything about the patients’ conditions matched this disorder except their age and the rapid onset. The onset of Parkinson’s disease is gradual and rarely becomes clinically evident until a person is over the age of 45. The heroin users had developed full-blown symptoms of advanced Parkinson’s disease within days. Langston suspected that the drug users had injected a new synthetic drug being sold as heroin and that this drug had triggered the acute onset of Parkinson’s disease.
This diagnosis proved to be correct. Parkinson’s disease results from cell death in the substantia nigra, a brainstem nucleus that is part of the basal ganglia. These cells are a primary source of the neurotransmitter dopamine. Langston could not see any structural damage on CT and MRI scans, but subsequent positron emission tomography (PET) studies confirmed hypometabolism of dopamine in the patients. Of more immediate concern, however, was how to treat the drug users. Langston adopted the universal treatment applied in Parkinson’s disease: He prescribed high doses of L-DOPA, a synthetic cousin of dopamine that is highly effective in compensating for the loss of endogenous dopamine. When Langston administered this medication to the drug abusers, they immediately showed a positive response. Their muscles relaxed, and they could move, although in a limited way.
Although this episode was tragic for the patients involved, the incident signified a breakthrough in research on Parkinson’s disease. Researchers tracked down the tainted drug and performed a chemical analysis; it turned out to be a previously unknown substance, bearing little resemblance to heroin but similar in structure to meperidine, a synthetic opioid that creates the sensations of heroin. On the basis of its chemical structure, it was given the name MPTP (1-methyl-4-phenyl- 1,2,3,6-tetrahydropyridine). Laboratory tests demonstrating that MPTP is selectively toxic for dopaminergic cells led to great leaps forward in medical research on the basal ganglia and on treatments for Parkinson’s disease. Before the discovery of this drug, it had been difficult to induce parkinsonism in nonhuman species. Primates do not develop Parkinson’s disease naturally, perhaps because their life expectancy is short. Moreover, because of its proximity to vital brainstem nuclei, the substantia nigra is difficult to access with traditional lesion methods. By administering MPTP, researchers could now destroy the substantia nigra and create a parkinsonian animal. These findings helped fuel the development of new treatment methods for Parkinson’s disease.
The MPTP story illustrates how neurological aberrations can elucidate the complicated patterns of connectivity in the motor structures of the CNS. In this chapter, we review the organization of the motor system. We describe how the brain produces coordinated movement and, at a higher level, how it selects actions to achieve our goals.
The Nobel laureate Charles Sherrington, a British physiologist, wrote, “Life’s aim is an act, not a thought” (1953). With this manifesto, Sherrington sought to emphasize that the ultimate goal of all cognition is action. Although people certainly need to be concerned with perception, attention, memory, and emotion, it was acting, not cogitating, that allowed our ancestors to survive and reproduce.
Scientists studying vision are fond of claiming that over 50 % of the brain is devoted to this one sensory system, but a motor control chauvinist could reasonably argue that over 50 % of the brain is devoted to the control of action. One such self-proclaimed chauvinist, Daniel Wolpert (echoing Charles Sherrington), goes so far as to claim that the only reason we have a brain is so that we can move in an adaptable manner (for an entertaining introduction to this idea, watch him at http://www.ted.com/talks/daniel_wolpert_the_real_reason_for_brains.html). According to these claims, well over 100 % of our brain acreage would be spoken for without even considering the other sensory systems or functions such as memory and language. Of course, as we will soon learn, an area can be involved in both vision and motor control. It might be easier to learn about brain systems by dividing chapters into simple headings like memory, perception, and action; but in reality, each of these divisions, both functionally and on a neural level, are integrated and not physically divisible. Just as Shakespeare spoke of one man playing many parts, one brain region can affect many functions. By focusing on the kinds of computations performed by different neural regions and systems, we come to see that perception and action are intimately interwoven, a theme that recurs in this chapter.
You might expect that our understanding of the motor system is very advanced. Unlike an internal process such as perception or memory, the output of the motor system can be directly observed from our actions. Nonetheless, many aspects of motor function remain elusive. Even a clear understanding of what the motor cortex encodes and how that code produces movement remains the subject of considerable debate.
We begin this chapter with a look at the anatomy and organization of the motor system. Following this, we develop a more detailed picture from a cognitive neuroscience perspective, focusing on the computational problems faced by the motor system: What are motor neurons encoding? How are motor goals represented? How are actions planned and selected? The chapter is peppered with discussions of movement disorders to illustrate what happens when particular regions of the brain no longer function properly; also included is an overview of exciting new treatment methods for some of these disorders. We close this chapter with a look at motor learning and expertise.
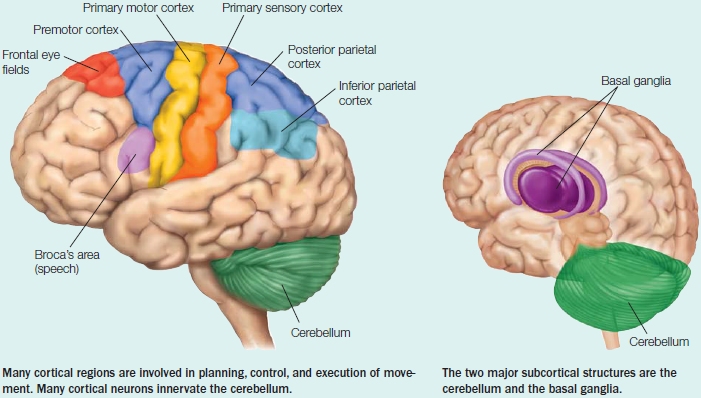
The Anatomy and Control of Motor Structures
The motor system is organized in a hierarchical structure with multiple levels of control that span the spinal cord, the subcortex, and the cerebral cortex (Scott, 2004). As Figure 8.2 illustrates, the lowest level of the hierarchy contains local circuits made up of motor neurons and interneurons in the spinal cord. The spinal mechanisms are the point of contact between the nervous system and muscles. They are also capable of producing simple reflexive movements. At the top of the hierarchy are premotor and association areas of the cortex. Processing within these regions is critical for planning an action based on an individual’s current goals, perceptual input, and past experience. Between the premotor and association areas and the spinal cord sit the primary motor cortex and brainstem structures, which with the assistance of the cerebellum and the basal ganglia, translate this action goal into a movement. These cortical and subcortical regions are highlighted in the Anatomical Orientation box. Because of this hierarchical structure, lesions at various levels of the motor system affect movement differently. In this section, along with the anatomy, we also discuss the deficits produced by lesions to particular regions. We begin at the bottom of the anatomical hierarchy and make our way to the top.
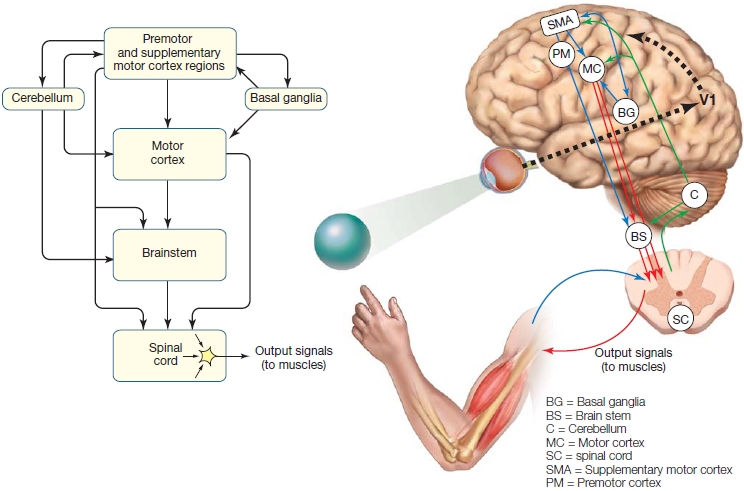
FIGURE 8.2 Overview of the motor pathways.
All connections to the arms and legs originate in the spinal cord. The spinal signals are influenced by inputs from the brainstem and various cortical regions, whose activity in turn is modulated by the cerebellum and basal ganglia. Thus control is distributed across various levels of a control hierarchy. Sensory information from the muscles is transmitted back to the brainstem, cerebellum, and cortex (not shown).
Muscles, Motor Neurons, and the Spinal Cord
Action, or motor movement, is generated by stimulating skeletal muscle fibers of an effector. An effector is a part of the body that can move. For most actions, we think of distal effectors—those far from the body center, such as the arms, hands, and legs. We can also produce movements with more proximal or centrally located effectors, such as the waist, neck, and head. The jaw, tongue, and vocal tract are essential effectors for producing speech; the eyes are effectors for vision.
All forms of movement result from changes in the state of muscles that control an effector or group of effectors. Muscles are composed of elastic fibers, tissue that can change length and tension. As Figure 8.3 shows, these fibers are attached to the skeleton at joints and are usually arranged in antagonist pairs, which enable the effector to either flex or extend. For example, the biceps and triceps form an antagonist pair that regulates the position of the forearm. Contracting or shortening the biceps muscle causes flexion about the elbow. If the biceps muscle is relaxed, or if the triceps muscle is contracted, the forearm is extended.
Muscles are activated by motor neurons, which are the final neural elements of the motor system. Alpha motor neurons innervate muscle fibers and produce contractions of the fibers. Gamma motor neurons are part of the proprioceptive system, important for sensing and regulating the length of muscle fibers. Motor neurons originate in the spinal cord, exit through the ventral root, and terminate in the muscle fibers. As with other neurons, an action potential in a motor neuron releases a neurotransmitter; for alpha motor neurons, the transmitter is acetylcholine. The release of transmitter does not modify downstream neurons, however. Instead, it makes the muscle fibers contract. The number and frequency of the action potentials and the number of muscle fibers in a muscle determine the force the muscle can generate. Thus, alpha motor neurons provide a physical basis for translating nerve signals into mechanical actions, changing the length and tension of muscles.

FIGURE 8.3 Muscles are activated by the alpha motor neurons.
An electromyogram (EMG) is recorded from electrodes placed on the skin over the muscle to measure electrical activity produced by the firing of alpha motor neurons. The input from the alpha motor neurons causes the muscle fibers to contract. Antagonist pairs of muscles span many of our joints. Activation of the triceps produces extension of the elbow; activation of the biceps produces flexion of the elbow.
Input to the alpha motor neurons comes from a variety of sources. Alpha motor neurons receive peripheral input from muscle spindles, sensory receptors embedded in the muscles that provide information about how much the muscle is stretched. The axons of the spindles form an afferent nerve that enters the dorsal root of the spinal cord and synapses directly on corresponding efferent alpha motor neurons. If the stretch is unexpected, the alpha motor neuron is activated, causing the muscle to return to its original length, or what is called the stretch reflex (Figure 8.4). Reflexes allow postural stability to be maintained without any help from the cortex. They also serve protective functions; for example, reflexes can contract a muscle to avoid a painful stimulus well before you consciously feel the pain.
Motor neurons are also innervated by spinal interneurons, which lie within the spinal cord. The interneurons are innervated both by afferent sensory nerves from the skin, muscles, and joints and by descending motor fibers (upper motor neurons) that originate in several subcortical and motor cortical structures. Thus, the signals to the muscles involve continual integration of sensory feedback with the motor commands from higher centers. This integration results in voluntary movement. The descending signals can be either excitatory or inhibitory. For example, descending commands that activate the biceps muscle produce flexion of the elbow. Because of this flexion, the triceps stretches. If unchecked, the stretch reflex would lead to excitation of the triceps and move the limb toward its original position. Thus, to produce movement (and demonstrate the size of your biceps), excitatory signals to one muscle, the agonist, are accompanied by inhibitory signals to the antagonist muscle via interneurons. In this way, the stretch reflex that efficiently stabilizes unexpected perturbations can be overcome to permit volitional movement.
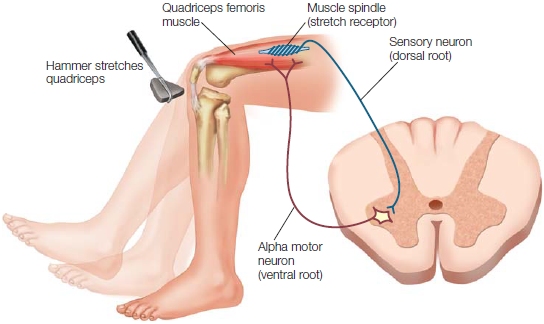
|
FIGURE 8.4 The stretch reflex.
When the doctor raps your knee, the quadriceps is stretched. This stretch triggers receptors in the muscle spindle to fire. The sensory signal is transmitted through the dorsal root of the spinal cord and directly activates an alpha motor neuron to contract the quadriceps. In this manner, the stretch reflex helps maintain the stability of the limb following an unexpected perturbation.
|
Subcortical Motor Structures
Moving up the hierarchy, we encounter many neural structures of the motor system located in the brainstem. The 12 cranial nerves, essential for critical reflexes associated with breathing, eating, eye movements, and facial expressions, originate in the brainstem. Many nuclei within the brainstem, including the vestibular nuclei, the reticular formation nuclei, and the substantia nigra, send direct projections down the spinal cord. These motor pathways are referred to collectively as the extrapyramidal tracts, meaning they are not part of the pyramidal tracts, the axons that travel directly from the cortex to the spinal segments (Figure 8.5). Extrapyramidal tracts are a primary source of indirect control over spinal activity modulating posture, muscle tone, and movement speed; they receive input from subcortical and cortical structures.
Cerebellum Figure 8.6 shows the location of two prominent subcortical structures that play a key role in motor control: the cerebellum and the basal ganglia. The cerebellum is a massive, densely packed structure containing more neurons than the rest of the central nervous system combined. Most of these neurons are contained in the layers of the cerebellar cortex. Inputs to the cerebellum primarily project to the cerebellar cortex. The output from the cerebellum originates in the deep cerebellar nuclei, projecting to brainstem nuclei and the cerebral cortex via the thalamus. An unusual feature of the cerebellum is that because the input from and output to the cortex both cross over to the contralateral side, the net effect is that the cerebellum has an ipsilateral organization: The right side of the cerebellum is associated with movements on the right side of the body, and the left side is associated with movements on the left side of the body.
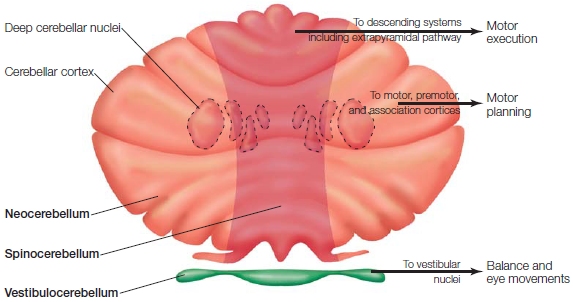
The cerebellum is made up of three regions, which appear to have followed different paths in phylogeny (Figure 8.7). Each region has unique anatomical inputs and outputs, and when lesioned, results in distinct clinical symptoms. The smallest and oldest region, the vestibulocerebellum, works with the brainstem vestibular nuclei to control balance and coordinate eye movements with body movements. For example, the vestibulo-ocular reflex (VOR) ensures that the eyes remain fixed on an object despite movements of the head or body. If the eyes were displaced with each movement, it would be difficult to monitor another organism or keep track of the location of a stimulus.
The medial region, the spinocerebellum, receives sensory information from the visual and auditory systems as well as proprioceptive information from the spinocerebellar tract. The output from the spinocerebellum innervates the spinal cord and nuclei of the extrapyramidal system. Lesions of the spinocerebellum can result in an unsteady gait and disturbances of balance. Cells in this region are especially sensitive to the effects of alcohol. Chronic alcohol abuse can cause persistent problems with balance. Even with acute alcohol use, cerebellar symptoms can be observed: Tests used by police on suspected drunk drivers are essentially assessing cerebellar function.
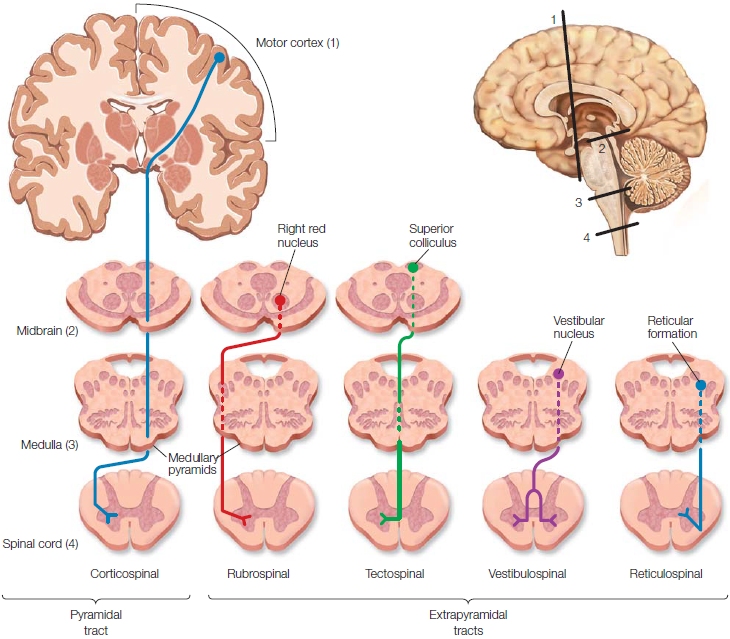
FIGURE 8.5 The brain innervates the spinal cord via the pyramidal and extrapyramidal tracts.
The pyramidal (corticospinal) tract originates in the cortex and terminates in the spinal cord. Almost all of these fibers cross over to the contralateral side at the pyramids. The extrapyramidal tracts originate in various subcortical nuclei and terminate in both contralateral and ipsilateral regions of the spinal cord.
The lateral zones of the cerebellar hemispheres constitute the newest region, the neocerebellum. This area is heavily innervated by descending fibers originating from many regions within the parietal and frontal lobes. Output from the neocerebellum projects back to the cortex via the thalamus, and the thalamic projections terminate in the primary motor, lateral premotor, and prefrontal cortices. Lesions to the neocerebellum produce ataxia, problems with sensory coordination of the distal limb movements, thus disrupting fine coordination. The classic test for this type of ataxia is touching the nose with a finger, which reveals the wavering, jerky movements of an intention tremor that occur while performing an intentional act (in contrast to resting tremors). Lesions to the most inferior regions of the neocerebellum produce subtler problems that may affect a range of more cognitive functions. These observations underscore the functional diversity of the cerebellum, inspiring current research efforts that challenge our traditional conceptions of the cerebellum as purely a “motor structure.” Using a range of cognitive neuroscience tools, evidence over the past twenty-five years has pointed to a role for the cerebellum in attention, language processing, planning, and more (Stoodley, 2012; Strick et al., 2009).
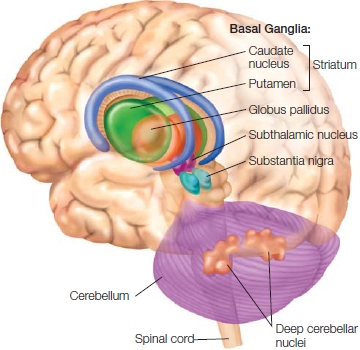
FIGURE 8.6 The basal ganglia and the cerebellum are two prominent subcortical components of the motor pathways.
The basal ganglia proper include the caudate, putamen, and globus pallidus, three nuclei that surround the thalamus. Functionally, however, the subthalamic nuclei and substantia nigra also are considered part of the basal ganglia. The cerebellum sits below the posterior portion of the cerebral cortex. All cerebellar output originates in the deep cerebellar nuclei.
Basal Ganglia The other major subcortical motor structure is the basal ganglia, a collection of five nuclei: the caudate nucleus and the putamen (referred to together as the striatum), the globus pallidus, the subthalamic nucleus, and the substantia nigra (see Figure 8.6). The organization of the basal ganglia bears some similarity to that of the cerebellum: Input is restricted mainly to the two nuclei forming the striatum, and output is almost exclusively by way of the internal segment of the globus pallidus and part of the substantia nigra. The remaining components (the rest of the substantia nigra, the subthalamic nucleus, and the external segment of the globus pallidus) modulate activity within the basal ganglia. Axons of the globus pallidus terminate in the thalamus, which in turn projects to motor and frontal regions of the cerebral cortex. Later we will see that the basal ganglia, with all of its inputs and outputs, plays a critical role in motor control, especially in the selection and initiation of actions.
Cortical Regions Involved in Motor Control
We will use the term motor areas to refer to cortical regions involved in voluntary motor functions, including the planning, control, and execution of movement. Motor areas include the primary motor cortex, the premotor cortex, and the supplementary motor area (see the Anatomical Orientation box). Other areas such as the posterior and inferior parietal cortex, as well as the primary somatosensory cortex, are also essential in producing movement.
The motor cortex regulates the activity of spinal neurons in direct and indirect ways. The corticospinal tract (CST) consists of axons that exit the cortex and project directly to the spinal cord (see Figure 8.5). The CST is frequently referred to as the pyramidal tract because the mass of axons resemble a pyramid as they pass through the medulla oblongata. CST axons terminate either on spinal interneurons or directly (monosynaptically) on alpha motor neurons. These are the longest neurons in the brain—some axons extend for more than 1 meter. Most corticospinal fibers originate in the primary motor cortex, but some originate in premotor cortex, supplemental motor area, and even somatosensory cortex.
As with the sensory systems, each cerebral hemisphere is devoted primarily to controlling movement on the opposite side of the body. About 80 % of the CST axons cross, or decussate, at the junction of the medulla and the spinal cord; another 10 % cross when they exit the spinal cord. Most extrapyramidal fibers also decussate. As we have already seen, the one exception to this crossed arrangement is the cerebellum.
|
FIGURE 8.7 The three divisions of the cerebellum.
These regions of the cerebellum are shown diagramatically along with their anatomical projections to the deep cerebellar nuclei and extracerebellar target regions.
|
Primary motor cortex The primary motor cortex (M1), or Brodmann area 4 (Figure 8.8), is located in the most posterior portion of the frontal lobe, spanning the anterior wall of the central sulcus and extending onto the precentral gyrus. M1 receives input from almost all cortical areas implicated in motor control. These areas include the parietal, premotor, supplementary motor, and frontal cortices as well as subcortical structures such as the basal ganglia and cerebellum. In turn, the output of the primary motor cortex constitutes the largest signal in the corticospinal tract.
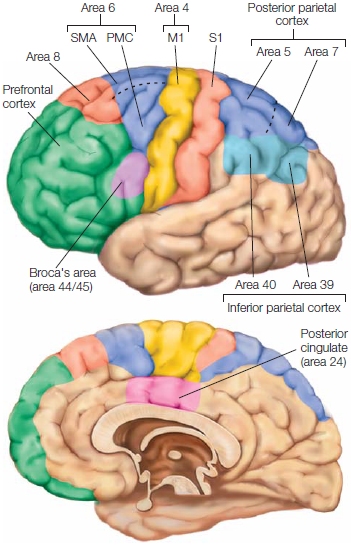
FIGURE 8.8 Motor areas of the cerebral cortex.
Brodmann area 4 is the primary motor cortex (M1). Area 6 encompasses the supplementary motor area (SMA) on the medial surface and premotor cortex (PMC) on the lateral surface. Area 8 includes the frontal eye fields. Inferior frontal regions (area 44) are involved in speech. Regions of parietal cortex associated with the planning and control of coordinated movement include S1, the primary somatosensory cortex, secondary somatosensory areas, and posterior and inferior parietal regions.
M1 includes two anatomical subdivisions, an evolutionarily older rostral region and a more recently evolved caudal region (Rathelot & Strick, 2009). The rostral part appears to be homologous across many species, but the more caudal part is thought to have evolved in a few species of Old World monkeys. It is present only in humans and some of our primate cousins. Unlike rostral corticospinal neurons that terminate on spinal interneurons, corticospinal neurons originating in the caudal region may terminate directly on alpha motor neurons. Interestingly, these motor neurons project to muscles of the upper limb. Functionally, this relatively recent adaptation is thought to provide more direct control of effectors essential for volitional movement. It allows greater dexterity as well as the ability to produce novel patterns of motor output.
M1 contains a crude somatotopic representation: Different regions represent different body parts. For example, an electrical stimulus applied directly to the medial wall of the precentral gyrus creates movement in the foot; the same stimulus applied at a ventrolateral site elicits tongue movement. It is possible to map this somatotopy non-invasively with transcranial magnetic stimulation (TMS), simply by moving the position of the coil over the motor cortex. Placing the coil a few centimeters off the midline will elicit jerky movements of the upper arm. As the coil is shifted laterally, the twitches shift to the wrist and then to hand movements.
Given the relatively crude spatial resolution of TMS (approximately 1 cm of surface area), the elicited movements are not limited to single muscles. Even with more precise stimulation methods, however, it is apparent that the somatotopic organization in M1 is not nearly as distinct as that seen in the somatosensory cortex. It is as if the map within M1 for a specific effector, such as the arm, were chopped up and thrown back onto the cortex in a mosaic pattern. Moreover, the representation of the effectors does not correspond to their actual size but reflects the importance of that effector for movement and the level of control required for manipulating it. Thus, despite their small size, the fingers span a large portion of the human motor cortex, thanks to their essential role in manual dexterity.
The preeminent status of the primary motor cortex for movement control is underscored by the knowledge that lesions to this area, or to the corticospinal tract, will produce a devastating loss of motor control. Lesions of the primary motor cortex usually result in hemiplegia, the loss of voluntary movements on the contralateral side of the body. Hemiplegia most frequently results from a hemorrhage in the middle cerebral artery; perhaps the most telling symptom of a stroke, it leaves the patient unable to move the affected limb. The problem is not a matter of will or awareness; the hemiplegic patient may exert great effort, but the limb will not move. Hemiplegia usually affects the most distal effectors, such as the fingers or hand.
Reflexes are absent immediately after a stroke that produces hemiplegia. Within a couple of weeks, though, the reflexes return and may become hyperactive and even spastic (resistant to stretch), reflecting a change in muscle tone. These changes result from a shift in control. Voluntary movement requires the inhibition of reflexive mechanisms. Without this, the stretch reflex would counteract the gesture. When the cortical influence is removed, primitive reflexive mechanisms take over.
Unfortunately, recovery from hemiplegia is minimal. Patients rarely regain significant control over the limbs of the contralateral side when the motor cortex has been damaged.
Nonetheless, scientists are using the tools and results from cognitive nueroscience to develop new treatment inventions to restore motor function. One approach is to look for ways that would promote neural recovery in the damaged hemisphere. For example, repetitive TMS over the lesioned cortex may stimulate neural plasticity (Kleim et al., 2006).
Other methods take a more behavioral approach, based on the idea that the brain may favor short-term solutions over long-term gains. Consider a patient with a hemiplegic right arm who has an itchy leg. The patient can scratch it quickly by using her left arm; to use the right would require considerable effort, even if the patient had recovered some ability to use this limb. Indeed, the situation may present a self-fulfilling prophecy: The advantage in using the left hand becomes more pronounced upon repeated use. This condition, in which the patient fails to use an affected limb even after significant recovery, is called learned disuse. To counteract this tendency, rehabilitation specialists use constraint-induced movement therapy (CIMT), a method that restrains patients from using their unaffected limb. For example, they might be required to wear a thick mitt on the unaffected limb, forcing them to use the affected limb if they need to grasp something. Two weeks of intensive CIMT has been found to produce substantial improvement in both strength and function of the paretic upper extremities, and the improvements are still evident 2 years later (Wolf et al., 2008).
Later in this chapter, we will review a more radical treatment approach for hemiplegia and paralysis, one that uses the neural signals of the patient’s cortex to directly control prosthetic devices.
Secondary Motor Areas Brodmann area 6, located just anterior to the primary motor cortex, contains the secondary motor areas (see Figure 8.8). Multiple somatotopic maps are found within the secondary motor areas (Dum & Strick, 2002)—although, as with M1, the maps are not clearly delineated and may not contain a full body representation. The lateral and medial aspects of area 6 are referred to as premotor cortex and supplementary motor area (SMA), respectively. Within premotor cortex, physiologists distinguish between ventral premotor cortex (PMv) and dorsal premotor cortex (PMd).
Secondary motor areas are involved with the planning and control of movement. One functional distinction between premotor cortex and SMA is whether the action is externally or internally guided. Premotor cortex has strong reciprocal connections with the parietal lobe, providing the anatomical substrate for external sensory-guided actions, such as grabbing a cup of coffee or catching a ball (see Chapter 6). SMA, in contrast, has stronger connections with medial frontal cortex, areas that we will see in Chapter 12 are associated with internally guided personal preferences and goals. For example, SMA might help decide which object to choose (e.g., coffee or soda), or with the planning of a sequence of learned actions (e.g., playing the piano).
Lesions to the secondary motor areas do not result in hemiparesis or hemiplegia. Because these regions are involved with the planning and guiding of movement, however, patients with lesions to these regions have problems in performing purposeful and coordinated movements. This disorder, known as apraxia—a loss of “praxis,” or skilled action—is a condition that affects motor planning. Patients with apraxia have no motor or sensory impairment. They have normal muscle strength and tone, and they do not exhibit movement disorders such as tremors. The patients can produce simple gestures, like opening and closing their fist or moving each finger individually. Nonetheless, they cannot link these gestures into meaningful actions, such as sequencing an arm and wrist gesture to salute. Apraxia is most commonly a result of left-sided lesions, yet the problems may be evident in gestures produced by either limb.
The symptoms and deficits seen in apraxia depend on the location of the lesion. Neurologists distinguish between two general subtypes of apraxia: ideomotor and ideational. In ideomotor apraxia, the patient appears to have a rough sense of the desired action but has problems executing it properly. If asked to pantomime how to comb his hair, the patient might knock his fist against his head repeatedly. Ideational apraxia is much more severe. Here, the patient’s knowledge about the intent of an action is disrupted. He may no longer comprehend the appropriate use for a tool. For example, one patient used a comb to brush his teeth, demonstrating by the action that he could make the proper gesture, but used the wrong object to do it.
Association Motor Areas As we saw in Chapter 6, the parietal cortex is a critical region for the representation of space. This representation is not limited to the external environment; somatosensory cortex provides a representation of the body and how it is situated in space. This information is critical to a person’s ability to move effectively. Think about a skill such as hitting a tennis ball. You need to track a moving object effectively; position your body so that you can swing the racquet to intersect the ball at the appropriate time and place; and, if you’re skilled, keep an eye on your opponent to attempt to place your shot out of her reach. Along the intraparietal sulcus in monkeys, neurophysiologists have identified distinct regions associated with eye movements, arm movements, and hand movements (Andersen & Buneo, 2002). Homologous regions have been observed in human imaging studies, leading to a functionally defined mosaic of motor areas within parietal cortex. Of course a skilled action, like playing tennis, will entail coordinated activity across all these effectors.
Given the importance of the parietal lobe in sensory integration, it should not be surprising that lesions there can also produce apraxia. Indeed, ideational apraxia is more often associated with parietal damage than with damage to secondary motor areas. What’s more, parietal damage may disrupt the ability to produce movement and lead to impairments in the recognition of actions produced by others, even if the patient’s sensory capabilities appear to be intact.
Harking back to our motor chauvinists, many other association areas of the cortex are implicated in motor function. Broca’s area, located within the posterior aspect of the inferior frontal gyrus in the left hemisphere (Hillis et al., 2004), and the insular cortex (medial to Broca’s area) are involved in the production of speech movements. Area 8 includes the frontal eye fields, a region (as the name implies) that contributes to the control of eye movements. The anterior cingulate cortex is also implicated in the selection and control of actions, evaluating the effort or costs required to produce a movement (see Chapter 12).
In summary, the motor cortex has direct access to spinal mechanisms via the corticospinal tract. Movement can also be influenced through many other connections. First, the primary motor cortex and premotor areas receive input from many regions of the cortex by way of corticocortical connections. Second, some cortical axons terminate on brainstem nuclei, thus providing a cortical influence on the extrapyramidal tracts. Third, the cortex sends massive projections to the basal ganglia and cerebellum. Fourth, the corticobulbar tract is composed of cortical fibers that terminate on the cranial nerves.
TAKE-HOME MESSAGES
- A part of the body that can move is referred to as an effector .
- Alpha motor neurons provide the point of translation between the nervous system and the muscular system, originating in the spinal cord and terminating on muscle fibers. Action potentials in alpha motor neurons cause the muscle fibers to contract.
- Extrapyramidal tracts are neural pathways that project from the subcortex to the spinal cord.
- The corticospinal or pyramidal tract is made up of descending fibers that originate in the cortex and project monosynaptically to the spinal cord.
- Two prominent subcortical structures involved in motor control are the cerebellum and basal ganglia.
- The primary motor cortex (Brodmann area 4) spans the anterior bank of the central sulcus and the posterior part of the central gyrus. It is the source of most of the corticospinal tract.
- Hemiplegia is a loss of the ability to produce voluntary movement. It results from damage to the primary motor cortex or the corticospinal tract, and the deficits are present in effectors contralateral to the lesion.
- Brodmann area 6 includes secondary motor areas. The lateral aspect is referred to as premotor cortex, and the medial aspect as supplementary motor area.
- The primary and secondary motor cortices contain somatotopic representations, although the maps are not as well defined as is seen in sensory cortices.
Computational Issues in Motor Control
We have seen the panoramic view of the motor system: how muscles are activated and which spinal, subcortical, and cortical areas shape this activity. Though we have identified the major anatomical components, we have only touched on their function. We now turn to some core computational issues that must be addressed when constructing theories about how the brain choreographs the many signals required to produce actions.
Central Pattern Generators
As described earlier, the spinal cord is capable of producing orderly movement. The stretch reflex provides an elegant mechanism to maintain postural stability even in the absence of higher-level processing. Are these spinal mechanisms a simple means for assembling and generating simple movements into more complicated actions?
In the late 1800s, Sherrington developed a procedure in which he severed the spinal cord in cats to disconnect the spinal apparatus from the cortex and subcortex (Sherrington, 1947). This procedure allowed Sherrington to observe the kinds of movements that could be produced in the absence of descending commands. As expected, stretch reflexes remained intact; in fact, these reflexes were exaggerated because inhibitory influences were removed from the brain. More surprisingly, Sherrington observed that these animals could alternate the movements of their hind limbs. With the appropriate stimulus, one leg flexed while the other extended; then the first leg extended while the other flexed. In other words, without any signals from the brain, the animal displayed movements that resembled walking. While such elementary movement capabilities are also present in people with spinal cord injuries, these individuals are unable to maintain their posture without descending control signals from the cortex and subcortex.
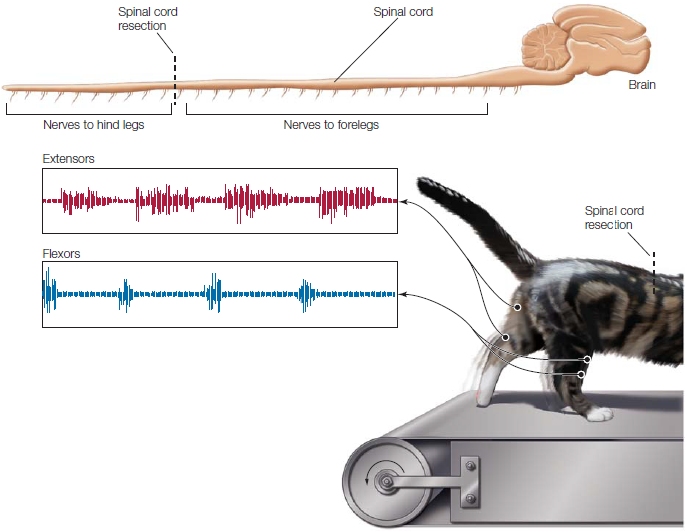
FIGURE 8.9 Movement is still possible following resection of the spinal cord.
In Brown’s classic experiment with cats, the spinal cord was severed so that the nerves to the hind legs were isolated from the brain. The cats were able to produce stereotypical rhythmic movements with the hind legs when supported on a moving treadmill. Because all inputs from the brain had been eliminated, the motor commands must have originated in the lower portion of the spinal cord.
One of Sherrington’s students, Thomas Graham Brown, went on to show that such movements did not even require any sensory feedback. Brown sectioned the spinal cord and then went a step further: He also cut the dorsal root fibers in the spinal cord, removing all feedback information from the effector. Even under these extreme conditions, the cat was able to generate rhythmic walking movements when put on a kitty treadmill (Figure 8.9). Thus, neurons in the spinal cord could produce an entire sequence of actions without any descending commands or external feedback signals.
These neurons have come to be called central pattern generators. They offer a powerful mechanism for the hierarchical control of movement. Consider, for instance, how the nervous system might initiate walking. Brain structures would not have to specify patterns of muscle activity. Rather, they would simply activate the appropriate pattern generators in the spinal cord, which in turn would trigger muscle commands. The system is truly hierarchical, because the highest levels are concerned only with issuing commands to achieve an action, whereas lower-level mechanisms translate the commands into a specific neuromuscular pattern to produce the desired movement. Central pattern generators most likely evolved to trigger actions essential for survival, such as locomotion. The production of other movements may have evolved using these mechanisms as a foundation. When we reach to pick up an object, for example, low-level mechanisms could automatically make the necessary postural adjustments to keep the body from tipping over as the center of gravity shifts.
Central Representation of Movement Plans
What exactly are cortical neurons coding, if not specific patterns of motor commands? To answer this question, we have to consider how actions are represented (Keele, 1986). Consider this scenario: You are busily typing at the computer and decide to pause and take a sip of coffee. To accomplish this goal, you must move your hand from the keyboard to the coffee cup. So how is this action coded in your brain? Well, it could be represented in at least two ways. First, by comparing the positions of your hand and the cup, you could plan the required movement trajectory—the path that would transport your hand from the keyboard to the cup. Alternatively, the action plan might simply specify the location of the cup (on the desk) and specify the motor commands that correspond to the limb being at that position (extended arm at 75 degrees), not how to get there. Of course, both forms of representations—trajectory based and location based—might exist in motor areas of the cortex and subcortex (see How the Brain Works: Where Is It? Assessing Location Through Perception and Action).
HOW THE BRAIN WORKS
Where Is It? Assessing Location Through Perception and Action
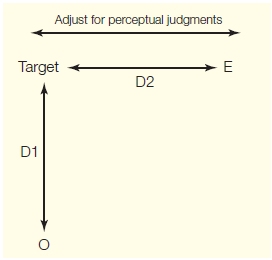
FIGURE 1 Perceptual judgment of distance.
Two people are needed for this demonstration. The observer, 0, stands at a fixed location in an open area. The experimenter, E, places a target at some point in the area. E walks along the perpendicular direction away from the target and stops when 0 judges that they are equidistant to the target D1 = D2). The results will be quite striking. When compared to the condition in which O is asked to walk to the target with the eyes closed.
To demonstrate that spatial information can be represented differently in systems involved in conscious perception and those associated with guiding action, try the experiment outlined in Figure 1. While standing in an open area, have a friend place an object 6 to 12 m from you. Then have your friend move along the perpendicular direction and stop him or her when you perceive that you are both equidistant from the object. Measure your accuracy. Now have your friend place the object in a new location, again 6 to 12 m away. When ready, close your eyes and walk forward, attempting to stop right over the object. Measure your accuracy.
Assuming that your performance matches that of the average person, you will notice a striking dissociation (Loomis et al., 1992). You will probably be quite inaccurate on the first task, underestimating the distance from you to the object. Yet on the second task, you should be very accurate. These results reveal a dissociation between two forms of judgment: one perceptual, the other motoric. In both situations the results suggest that separate representational systems underlie judgments of location and distance. Although location judgments are accurate, the representation of distance is subject to perceptual distortions. Our perception of distance is highly compressed: Things almost always are farther away than they appear. (Could this be a “safety” mechanism to ensure that we ready ourselves for an approaching predator?) As this experiment demonstrates, however, our action systems are not similarly fooled. Little, if any, compression of distance occurs when we move to a target location.
In an early study attempting to understand the neural code for movements, Emilio Bizzi and his colleagues (1984) at the Massachusetts Institute of Technology performed an experiment to test whether trajectory and/ or location were being coded. The experiments involved monkeys who had, through a surgical procedure, been deprived of all somatosensory, or afferent, signals from the limbs. These de-afferented monkeys were trained in a simple pointing experiment. On each trial, a light appeared at one of several locations. After the light was turned off, the animal was required to rotate its elbow to bring its arm to the target location—the point where the light had been.
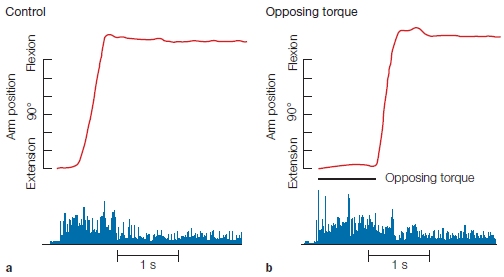
FIGURE 8.10 Endpoint control.
De-afferented monkeys were trained to point in the dark to a target indicated by the brief illumination of a light. The top traces (red) show the position of the arm as it goes from an initial position to the target location. The bottom traces (blue) show the EMG activity in the biceps. (a) In the control condition, the animals were able to make the pointing movements accurately, despite the absence of all sources of feedback. (b) In the experimental condition, an opposing force was applied at the onset of the movement, preventing the arm from moving (bar under the arm position trace). Once this force was removed, the limb rapidly moved to the correct target location. Because the animal could not sense the opposing force, it must have generated a motor command corresponding to the target location.
The critical manipulation included trials in which an opposing torque force was applied just when movement started. These forces were designed to keep the limb at the starting position for a short time. Because the room was dark and the animals were de-afferented, they were unaware that their movements were counteracted by an opposing force. The crucial question was, where would the movement end once the torque force was removed? If the animal had learned that a muscular burst would transport its limb a certain distance, applying an opposing force should have resulted in a movement trajectory that fell short of the target. If, however, the animal generated a motor command specifying the desired position, it should have achieved this goal once the opposing force was removed. As Figure 8.10 shows, the results clearly favor the latter location hypothesis. When the torque motor was on, the limb stayed at the starting location. As soon as it was turned off, the limb rapidly moved to the correct location. This experiment provided dramatic evidence showing that central representations can be based on a location code.
Although this experiment provides impressive evidence of location planning, it doesn’t mean that location is the only thing that is being coded. It just means that it is one of the things being coded. We know that you can also control the form with which a movement is executed. For example, in reaching for your coffee cup, you could choose simply to extend your arm. Alternatively, you might rotate your body, reducing the distance the arm has to move. If the coffee cup were tucked behind a book, you could readily adjust the reach to avoid a spill. Indeed, for many tasks, such as dodging a predator or being in a tango competition, the trajectory and type of movement are as important as the final goal. So although endpoint control reveals a fundamental capability of the motor control system, distance and trajectory planning demonstrate additional flexibility in the control processes.
Hierarchical Representation of Action Sequences
We must also take into account that most of our actions are more complex than simply reaching to a location in space. More commonly, an action requires a sequential set of simple movements. In serving a tennis ball, we have to toss the ball with one hand and swing the racquet with the other so that it strikes the ball just after the apex of rotation. In playing the piano, we must strike a sequence of keys with appropriate timing and force. Are these actions simply constructed by the linking of independent movements, or are they guided by hierarchical representational structures that govern the entire sequence? The answer is that they are guided. Hierarchical representational structures organize movement elements into integrated chunks. Researchers originally developed the idea of chunking when studying memory capacity, but it has also proven relevant to the representation of action.
Donald MacKay (1987) of the University of California, Los Angeles, developed a behavioral model to illustrate how hierarchical ideas could prove insightful for understanding skilled action. At the top of the hierarchy is the conceptual level (Figure 8.11), corresponding to a representation of the goal of the action. In this example, the man’s intention (goal) is to accept the woman’s invitation to dance. At the next level, this goal must be translated into an effector system. He could make a physical gesture or offer a verbal response. Embedded within each of those options are more options. He can nod his head or extend his hand, or if he has the gift of gab, he can select one sentence from a large repertoire of potential responses: “I was hoping you would ask”; or “You will have to be careful, I have two left feet.” Lower levels of the hierarchy then translate these movement plans into patterns of muscular activation. For example, a verbal response entails a pattern of activity across the speech articulators, and extension of the hand requires movements of the arm and fingers.
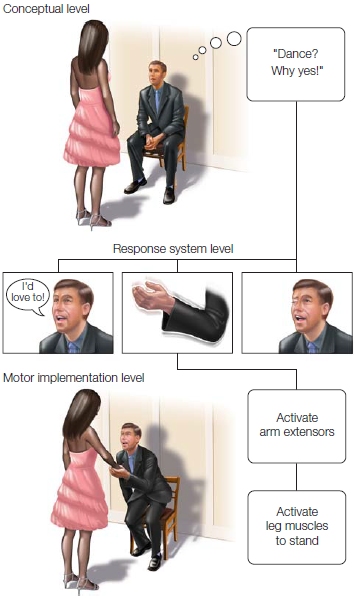
FIGURE 8.11 Hierarchical control of action.
Motor planning and learning can occur at multiple levels. At the lowest level are the actual commands to implement a particular action. At the highest level are abstract representations of the goal for the action. Multiple actions can usually achieve the same goal.
The hierarchical properties of this model are explicit. Each level corresponds to a different form for representing the action. Actions can be described in relation to the goals to be achieved (accepting the invitation), and this level need not be tied to a specific form of implementation (nodding or verbalizing). The two forms of responding, however, share a level of representation. In a similar fashion, when we convey a linguistic message by speaking or by writing, a common level of representation is on both the conceptual and the lexical levels. Higher levels in the hierarchy need not represent all of the information.
Viewing the motor system as a hierarchy enables us to recognize that motor control is a distributed process. Just like in a large corporation where the chief executive, sitting at the top of the organizational hierarchy, is unconcerned with what is going on in the shipping department, the highest levels of the motor hierarchy might not be concerned with the details of a movement.
Hierarchical organization also can be viewed from a phylogenetic perspective. Unlike humans, many animals without a cerebral cortex are capable of complex actions: The fly can land with near-perfect precision; the lizard can flick its tongue at the precise moment to snare its evening meal. We might consider the cortex as an additional piece of neural machinery superimposed on a more elementary control system. Movement in organisms with primitive motor structures is based primarily on simple reflexive actions. A blast of water against the abdominal cavity of the sea slug automatically elicits a withdrawal response. More highly evolved motor systems, however, have additional layers of control that can shape and control these reflexes. For example, brainstem nuclei can inhibit spinal neurons so that a change in a muscle length does not automatically trigger a stretch reflex.
In a similar way, the cortex can provide additional means for regulating the actions of the lower levels of the motor hierarchy, offering an organism even greater flexibility in its actions. We can generate any number of movements in response to a sensory signal. As a ball comes whizzing toward him, a tennis player can choose to hit a crosscourt forehand, go for a drop shot, or pop a defensive lob. Cortical mechanisms also enable us to generate actions that are minimally dependent on external cues. We can sing aloud, wave our hands, or pantomime a gesture. Reflecting this greater flexibility, it is no surprise that the corticospinal tract is one of the latest evolutionary adaptations, appearing only in mammals. It affords a new pathway that the cerebral hemispheres can take to activate ancient motor structures.
Theories about how the motor system functions need to incorporate two observations: Pattern generators produce fixed action patterns but don’t require cortical input; nonetheless, movements are flexible and not mechanical. Somehow those fixed action patterns are modified into more complex, goal-oriented movements by inputs from multiple areas of the motor cortex and brainstem. At higher levels, central representations are concerned with spatial goals and planning the more abstract components of the movement. They are not concerned with the detailed pattern of muscular contractions.
TAKE-HOME MESSAGES
- Neurons within the spinal cord can generate an entire sequence of actions without any external feedback signal. These circuits are called central pattern generators.
- Descending motor signals modulate the spinal mechanism to produce voluntary movements.
- The motor system is hierarchically organized. Subcortical and cortical areas represent movement goals at various levels of abstraction.
Physiological Analysis of Motor Pathways
So far in this chapter, we have stressed two critical points on movement: First, as with all complex domains, motor control depends on several distributed anatomical structures. Second, these distributed structures operate in a hierarchical fashion. We have seen that the concept of hierarchical organization also applies at the behavioral level of analysis. The highest levels of planning are best described by how an action achieves an objective; the lower levels of the motor hierarchy are dedicated to translating a goal into a movement. We now turn to the problem of relating structure to behavior: What are the functional roles of the different components of the motor system? In this section, we take a closer look at the neurophysiology of motor control to better understand how the brain produces actions.
Neural Coding of Movement
Neurophysiologists have long puzzled over how best to describe cellular activity in the motor structures of the CNS. Stimulation of the primary motor cortex, either during neurosurgery or via TMS, can produce discrete movements about single joints, providing a picture of the somatotopic organization of the motor cortex. This method, however, does not provide insight into the activity of single neurons, nor can it be used to study how and when cells become active during volitional movement. To address these issues, we have to record the activity of single cells and ask what parameters of movement are coded by such cellular activity. For example, is cellular activity correlated with parameters of muscle activity such as force, or with more abstract entities such as movement direction or desired final location?
In a classic series of experiments, Apostolos Georgopoulos (1995) and his colleagues studied this question by recording from cells in various motor areas of rhesus monkeys. The monkeys were trained with the apparatus shown in Figure 8.12 on what has come to be called the center-out task. The animal initiates the trial by moving the lever to the center of the table. After a brief hold period, a light illuminates one of eight surrounding target positions, and the animal moves the lever to this position to obtain a food reward. This movement is similar to a reaching action and usually involves rotating two joints, the shoulder and the elbow.
The results of these studies convincingly demonstrate that the activity of the cells in the primary motor cortex correlates much better with movement direction than with target location. Figure 8.12a shows a neuron’s activity when movements were initiated from a center location to eight radial locations. This cell was most strongly activated (red arrows in Figure 8.12a) when the movement was toward the animal. Figure 8.12b shows results from the same cell when movements were initiated at radial locations and always ended at the center position. In this condition, the cell was most active (Figure 8.12b, red arrows) for movements initiated from the most distant position; movement was again toward the animal. Many cells in motor areas show directional tuning, or exhibit what is referred to as a preferred direction. This tuning is relatively broad. For example, the cell shown in Figure 8.12 shows a significant increase in activity for movements in four of the eight directions. An experimenter would be hard-pressed to predict the direction of an ongoing movement if he were observing only the activity of this individual cell.
We can assume, however, that activity is distributed across many cells, each with their unique preferred direction. To provide a more global representation, Georgopoulos and his colleagues introduced the concept of the population vector (Figure 8.13). The idea is quite simple: Each neuron can be considered to be contributing a “vote” to the overall activity level. The strength of the vote will correspond to how closely the movement matches the cell’s preferred direction: If the match is close, the cell will fire strongly; if the match is poor, the cell will fire weakly or even be inhibited. Thus, the activity of each neuron can be described as a vector, oriented to the cell’s preferred direction with a strength equal to its firing rate. The population vector is the sum of all the individual vectors.
The population vector has proved to be a powerful tool in motor neurophysiology. With relatively small numbers of neurons (e.g., 30–50), the population vector provides an excellent predictor of movement direction. The population vector is not limited to simple 2-D movements; it also has proven effective at representing movements in 3-D space. Interestingly, neural activity in many motor areas appears to be correlated with movement direction.
It is important to keep in mind that the physiological method is inherently correlational. Directional tuning is prevalent in motor areas, but this does not mean that direction is the key variable represented in the brain. Note that the experiment outlined in Figure 8.12 contains a critical confound. We can describe the data in terms of movement direction, interpreting the results to show that the cell is active for movements toward the animal. To move in this direction, the animal activates the biceps muscle to produce flexion about the elbow. From these data, we do not know if the cell is coding direction, or the level of biceps activation when the elbow is being flexed, or some other parameter correlated with these variables. Subsequent experiments have addressed this problem. The results are, as so often happens when looking at the brain, complex. Within any given area, a mixture of representations is found. The activity of some cells is best correlated with external movement direction, and the activity of other cells with parameters more closely linked to muscular activation patterns (Kakei et al., 1999).
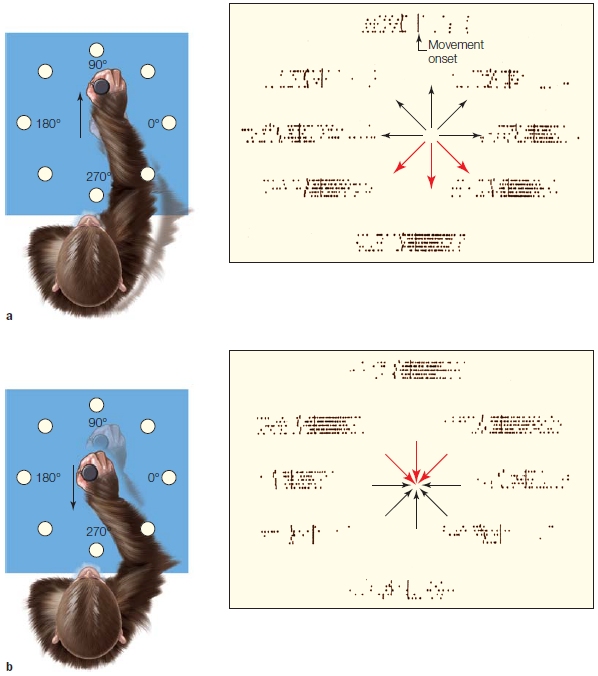
FIGURE 8.12 Motor cortex activity is correlated with movement direction.
(a) The animal was trained to move a lever from the center location to one of eight surrounding locations. The activity of a motor cortex neuron is plotted next to each target location. Each row represents a single movement, and the dots correspond to action potentials. The data are aligned by movement (vertical bar). (b) Here, movements originated at the eight peripheral locations and always terminated at the center location. The activity for the neuron is now plotted next to the starting locations. The neuron is most active (i.e., greatest density of dots) for movements in the downward direction (red arrows), regardless of starting and final locations.
Alternative Perspectives on Neural Representation of Movement
The population vector is dynamic and can be calculated continuously over time. Indeed, after defining the preferred direction of a set of neurons, we can calculate the population vector from the activation of that set of neurons even before the animal starts to move. To do this, and provide a way to dissociate planning- and movement-related activity, experimenters frequently impose a delay period. The animal is first given a cue indicating the direction of a forthcoming movement and then required to wait for a “go” signal before initiating the movement (Figure 8.14). This procedure reveals that the population vector shifts in the direction of the upcoming movement well before the movement is produced, suggesting that at least some of the cells are involved in planning the movement and not simply recruited once the movement is being executed. In fact, by looking at the population vector, which was recorded more than 300 ms before the movement, the direction of the forthcoming movement can be precisely predicted. This result may not sound like that big of a deal to you, but it put motor researchers into a frenzy—although not until about 10 years after Georgopolous’s initial studies on the population vector. With hindsight, can you see why? As a hint, consider how this finding might be used to help people with spinal cord injuries. We will explore this a bit later in the section called “The Brain–Machine Interface.”
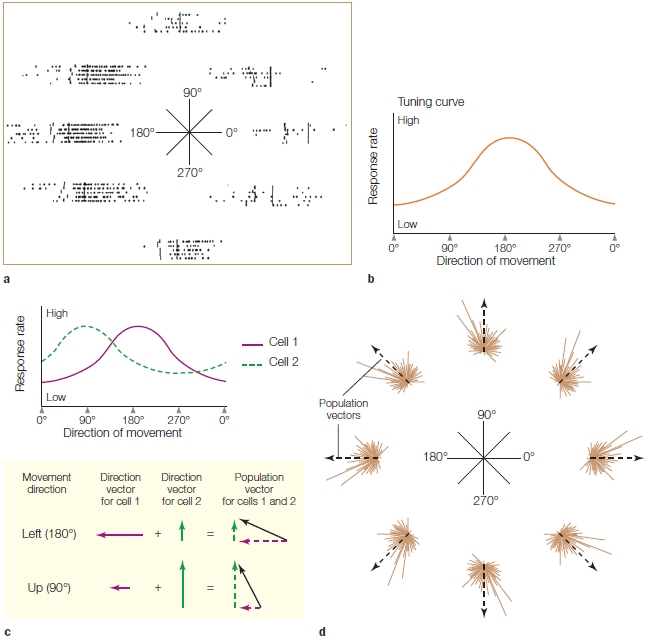
FIGURE 8.13 The population vector provides a cortical representation of movement.
The activity of a single neuron in the motor cortex is measured for each of the eight movements (a) and plotted as a tuning profile (b). The preferred direction for this neuron is 180°, the leftward movement. (c) Each neuron’s contribution to a particular movement can be plotted as a vector. The direction of the vector is always plotted as the neuron’s preferred direction, and the length corresponds to its firing rate for the target direction. The population vector (dashed line) is the sum of the individual vectors. (d) For each direction, the solid lines are the individual vectors for each of 241 motor cortex neurons; the dotted line is the population vector calculated over the entire set of neurons. Although many neurons are active during each movement, the summed activity closely corresponds to the actual movements.
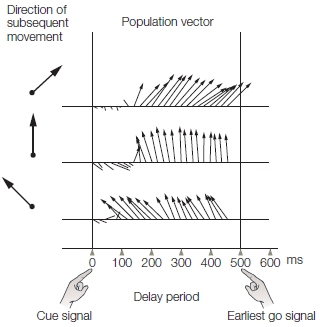
FIGURE 8.14 The direction of the population vector predicts the direction of a forthcoming movement.
At the cue, one of the eight targets is illuminated, indicating the direction for a subsequent movement. The animal must refrain from moving until the go signal (500 ms later in this example). The population vector was calculated every 20 milliseconds. The population vector is oriented in the direction for the planned movement, even though EMG activity is silent in the muscles during the delay period.
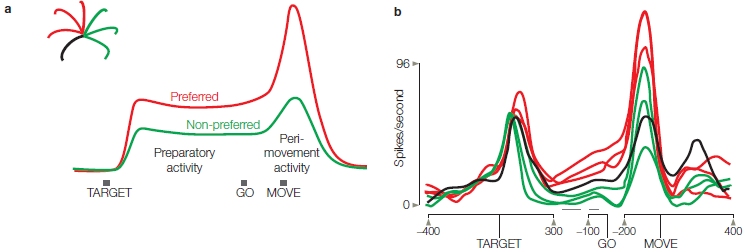
Even though directional tuning and population vectors have become cornerstone concepts in motor neurophysiology, it is also important to consider that many cells do not show strong directional tuning. Even more puzzling, the tuning may be inconsistent: The tuning exhibited by a cell before movement begins may shift during the actual movement (Figure 8.15a). What’s more, many cells that exhibit an increase of activity during the delay phase show a brief drop in activity just before movement begins (Figure 8.15b), or a different firing pattern in preparation and execution of a movement (Figure 8.15c). This result is at odds with the assumption that the planning phase is just a weaker, or subthreshold version of the cell’s activity during the movement phase.
What are we to make of these unexpected findings, in which the tuning properties change over the course of an action? Mark Churchland and his colleagues (2012) suggest that we need a radically different perspective on motor neurophysiology. Rather than viewing neurons as static representational devices (e.g., with a fixed directional tuning), we should focus on the dynamic properties of neurons, recognizing that movement arises as the set of neurons move from one state to another. By this view, we might see that neurons wear many hats, coding different features depending on time and context. There need not be a simple mapping from behavior to neural activity. Indeed, given the challenge of using limbs with complex biomechanics to interact with a wide range of objects and environments, we might expect the nervous system to have evolved such that information is represented in a multidimensional format, coding a wide range of variables such as force, velocity, and context. This form of representation may be harder for the experimenter to decode, but it is likely an important adaptation that gives the motor system maximum flexibility (not to mention job stability for neurophysiologists).
|
|
FIGURE 8.15 Planning- and execution-related activity are not always correlated.
(a) Schematic of what would be expected if neural activation during movement execution was an amplified version of that observed during movement planning. This neuron is more active when planning movements toward the upper left region of the workspace (red) compared to when the movement will be to the right (green). This planning-related difference is maintained after the “Go” signal when the animal executes the movement. (b) A neuron in motor cortex showing a firing pattern similar to the idealized neuron. (c) A different neuron in motor cortex that shows a different preferred direction during the planning phase compared to the execution phase (reversal or red-green pattern right around the time of movement onset).
|

|
Although scientists refer to one part of the brain as motor cortex and another region as sensory cortex, we know that these areas are closely entwined with one another. People produce movements in anticipation of their sensory consequences: We increase the force used to grip and lift a full cup of coffee in anticipation of the weight we expect to experience. Similarly, we use sensory information to adjust our actions. If the cup is empty, we quickly reduce the grip force to avoid moving the cup upward too quickly. Physiologists observe this interdependency and have recognized for some time that the motor cortex isn’t just “motor,” and the sensory cortex isn’t just “sensory.” For example, in rats, the neurons that control whisker movements are predominantly in somatosensory cortex.
In monkeys, sensory inputs rapidly reshape motor activity (reviewed in Hatsopoulos & Suminski, 2011). In fact, some evidence suggests that the directional tuning of some motor cortex neurons is more about “sensory” tuning. Consider the same shoulder movement induced by two different sensory events. One is caused by a nudge to the elbow and the other following a nudge to the shoulder. As early as 50 ms, well before the sensory signals in sensory cortex would have been processed and sent to the motor system, M1 neurons show differential responses to the two types of nudges. It appears that the sensory information was processed within M1 directly, allowing for fast, nearly real-time feedback (Pruszynski et al., 2011a, b).
Taken together, the neurophysiological evidence points to a more nuanced picture than we might have anticipated from our hierarchical control model. Rather than a linkage of different neural regions with specific levels in a processing hierarchy, one that moves from abstract to more concrete representations, the picture reveals an interactive network of motor areas that represent multiple features. This complexity becomes even more apparent in the next section, when we turn our attention to motor planning.
TAKE-HOME MESSAGES
- Motor neurophysiologists correlate cellular activity in motor cortex with the animal’s behavior.
- A common observation is that neurons in motor areas exhibit a preferred direction, in which the firing rate is strongest for movements in a limited set of directions.
- The population vector is a representation based on combining the activity of many neurons.
- Population vectors that provide a close match to behavior can be constructed from many motor areas, although this does not mean that all of these cells represent movement direction.
- Before movement even begins, the population vector is a reliable signal of the direction of a forthcoming movement. This finding indicates that some cells are involved in planning movements as well as executing movement.
- Neurons have dynamic properties, coding different features depending on time and context. There need not be a simple mapping from behavior to neural activity.
- The heterogeneity of responses exhibited by neurons in M1 includes both motor and sensory information.
Goal Selection and Action Planning
We now understand that the neural codes found in motor areas can be abstract, more related to the goals of an action than to the specific muscle patterns required to produce the movement needed to achieve that goal. Using the current context, including sensory information and feedback, the motor cortex may have more than one option for achieving that goal. In this section, we will look at how we select goals and plan motor movements to achieve them.
Consider again the situation where you are at your computer, working on a paper, with a steaming cup of coffee on your desk. You may not realize it, but you are faced with a problem that confronts all animals in their environment: deciding what to do and how to do it. Should you continue typing or sip your coffee? If you choose the coffee, then some intermediate goals must be attained—for example, reaching for the cup, grasping the cup, and bringing it to your mouth—to achieve the overarching goal of a swig of coffee. Each step requires a set of gestures, but in each case there is more than one way to perform them. For example, the cup is closer to your left hand, but your right hand is more trustworthy; which to use? Decisions must be made at multiple levels. We have to choose a goal, choose an option for achieving the goal, and choose how to perform each intermediate step.
|
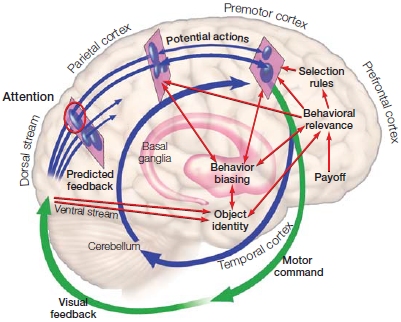
FIGURE 8.16 Sketch of the affordance competition hypothesis in the context of visually guided movement.
Schematic of the processes and pathways when choosing to reach for one object among a display of many objects. The multiple pathways from visual cortex across the dorsal stream correspond to action plans for reaching to the different objects. The thickness of the arrows and circles indicate the strength for each competing plan. Selection is influenced by many sources (red arrows). The movement (green arrow) results in visual feedback of the action and results in the competition starting anew, but now in a different context.
|
|
Action Goals and Movement Plans
Paul Cisek of the University of Montreal (2007) offers one hypothesis for how we set goals and plan actions. It incorporates many of the ideas and findings that we are going to look at, providing a general framework for action selection. His affordance competition hypothesis is rooted in an evolutionary perspective. This hypothesis considers that the brain’s functional architecture has evolved to mediate real-time interactions with the world. Affordances are the opportunities for action defined by the environment (Gibson, 1979). Our ancestors, driven by internal needs such as hunger and thirst, evolved in a world where they engaged in interactions with a changing, and sometimes hostile, environment that held a variety of opportunities and demands for action. To survive and reproduce, early humans had to be ever ready, anticipating the next predator or properly positioning themselves to snag available prey or ripe fruit. Many interactions don’t allow time for carefully evaluating goals, considering options, and then planning the movements—what’s known as serial processing.
A better survival strategy is to develop multiple plans in parallel. Cisek’s affordance competition hypothesis proposes that the processes of action selection (what to do) and specification (how to do it) occur simultaneously within an interactive neural network, and they evolve continuously. Even when performing one action, we are preparing for the next. The brain uses the constant stream of sensory information arriving from the environment through sensorimotor feedback loops to continuously specify and update potential actions and how to carry them out. That’s the affordance part. This sensory information is constrained by our internal drive states, longer-range goals, expected rewards, and anticipated costs, and we use all this information to assess the utility of the different actions. This is the competition part. At some point, one option wins out over the other competitors. An action is selected and executed.
This selection process involves many parts of the motor pathway, where interactions within frontoparietal circuits have a prominent role (see Figure 8.16). This schema implies that decision-making processes are embedded in the neural systems associated with motor control, not carried out by some sort of detached central control center. Is there any evidence supporting this? Let’s start with the notion that an action has multiple goals, and each goal is linked with the plan to accomplish it.
Cisek (2005) developed his model based on evidence obtained in single-cell recordings from the premotor cortex of monkeys. In each trial of his study, the animal was presented with two targets, either of which it could reach with its right arm. After a delay period, a cue indicated the target location for the current trial. During this delay period, neural signatures for both movements could be observed in the activity of premotor neurons, even though the animal had yet to receive a cue for the required action. These signatures can be viewed as potential action plans. With the onset of the cue, the decision scales were tipped. Activity associated with movement to that target became stronger, and activity associated with the other movement became suppressed. Thus, following the cue, the initial dual representation consolidated into a single movement (Figure 8.17). In a variant of this task, only one target is presented. Even here, though, researchers can observe the simultaneous specifications of multiple potential actions in the anterior intraparietal area. In this case, the multiple representations are for different ways the goal could be reached (Baumann et al., 2009). So it also appears that goals can have more than one plan, and the plans to attain them are coupled.
|
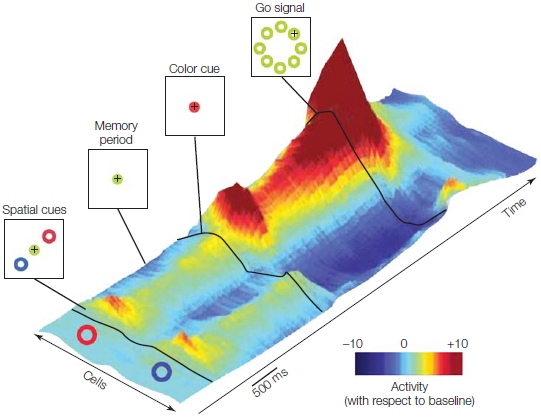
FIGURE 8.17 3-D representation of activity in a population of neurons in the dorsal premotor cortex.
Preferred direction of the cells is represented along the bottom left of the figure and time along the bottom right. When the two cues appear, the firing rate increases in neurons tuned to either target. When the color cue appears, indicating the target, activity increases for cells tuned to this direction and decrease for cells tuned to the other direction.
|
Representational Variation Across Motor Areas of the Cortex
Other cells in premotor cortex have been shown to represent action goals more abstractly. For example, some neurons discharge whenever the monkey grasps an object, regardless of the effector used. It could be the right hand, the left hand, the mouth, or both hand and mouth. Giacomo Rizzolatti of the University of Parma, Italy, proposed that these neurons form a basic vocabulary of motor acts (Rizzolatti et al., 2000). Some cells are preferentially activated when the animal reaches for an object with its hand; others become active when the animal makes the same gesture to hold the object; and still others, when the animal attempts to tear the object—a behavior that might find its roots in the wild, where monkeys break off tree leaves. Therefore, cellular activity in this area might reflect not only the trajectory of a movement, but also basic gestural classes of actions such as reaching, holding, and tearing.
As described earlier, Brodmann area 6 includes premotor cortex on the lateral surface and supplementary motor area on the medial surface. We noted that one distinction between these two secondary regions was in terms of their integration of external and internal information. Lateral premotor is more heavily connected with parietal cortex, and this finding is consistent with a role for this region in sensory-guided action. The supplementary motor area (SMA), with its strong connections to medial frontal cortex, is likely biased to influence action selection and planning based on internal goals and personal experience (see Chapter 12).
The SMA has also been hypothesized to play an important role in more complex actions such as those involving sequential movements or those requiring coordinated movements of the two limbs. Usually, skilled behavior requires a precise interplay of both hands. The two hands may work in a similar fashion, as when we push a heavy object or row a boat. In other tasks, however, the two hands take on different, complementary roles, as when we open a jar or tie our shoes. Damage to the SMA, in both monkeys and humans, can lead to impaired performance on tasks that require integrated use of the two hands, even though the individual gestures performed by either hand alone are unaffected (Wiesendanger et al., 1996). If a person is asked to pantomime opening a drawer with one hand and to retrieve an object with the other, both hands may mime the opening gesture. Again, this deficit fits with the idea of a competitive process in which an abstract goal—to retrieve an object from the drawer—is activated and a competition ensues to determine how the required movements are assigned to each hand. When the SMA is damaged, the assignment process is disrupted and execution fails, even though the person is still able to express the general goal.
Lesions of the SMA can also result in alien hand syndrome, a condition in which one limb produces a seemingly meaningful action but the person denies responsibility for the action. For example, the person may reach out and grab an object and then be surprised to find the object in her hand. In more bizarre cases, the two hands may work in opposition to one another, a condition that is especially prevalent after lesions or resection of the corpus callosum. One patient described how her left hand would attempt to unbutton her blouse as soon as she finished getting dressed. When she was asked to give the experimenter a favorite book, her left hand reached out and snagged the closest book, whereupon she exclaimed with surprise, “Oh, that’s not the one!” These behaviors provide further evidence of motor planning as a competitive process, one that can entail a competition not just between potential targets of an action (e.g., the coffee cup or the computer keyboard) but also between the two limbs (see How the Brain Works: Patting Your Head While Rubbing Your Stomach).
As we might expect, given its role in spatial representation, planning-related activity is also evident in the parietal lobe. When a spatial target is presented to a monkey, neurons begin to discharge in at least two regions within posterior parietal cortex (PPC), the lateral intraparietal (LIP) area, and the medial intraparietal (MIP) area (Calton et al., 2002; Cui & Andersen, 2007). When an arm movement is used to point to the target, the activity becomes stronger in MIP than LIP. If, however, the animal simply looks at the target, activity becomes stronger in LIP than MIP. Besides demonstrating effector specificity within the PPC, these findings also emphasize that plans for both reaching and eye movements are simultaneously prepared, consistent with the affordance competition hypothesis. Effector specificity within the parietal lobe has also been identified in humans with the aid of fMRI, which shows that different regions of the intraparietal sulcus are activated for eye and arm movements (Tosoni et al., 2008).
Together, these results help reveal how action selection and movement planning evolve within parietofrontal pathways. In general, we see many similarities between posterior parietal cortex and premotor regions. For example, cells in both regions exhibit directional tuning, and population vectors derived from either area provide an excellent match to behavior.
These areas, however, also have some interesting differences. One difference is seen in the reference frame for movement. To take our coffee cup example, we need to recognize that reaching requires a transformation from vision-centered coordinates to hand-centered coordinates. Our eyes can inform us of where objects lie in space. To reach that object with the hand, however, we need to define the position of the object with respect to the hand, not the eyes. Moreover, to sense hand position, we don’t have to look at our hands. Somatosensory information is sufficient. You can prove this to yourself by trying to reach for something with the starting position of your hand either visible or occluded. Your accuracy is just as good either way. Physiological studies suggest that representations within parietal cortex tend to be in an eye-centered reference frame, whereas those in premotor cortex are more hand-centered (Batista et al., 1999). Thus parietofrontal processing involves a reference frame transformation.
Another intriguing difference between parietal and premotor motor areas comes from a fascinating study that attempted to identify where intentions are formed and how we become aware of them (Desmurget et al., 2009). The study employed direct brain stimulation during neurosurgery. When the stimulation was over posterior parietal cortex, the patients reported that they experienced the intention or desire to move, making comments such as “I felt a desire to lick my lips.” In fact, if the stimulation level was increased, the intention was replaced with the perception that they had actually performed the movement. This experience, however, was illusory. The patients did not produce any overt movement, and even careful observation of the muscles showed no activity. In contrast, stimulation of the dorsal premotor cortex triggered complex multi-joint movements such as arm rotation or wrist flexion, but here the patients had no conscious awareness of the action and no sense of movement intention. It is unclear what to make of this striking dissociation. These researchers suggested that the posterior parietal cortex is more strongly linked to motor intention, the movement goals, and premotor cortex to movement execution. The signal we are aware of when making a movement does not emerge from the movement itself but rather from the prior conscious intention and predictions we make about the movement in advance of action.
HOW THE BRAIN WORKS
Patting Your Head While Rubbing Your Stomach
Recall the childhood challenge to pat your head while rubbing your stomach? Then you already know this apparently simple task is not so easy. It’s nearly impossible to generate the conflicting spatial trajectories—moving one hand up and down while using the other to make a circular movement. The two movements compete. We fail to map one direction for one hand and the other direction for the opposite hand. Eventually one of the movements dominates, and we end up rubbing both the head and the stomach or patting both of them. Based on the selection hypothesis outlined in this chapter, we can think of this bimanual conflict as competition between two movement goals. Each task activates both hemispheres, and we cannot keep the crosstalk created by these activation patterns from interfering with the movements.
If this hypothesis is correct, spatial interference should be eliminated when each movement goal is restricted to a single hemisphere and the pathways connecting the two hemispheres are severed. To test this idea, Elizabeth Franz and her colleagues (1996) at the University of California, Berkeley, tested a patient whose corpus callosum had been resected. The stimuli for this bimanual movement study were a pair of three-sided figures whose sides followed either a common axis or perpendicular axes. The stimuli were projected briefly—one stimulus appeared in the left visual field, the other in the right visual field. After viewing the stimuli, the participants were instructed to produce the two patterns simultaneously, using the left hand for the pattern projected in the left visual field and the right hand for the pattern in the right visual field. The brief presentation was used to ensure that each stimulus was isolated to a single hemisphere in the split-brain patient. In control participants, rapid transfer of information via the corpus callosum was expected.
As Figure 1, top shows, control participants had little difficulty producing bilateral movements when the segments of the squares followed a common axis of movement (upper left). When the segments required movements along perpendicular axes, however (lower left), their performance deteriorated dramatically. Long pauses occurred before each segment, and trajectories frequently deviated from the target—something you can demonstrate to yourself by trying this task.
In contrast, the split-brain patient’s performance (right column) did not differ significantly between the two movements. He initiated and completed movements in the two conditions with comparable speed, and the movements were accurate in both. Indeed, in a second experiment, this patient simultaneously drew a square with the left hand and a circle with the right hand. Each hemisphere produced the pattern with no signs of interference from demands presented to the opposite hemisphere.
These results indicate that the callosotomy procedure yields a spatial uncoupling in bimanual movements. Another striking observation was that, even for the splitbrain patient, the actions of the two hands were not independent of one another. As with the control participants, the two hands moved in synchrony. They initiated and terminated the segments of the squares at approximately the same time. This temporal coupling was seen more clearly when participants were asked to produce oscillatory movements in which each hand moved along a single axis. Regardless of whether the two hands followed a common axis (e.g., both horizontal or both vertical) or perpendicular axes (e.g., one horizontal and the other vertical), the two hands reversed direction at the same time.
This study provided valuable insights into the neural structures underlying bimanual coordination. First, the spatial goals for bimanual movements are coordinated via processing across the corpus callosum. When a task requires conflicting directions of movement, interference is extensive as long as the callosal connections are intact.
Second, these connections are not necessary for the temporal coupling of movement. Perhaps the initiation of movement is regulated either by a single hemisphere or by subcortical mechanisms. Third, the dissociation of spatial and temporal coupling emphasizes a distributed view of how the motor system’s neural structures contribute to coordination. The neural structures that represent the spatial goals are separate from those involved in initiating the movements selected to meet these goals.
FIGURE 1 Bimanual movements following resection of the corpus callosum.
While looking at a central fixation point, participants were briefly shown the two patterns. They were instructed to simultaneously draw the pattern on the left with the left hand and the one on the right with the right hand. Normal participants (left column) were able to draw the patterns that shared a common axis but had severe difficulty when the orientation of the two figures differed by 90°. The split-brain patient (right column) performed equally well in both conditions.
This idea is further supported by an fMRI study conducted by Scott Grafton and colleagues at the University of California, Santa Barbara (Hamilton & Grafton, 2007). They questioned whether motor representations in parietal regions correspond to the nuts and bolts of the movements per se, or the grander intentions concerned with the goals and outcome of the action. This study took advantage of the widely studied repetition suppression (RS) effect. RS was first described in studies of visual perception: When a stimulus is repeated, the blood oxygen level– dependent (BOLD) response to the second presentation of the stimulus is lower than that to the initial presentation. In applying this fMRI method to action perception, the researchers asked whether the RS effect was linked to the goal of an action, the specific movement, or a combination of these factors (Figure 8.18). To test this, participants were shown videos of short action clips. The videos showed a box that could be opened by sliding the cover forward or backward. In this way, the researchers could present pairs of video clips in which either the same goal was achieved (e.g., closing the cover) by two different actions, in which one clip showed sliding forward and the other backward; or the same movement was made, but resulted in two different goals, one resulting in an open box and the other a closed box. The results showed that RS in the right inferior parietal cortex was related to the action goal, whereas RS in left frontal cortex was related to the movement (Figure 8.19), providing a slick demonstration of goal-based processing in parietal cortex and movement-based processing in frontal cortex.
TAKE-HOME MESSAGES
- The affordance competition hypothesis proposes that the processes of action selection (what to do) and specification (how to do it) occur simultaneously within an interactive neural network that continuously evolves from planning to execution.
- Action selection involves a competitive process.
- Rather than view selection and planning as serial processes, neural activity reveals that there is parallel activation of multiple goals and movement plans.
- Supplementary motor area is important for coordinating motor behavior in time (sequential movements) and between limbs (bimanual coordination).
- Parietal motor areas also show topography: Different regions of the intraparietal cortex are associated with hand, arm, and eye movements.
- Parietal motor representations are more goal oriented, whereas premotor-motor representations are more closely linked to the movement itself.
- Conscious awareness of movement appears to be related to the neural processing of action intention rather than the movement itself.
The Brain–Machine Interface
FIGURE 8.18 A set of stimuli for inducing repetition suppression.
Participants watched a series of movie clips of a hand opening or closing a box. In this example, the initial clip shows the hand moving forward to open the box. In subsequent clips, the outcome was either repeated or novel, and the kinematics (direction of motion) was either repeated or novel relative to the previous clip. Repetition suppression effects were measured by comparing the BOLD response over successive clips.
Can neural signals be used to control a movement directly with the brain, bypassing the intermediate stage of muscles? For instance, could you plan an action in your motor cortex (e.g., let’s fold the laundry), somehow connect those motor cortex neurons to a computer, and send the planned action to a robot, which would fold the laundry? Sounds extraordinary? Yet it is happening. The process is called a brain– machine interface (BMI). It uses decoding principles (see Chapter 6) to control brain–machine interface systems, which have incredible potential to improve the lives of people with spinal cord injuries, amputations, and other diseases that have affected their ability to move at will.
Early Work on Brain–Machine Interface Systems
John Chapin of the State University of New York (Chapin et al., 1999) provided one of the first demonstrations of the viability of a BMI by using a simple motor task in a highly motivated population: thirsty rats. He first trained the rats to press a button that caused a lever arm to rotate. The lever was connected to a computer, which measured the pressure on the button and used this signal to adjust the position of a robot arm. One end of the lever contained a small well; if positioned properly, a few drops of water would fill the well. Thus, by learning to vary the pressure of the button press, the rat controlled the lever arm and could replenish the water and then spin the lever to take a drink (Figure 8.20). Chapin recorded from neurons in the motor cortex during this task, measuring the correlation between each neuron and the force output the rat used to adjust and move the lever. Once the rat’s behavior had stabilized, Chapin could construct an online population vector, one that matched the animal’s force output rather than movement direction. With as few as 30 or so neurons, the match between the population vector and behavior was excellent.
Here is where things get interesting. Chapin then disconnected the input of the button to the computer and instead used the output of the time-varying population vector as input to the computer to control the position of the lever arm. The rats still pushed the button, but that no longer controlled the lever; it was now controlled by their brain activity. If the activity level in the vector was high, the arm swiveled in one direction; if low, it swiveled in the other direction, or even stopped the lever arm entirely. Amazingly, population vectors generated from as few as 25 neurons proved sufficient for the rats to successfully control the robot arm to obtain water.
FIGURE 8.19 Brain regions showing repetition suppression effects for repeated outcomes and movements.
Voxels showing RS in inferior frontal gyrus (IFG) and inferior parietal lobe (IPL) in the right and left hemispheres. RS was strongest in left IFG when the movement was repeated and strongest in right IPL when the outcome was repeated.
As impressive as this result was, Chapin could not, of course, tell the animals about the shift from arm control to brain control. Unaware of the switch to BMI, the rats continued to press and release the button. Over time, though, the animals became sensitive to the lack of a precise correlation between their arm movements and the lever position (the correlation was not perfect). Amazingly, they continued to generate the cortical signals necessary to control the lever, but they also stopped moving their limb. They learned they could kick back, relax, and simply think about pushing the button with the precision required to satiate their thirst.
Over the past 20 years, research on brain–machine interface (BMI) systems has skyrocketed. Three elements are required: microelectrode arrays implanted on the cortex to record neural activity, a computer with decoding algorithms, and a prosthetic effector. In the first primate studies, monkeys were trained to control the two-dimensional position of a computer cursor. With more sophisticated algorithms, these animals have learned to use BMI systems that control a robotic arm with multiple joints, moving the prosthetic limb through three-dimensional space to grasp food and bring it to their mouth (Velliste et al., 2008). Videos are available at http://motorlab.neurobio.pitt.edu/multimedia.php. Besides controlling BMI with output from primary motor cortex, BMI also works with cells in premotor, supplementary motor, and parietal cortex (Carmena et al., 2003). The control algorithms have also become more advanced, adopting ideas from work on computer learning. Rather than use a serial process in which the directional tuning of the neurons is fixed during the initial free-movement stage, researchers now use computer algorithms that allow the tuning to be updated by real-time visual feedback as the animal learns to control the BMI device (D. Taylor et al., 2002).
FIGURE 8.20 Rats can be trained to use a lever to control a robot arm that delivers them drops of water.
Neurons in the rat’s primary motor cortex are recorded while the animal presses the lever. A population vector is constructed, representing the force exerted by the animal. A switch is then activated so that the position of the lever is now based on the population vector. The rat soon learns that he does not have to press the lever to retrieve the water.
Making Brain–Machine Interface Systems Stable
One major challenge facing BMI researchers is how to establish a stable control system, one that can last for years. In a typical experiment, the animal starts each daily session by performing real movements to allow the researcher to construct the tuning profiles of each neuron. The process is rather like a daily recalibration. Once the neuron profiles are established, the BMI system is implemented. This approach, though, is not practical for BMI use as a clinical treatment. First, it is very difficult to record a fixed set of neurons over a long period of time. Moreover, construction of neuron profiles using real movements won’t be possible for BMI to be useful for paralyzed individuals or people who have lost a limb.
To address this issue, researchers have looked at both the stability and flexibility of neural representations. Karunesh Ganguly and Jose Carmena (2009) at the University of California, Berkeley, implanted a grid of 128 microelectrodes in the motor cortex of a monkey. This device allowed them to make continuous daily recordings. Although the signal from some electrodes would change from day to day, a substantial number of neurons remained stable for days (Figure 8.21). Using the output from this stable set, a BMI system successfully performed center-out reaching movements over a 3-week period. The animals achieved close to 100 % accuracy in reaching the targets, and the time required to complete each movement became much shorter over the 3-week period. This result suggested that with a stable decoder, the motor cortex neurons used a remarkably stable activation pattern for prosthetic control.
The shocker came in the next experiment. Using these well-trained animals, researchers randomly shuffled the decoder. For example, if a neuron had a preferred direction of 90 degrees, the algorithm was altered so that the output of this neuron was now treated as if it had a preferred direction of 130 degrees. This new “stable” decoder, of course, played havoc with BMI performance. The monkey would think “move up,” and the cursor would move sideways. Over a few days of practice, however, the monkey was able to adapt to the new decoder, again reaching nearperfect performance (Figure 8.21c). With visual feedback, the animal could learn to use a decoder unrelated to arm movements. As long as the algorithm remained stable, it could actually reshape the decoder. Even more impressive, when the original decoder was reinstated, the animal again quickly adapted. Interestingly, with this adaptive system, the tuning functions of each neuron varied from one context to the next and even deviated from their shape during natural movement (Figure 8.21d). It appears, then, that long-term neuroprosthetic control leads to the formation of a remarkably stable cortical map that is readily recalled and resistant to the storage of a second map.
FIGURE 8.21 Stability and flexibility of performance and neutral activity during BMI control.
(a) Recordings were made for 19 consecutive days from an ensemble of neurons in motor cortex. Directional tuning for two neurons show remarkable stability across Sessions 9–19. (b) Using a fixed decoder based on the output of the neural ensemble, the monkey learns to successfully move a cursor under BMI control in the center-out task. Accuracy becomes near perfect within a few days and the time required on each trial becomes much faster. (c) Performance with a shuffled decoder. The input to the BMI algorithm was randomly shuffled in Session 20 and the animal failed to reach any targets. With continued use of the shuffled decoder, however, the animal quickly became proficient at reaching the target. (d) Tuning functions for three neurons when used in original decoder (blue) or shuffled decoder (red). Tuning functions for some neurons shifted dramatically for the two contexts. With practice, the animal could successfully control the cursor with either decoder.
These results hold great promise for the translation of BMI research into the clinic. They demonstrate that the representation of individual neurons can be highly flexible, adapting to the current context. Such flexibility is essential for ensuring that the system will remain stable over time, and it is also essential for using a single BMI system to control a host of devices such as computer cursors or eating utensils. It is reasonable to assume that a single set of neurons can learn to incorporate the different challenges presented by devices that have no friction or mass (the position of a mouse on a computer screen) to ones with large mass and complicated moving parts (a prosthetic arm or a robot).
There is great urgency to get BMI ideas into clinical practice. The numbers of patients who would benefit from such systems are huge. In the United States alone, over 5.5 million people suffer some form of paralysis, either from injury or disease, and 1.7 million have limb loss. This need has motivated some scientists to move toward clinical trials in humans. John Donoghue and his colleagues at Brown University presented the first such trial, working with a patient, M.N., who became quadriplegic following a stab wound that severed his spinal cord. The researchers implanted an array of microchips in the patient’s motor cortex (Hochberg et al., 2006). Despite 3 years of paralysis, the cells were quite active. Moreover, the firing level of the neurons varied as M.N. imagined different types of movements. Some units were active when he imagined making movements that involved the shoulder, others while imagining moving his hand. The researchers were also able to determine the directional tuning profiles of each neuron, asking M.N. to imagine movements over a range of directions.
From this data, they created population vectors and used them as control signals for BMI interface devices. Using the output of approximately 100 neurons, M.N. was able to move a cursor around a computer screen (Figure 8.22). His responses were relatively slow and the path of the cursor somewhat erratic. Nonetheless, M.N. could control the cursor to open his e-mail, use software programs to make drawings, or play computer games such as PONG. When connected to a prosthetic limb, M.N. could control the opening and closing of the hand, a first step to performing much more complicated tasks. Another patient has learned, after months of training, to use a BMI system to control a robotic arm to reach and grasp objects (Hochberg et al., 2012). (Video clips of people using BMI systems can be seen at http://www.nature.com/nature/journal/v442/n7099/suppinfo/nature04970.html and http://www.nature.com/nature/journal/v485/n7398/full/nature11076.html#/supplementary-information.)
|
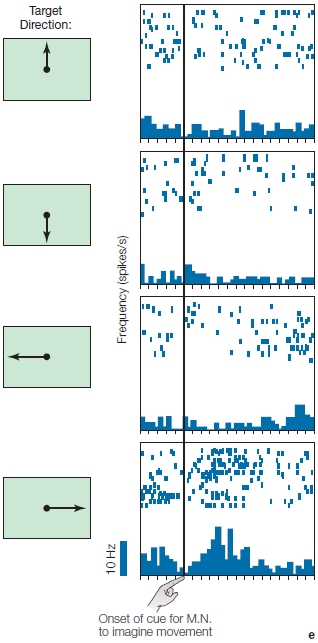
|
|
FIGURE 8.22 Brain–machine interface used by M.N.
(a) The size of the implanted electrode device in relation to a U.S. penny. (b) A magnified image of the recording electrode array. (c) The location in the precentral gyrus where the electrode array was implanted. (d) The subject M.N. with the implanted device. He is controlling a cursor on the computer screen with his neural activity. (e) The firing of one cell during four different conditions in which M.N. was cued to imagine moving his hand up, down, left, or right. The cell shown here fired best when M.N. imagined moving his hand to the right; other cells fired selectively when M.N. imagined moving his hand left, up, or down. When information from all of the cells recorded from the implanted electrode was combined, the desired direction of movement could be predicted. Once the BMI device learned how the pattern of M.N.’s activity correlated with the desire to move in these directions, M.N. could begin to use his intentions to move a cursor wherever he chose. Using this technology, M.N. was also able to open simulated e-mails, operate a television, open and close a prosthetic hand, and move a robotic arm. Such technology holds great promise for people like M.N., who cannot otherwise physically interact with their environment.
|
BMI research is still in its infancy. This work, though, provides a compelling example of how basic findings in neuroscience—the coding of movement direction and population vector representations—can be combined with principles from bioengineering to develop vital clinical therapies.
TAKE-HOME MESSAGES
- Brain–machine interface systems use neural signals to directly control robotic devices such as a computer cursor or a prosthetic device.
- BMIs offer a promising avenue for rehabilitation of people with severe movement disorders such as those resulting from spinal cord injury.
- Early BMI systems required two phases. In the first phase, neural activity was recorded while the animal produced movement and the tuning properties (such as preferred direction) were recorded. In the second phase, the output from these neurons was used to control an interface device.
- Current studies are exploring how decoders can be adapted through experience in BMI control and are looking at the stability of such systems over extended periods of time. Advances on these problems are essential for building BMI systems that will be useful in clinical settings.
Movement Initiation and the Basal Ganglia
With multiple action plans dueling it out in the cortex, how do we decide on which movement to execute? We can’t use our right arm to simultaneously type on the computer keyboard and reach for a cup of coffee. Parallel processing works fine for planning, but at some point, the system must commit to a particular action.
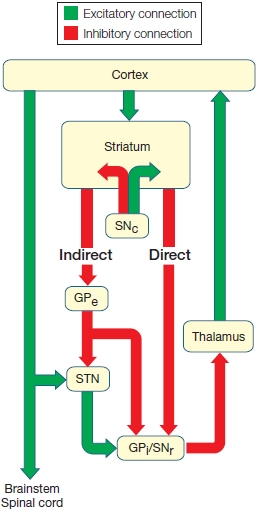
FIGURE 8.23 Wiring of the direct and indirect pathways in the basal ganglia.
Green links indicate excitatory projections, and red links indicate inhibitory projections. Inputs from the cortex project primarily to the striatum. From here, processing flows along two pathways. The direct pathway goes to the output nuclei: the internal segment of the globus pallidus (GPi) and the pars reticularis of the substantia nigra (SNr). The indirect pathway includes a circuit through the external segment of the globus pallidus (GPe) and the subthalamic nucleus (STN) and then to the output nuclei. The output projections to the thalamus are relayed to the cortex, frequently terminating close to the initial source of input. The dopaminergic projections of the pars compacta of the substantia nigra (SNc) modulate striatal activity by facilitating the direct pathway via the D1 receptors and inhibiting the indirect pathway via the D2 receptors. The output of the basal ganglia also inhibits other subcortical structures such as the superior colliculus (not shown).
The basal ganglia appear to play a critical role in movement initiation. To understand this, it is important to examine the neuroanatomical wiring of this subcortical structure which is diagrammed in Figure 8.23. Almost all of the afferent fibers to the basal ganglia terminate in two of the nuclei, the caudate and putamen, or what are collectively referred to as the striatum. These input fibers originate across much of the cerebral cortex, including sensory, motor, and association cortices. The basal ganglia have two output pathways, which originate in the internal segment of the globus pallidus (GPi) and the pars reticularis of the substantia nigra (SNr). SNr axons project to and terminate primarily in the superior colliculus and provide a crucial signal for the initiation of eye movements. GPi axons, on the other hand, terminate in thalamic nuclei, which in turn project to the motor cortex, supplementary motor area, and prefrontal cortex.
Processing within the basal ganglia takes place along two pathways (DeLong, 1990). The direct pathway involves fast, direct, inhibitory connections from the striatum to the GPi and SNr. The indirect pathway takes a slower, roundabout route to the GPi and SNr. Striatal axons inhibit the external segment of the globus pallidus (GPe), which in turn inhibits the subthalamic nucleus and GPi. The output from the basal ganglia via the GPi and SNr is also inhibitory. Indeed, these nuclei have high baseline firing rates, producing strong tonic inhibition of the motor system via their inhibitory projection to the thalamus or the superior colliculi, a region important for eye movements.
The final internal pathway of note is the projection from the pars compacta of the substantia nigra (SNc) to the striatum, known as the dopamine pathway. Interestingly, this pathway has opposite effects on the direct and indirect pathways, despite having a common transmitter, dopamine. The substantia nigra excites the direct pathway by acting on one type of dopamine receptor (D1) and inhibits the indirect pathway by acting on a different type of dopamine receptor (D2).
The Basal Ganglia as a Gatekeeper
Tracing what happens when cortical fibers activate the striatum can help us understand basal ganglia function. Via the direct pathway, target neurons in the output nuclei (GPi and SNr) of the basal ganglia are inhibited, thus encumbering the connection to the thalamus. This results in excitation of the thalamus and cortical motor areas. On the other hand, striatal activation along the indirect pathway results in increased excitation of the output nuclei, leading to increased inhibition of the cortex. It appears, then, that the direct and indirect pathways are at odds with one another. If processing along the indirect pathway is slower, however, the basal ganglia can act as a gatekeeper of cortical activity; less inhibition from the direct pathway is followed by more inhibition from the indirect pathway. The nigrostriatal fibers of the dopamine pathway enhance the direct pathway while reducing the effects of the indirect pathway.
Seen in this light, the basal ganglia can be hypothesized to play a critical role in the initiation of actions (Figure 8.24). As we argued earlier in this chapter, processing in the cortical motor areas can be viewed as a competitive process in which candidate actions compete for control of the motor apparatus. The basal ganglia are positioned to help resolve the competition. The strong inhibitory baseline activity keeps the motor system in check, allowing cortical representations of possible movements to become activated without triggering movement. As a specific motor plan gains strength, the inhibitory signal is decreased for selected neurons. This movement representation breaches the gate, thus winning the competition.
Interestingly, computational analyses demonstrate that the physiology of the direct pathway in the basal ganglia is ideally designed to function as a winner-take-all system—a method for committing to one action plan from among the various alternatives. Greg Berns and Terry Sejnowski (1996) of the Salk Institute in La Jolla, California, evaluated the functional consequences of all possible pair-wise connections of two synapses, either of which could be excitatory or inhibitory. By their analysis, a series of two successive inhibitory links is the most efficient way to make a selected pattern stand out from the background. With this circuit, the disinhibited signal stands out from a quiet background. In contrast, with a pair of excitatory connections the selected pattern has to raise its signal above a loud background. Similarly, a combination of inhibitory and excitatory synapses in either order is not efficient in making the selected pattern distinct from the background. Berns and Sejnowski noted that the double inhibition of the direct pathway is relatively unique to the basal ganglia. This arrangement is particularly useful for selecting a response in a competitive system. For example, consider if you were at the beach, searching for a friend’s kayak on the horizon. If the ocean is filled with all sorts of sailing vessels, your task is challenging. But if the waters are empty that afternoon, it will be easy to detect the kayak as it comes around the point. Similarly, a new input pattern from the striatum will stand out much more clearly when the background activity is inhibited.
FIGURE 8.24 Computational model of the basal ganglia’s role in movement initiation.
The inhibitory output of the basal ganglia keeps potential responses in check until activation for one of the options reaches a threshold, resulting in the initiation of that movement. By this model, “selection” occurs even though the basal ganglia need not evaluate the possible choices, but rather, only monitors their activation level.
As mentioned earlier, dopamine has opposite effects on the direct and indirect pathway. Dopamine has long been known to be a critical neurotransmitter in signaling reward. Dopamine receptors are found in many brain regions, but they are especially prevalent in the striatum (see Chapter 12 for a detailed discussion of dopamine and reward). The direct pathway has D1 receptors, which are excitatory and produce excitatory postsynaptic potentials (EPSPs); the indirect pathway has D2 receptors, which are inhibitory and produce IPSPs. The net result is that dopamine release has the effect of promoting selected actions represented in the direct pathway and discouraging nonselected actions via the indirect pathway. Thus, rewarded actions are more likely to occur in the future, providing a link between movement initiation, reward, and motor learning (see “Contributions of the Basal Ganglia to Learning and Cognition”).
Disorders of the Basal Ganglia
Looking at the basal ganglia circuits in Figure 8.23 makes it clear that lesions in any part of the basal ganglia interfere with coordinated movement, but the form of the problem would vary considerably depending on the location of the lesion. For instance, Huntington’s disease is a hereditary neurodegenerative disorder that appears during the fourth or fifth decade of life. The onset is subtle, usually a gradual change in mental attitude in which the patient is irritable, absentminded, and loses interest in normal activities. Within a year, movement abnormalities are noticed: clumsiness, balance problems, and a general restlessness. Involuntary writhing movements, or chorea, gradually dominate normal motor function. The patient may adopt contorted postures, and his arms, legs, trunk, and head may be in constant motion.
We can understand the excessive movements, or hyperkinesia, seen with Huntington’s disease by considering how the pathology affects information flow through the basal ganglia. The striatal changes occur primarily in inhibitory neurons forming the indirect pathway. As shown in Figure 8.25a, these changes lead to a reduced output from the basal ganglia, and thus greater excitation of thalamic neurons, which in turn excite the motor cortex. Later in the disease, many regions of the brain area are affected. But atrophy is most prominent in the basal ganglia, where the cell death rate is ultimately as high as 90% in the striatum.
The genetic origin of Huntington’s disease is briefly reviewed in Chapter 3. This fatal disease has no cure, and patients usually die within 12 years of onset. At autopsy, the brain of a Huntington’s disease patient typically reveals widespread pathology in cortical and subcortical areas. These changes are also evident from imaging studies performed as the disease unfolds.
Parkinson’s disease, the most common and well-known disorder affecting the basal ganglia, is the result of the loss of dopaminergic neurons in the substantia nigra pars compacta (SNc; Figure 8.26). As with most brain tissue, dopaminergic neurons in the substantia nigra (SNc) atrophy with age. Parkinsonian symptoms become manifest when too many of these neurons are lost (Figure 8.26b).
Symptoms of Parkinson’s disease related to the basal ganglia include disorders of posture and locomotion, hypokinesia, and bradykinesia. Hypokinesia refers to an absence of or reduction in voluntary movement. Parkinson’s patients act as if they are stuck in a posture and cannot change it. This problem, which we might think of as a stuck or blocked gate, is especially evident when the patients try to initiate a new movement. Many patients develop small tricks to help them overcome the hypokinesia. For example, one patient walked with a cane, not because he needed help maintaining his balance, but because it was a visual target that helped him to get a jump start. When he wanted to walk, he placed the cane in front of his right foot and kicked it—which caused him to overcome inertia and commence his walking. Once started, the movements are frequently slow, or bradykinetic.
Look at Figure 8.25b. Parkinson’s disease primarily reduces the inhibitory activity along the direct pathway. With no excitatory SNc input into the striatum, the output along the direct pathway decreases and the inhibitory output from the GPi to the thalamus increases. At the same time, decreased SNc input inhibits the indirect pathway. The net physiological effect is increased thalamic inhibition, either because GPe produces less inhibition of GPi or because the subthalamic nucleus (STN) increases its excitation of the GPi. The net result of all these effects is reduced excitation of the cortex due to the excessive thalamic inhibition. The cortex may continue to plan movements, but without normal functioning basal ganglia, the ability to quickly initiate a movement is compromised. Once movement is initiated, it is frequently slow.
|
FIGURE 8.25 Differential neurochemical alterations in Huntington’s and Parkinson’s diseases.
As in Figure 8.23, green links indicate excitatory projections, and red links indicate inhibitory projections. (a) In Huntington’s disease, the inhibitory projection along the indirect pathway from the striatum to the external segment of the globus pallidus (GPe) is reduced. The net consequence is reduced inhibitory output from the internal segment of the globus pallidus (GPi) and thus an increase in cortical excitation and movement. (b) Parkinson’s disease primarily reduces the inhibitory activity along the direct pathway, resulting in increased inhibition from the GPi to the thalamus and thus a reduction in cortical activity and movement.
|
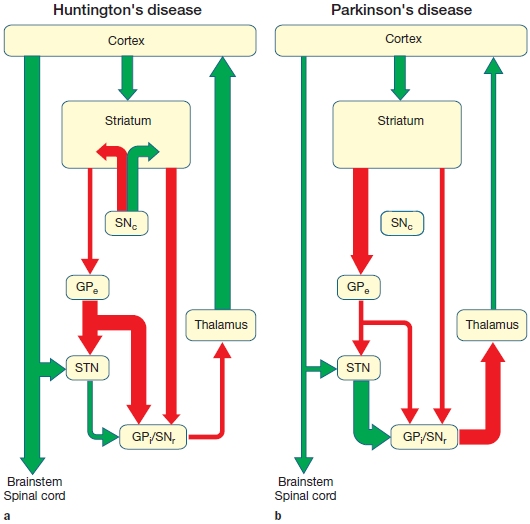
|
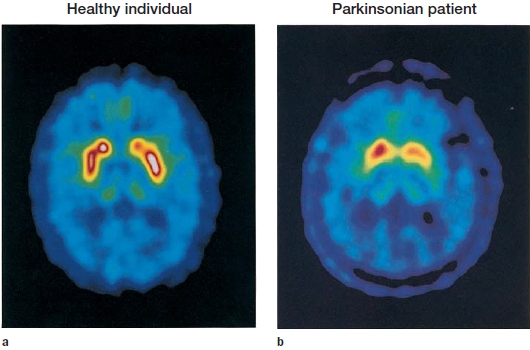
FIGURE 8.26 Radioactive tracers to label the distribution of specific neurotransmitters with PET.
Healthy individuals and Parkinson’s disease patients were injected with a radioactive tracer, fluorodopa (seen as yellow, red, and orange). This agent is visible in the striatum, reflecting the dopaminergic projections to this structure from the substantia nigra. Compare the greater uptake in the scan from a healthy person (a) to the uptake in a patient’s scan (b).
Parkinson’s patients get stuck in one position or posture and have difficulty shifting to a new one. After a number of years, they also show cognitive problems, performing below normal on various tests of neuropsychological function. This may be either secondary to effects of chronic L-DOPA therapy or the result of reduced dopaminergic input to the cerebral cortex. Their cognitive deficits, however, could be at the heart of both the motor and cognitive problems of these patients. Perhaps the basal ganglia perform an operation that is critical for shifting from one movement to another as well as from one idea (mental set) to another.
To test this idea, Steven Keele and his colleagues at the University of Oregon (Hayes et al., 1998) developed two tasks: one required a motor shifting operation (Figure 1) and the other a cognitive shift. For the motor task, patients were taught two sequences of three key presses (1-2-3 and 1-3-2). After this training phase, the patients were required to produce a six-element sequence composed of either the two sequences in succession or two repetitions of one of the sequences. As predicted, in the shifting sequence condition (from the 1-2-3 to 1-3-2), the responses for the Parkinson’s patients were especially slow at the switching point, the transition from the third to the fourth element. Note that, in both the repetition and the shifting condition, the fourth element requires exactly the same response: a finger press with the index finger. In the shifting condition, however, this response is part of a different sub-sequence.
FIGURE 1 Motor and cognitive tests of set shifting.
(a) In the motor task, participants performed two successive sequences that were either identical or different. Although the movement at the transition point was the same in both the no-shift and the shift conditions, Parkinson’s patients were much slower in the latter condition. (b) In the cognitive task, participants had to respond to either the color or the shape of a stimulus. Trials were paired such that the second response was either the same dimension (no shift) or the other dimension (shift). As in the motor task, Parkinson’s patients were especially slow when they had to shift.
For the cognitive task, patients were trained on reaction time tasks involving either color or shape discrimination. After training on each dimension, pairs of trials were introduced in which the two responses were either along the same dimension (e.g., color–color for both trials) or required a shift from one dimension to the other (e.g., shape on one trial switching to color on the next). As in the motor task, the Parkinson’s patients were significantly slower when they had to shift dimensional sets. This problem cannot be attributed to a motor deficit, because the motor responses (pressing a key) on the second trial were identical in all conditions.
The shifting hypothesis offers a unified framework for understanding basal ganglia function in both action and cognition. Located in a position to monitor activation across wide regions of the cortex, the basal ganglia are able to orchestrate a shift between different actions or between different mental sets. The shifting hypothesis is also relevant when thinking about the more general role of the basal ganglia and dopamine in reinforcement learning.
Behaviors have consequences, and when consequences affect the probability that a behavior will or will not be repeated, we call that a reinforcement contingency. We know that when a consequence is rewarding, we alter our behavior to repeat the reward, just like a dog does when he is rewarded with a treat. Dopamine neurons encode both present rewards and the prediction of future rewards (Chapter 12). Thus, a rewarding consequence, such as a winning crosscourt forehand in tennis, will result in the release of dopamine in the striatum. It can be hypothesized that dopamine modifies the input–output channels in the basal ganglia, biasing the system to produce certain responses over others. This makes it more likely that the same response will be initiated when the rewarded input pattern is reactivated in the future (Figure 2). In fact, corticostriatal synaptic plasticity is strongly modulated by dopamine (Reynolds & Wickens, 2000). The next time the tennis ball whizzes by from the same direction, your arm powers back in the previously successful pattern. Thus, by biasing behavior and making it more likely that an animal will shift to the newly rewarded action when it runs across the same circumstances again, the dopamine neurons of the basal ganglia facilitate reinforcement learning.

FIGURE 2
The ability to alter responses according to probable outcomes is essential for producing novel behavior or for combining patterns of behavior into novel sequences. We can now see a link between basal ganglia dysfunction and psychiatric disorders characterized by the repetitive production of stereotyped movement patterns. Examples are Tourette’s syndrome, where a simple tic or a hand brushing across the face may be seen, and obsessive-compulsive disorder, where an entire behavioral sequence, such as hand washing, can be performed over and over. A failure to shift may result in the repeated production of a single pattern—or in an absence of movement, the problem of the patient with Parkinson’s disease. In either case, basal ganglia dysfunction makes it difficult to select new actions that arise when sensory input or internal goals change.
One of the great breakthroughs in neurology occurred in the 1950s with the development of L-DOPA, a synthetic precursor of dopamine. L-DOPA can cross the blood– brain barrier and be metabolized to create dopamine, providing a replacement therapy for the loss of endogenous dopamine. This therapy provided a tremendous benefit to people with Parkinson’s disease and, in fact, continues to do so today. Almost all people who are diagnosed with Parkinson’s are put on some form of L-DOPA therapy, providing a simple medication protocol that considerably improves their motor problems. Over time, however, the efficacy of the drug may change. Many patients develop drug-induced movement disorders, or hyperkinesias—excessive, involuntary movements that are as debilitating as the symptoms of the disease. Moreover, the medication does not prevent the loss of dopamine-producing neurons, so the disease continues to progress until at some point patients may no longer be responsive to L-DOPA therapy.
Due to the limitations of drug therapy, clinicians have sought to develop alternative or supplemental treatments for Parkinson’s disease. For instance, neurosurgeons have devised interventions that seek to restore the balance of inhibitory and excitatory circuits between the basal ganglia and the cortex. The hyperactivity of the globus pallidus that occurs when inhibitory striatal signals are attenuated by the disease can be reduced by pallidotomy, a procedure in which small lesions are made in the globus pallidus. This procedure has proven effective in many patients. The pallidus, however, is quite large, and identifying the best location for the lesions is problematic. What’s more, significant risks are associated with the procedure (de Bie et al., 2002).
FIGURE 8.27 Deep-brain stimulation for Parkinson’s disease is achieved by implanting electrodes in the subthalamic nucleus of the basal ganglia.
A pacemaker-like device is connected to the electrodes and implanted subcutaneously. The electrodes can then be stimulated by the pacemaker at regular intervals, leading to improvement in many of the symptoms of Parkinson’s disease.
An alternative approach that has gained widespread acceptance over the past decade involves another surgical method, deep-brain stimulation (DBS; Figure 8.27). DBS consists of implanting an electrode into a targeted neural region; for Parkinson’s disease, this is usually the STN, although some patients receive implants in the globus pallidus and others in the thalamus. A current is then passed through the electrode at high frequencies. This stimulation alters activity in the targeted region and throughout the circuit.
Why DBS works on Parkinson’s disease remains a mystery (Gradinaru et al., 2009). It is unclear which circuit elements are responsible for the therapeutic effects. The stimulation level is usually quite high, also creating unnatural activity levels in the nearby basal ganglia circuitry. As can be seen in Figure 8.23, stimulation of the STN should increase excitation of the globus pallidus and result in increased inhibition of the thalamus. Thus, DBS might have been expected to exacerbate parkinsonian symptoms. The mystery is that it doesn’t. One hypothesis is that the periodic output of the DBS stimulator provides a mechanism to normalize neural oscillations between the basal ganglia and cortex. By this view, it is not the overall level of activity that is important, but the pattern of activity.
Whatever the actual mechanism, it is now clear that DBS can be a very effective treatment for people with advanced Parkinson’s disease and for some individuals who do not respond to drug therapy. Indeed, the effects can be dramatic. With the stimulator off, the patient may be frozen in place, only able to initiate locomotion with great effort—and even then, taking tiny, shuffling steps. Turn on the device, wait 10 minutes, and the person is sprinting down the hallway. DBS has proven extremely popular: In its first decade of use, the procedure was performed on over 75,000 patients.
DBS is now used to treat a host of movement disorders such as tremor and dystonia (involuntary muscle spasms and twisting of the limbs). Clinical trials are now under way for many other uses, including chronic headache, Alzheimer’s disease, and even drug addiction (Lyons, 2011). Much of the focus here is on comparing the efficacy of different implant locations in the treatment of these disorders. As with Parkinson’s disease, we lack a clear understanding of why the treatment works in many of these cases (nor do we have enough data to verify the long-term benefit). But the demand for effective treatments is great. Sometimes it is beneficial to test new procedures once they have been deemed safe, even if we are unsure of their clinical efficacy.
TAKE-HOME MESSAGES
- The output from the basal ganglia, via thalamic projections, influences activity in the cortex, including the motor cortex.
- All of the output signals from the basal ganglia are inhibitory. Thus, in the tonic state, the basal ganglia dampen cortical activity.
- Movement initiation requires disinhibition: The striatal projection to the GPi inhibits an inhibitory signal, resulting in excitation at the cortex.
- Striatal neurons influence the output nuclei of the basal ganglia via the direct pathway and the indirect pathway.
- Dopamine is produced in the substantia nigra pars compacta, a brainstem nucleus that projects to the striatum. It has an excitatory effect on the direct pathway and an inhibitory effect on the indirect pathway.
- Parkinson’s disease results from cell death in dopamine-producing cells in the substantia nigra.
- Parkinson’s disease includes disorders of posture and locomotion, hypokinesia (the absence or reduction of voluntary movement), and bradykinesia (slowness in initiating and executing movement).
- The drug L-DOPA is used in treating Parkinson’s disease because it can compensate for the loss of endogenous dopamine.
- Deep-brain stimulation is a surgical technique in which electrodes are implanted in the brain. This procedure has become a novel treatment for Parkinson’s disease. Implants usually are placed in the subthalamic nucleus.
- The basal ganglia may play a general function in state changes. For the motor system, a state change would correspond to the initiation of a new movement. In the cognitive system, a state change could be a change in mental set, such as when we change from one goal to another. Dopamine acts as a reinforcement signal to bias some states over others.
Action Understanding and Mirror Neurons
Defining where perception ends in the brain and action starts may be an impossible task. Perceptual systems have evolved to support action; likewise, actions are produced in anticipation of sensory consequences. For a monkey in the wild, seeing a ripe banana on a tree engages the action systems required to retrieve the food—movements that allow the animal to climb skillfully among the branches and that result in the satisfying taste of the fruit.
A serendipitous observation in the laboratory of Giacomo Rizzolatti provided some of the most compelling evidence of the links between perception, action, and cognition, helping to launch one of the most exciting areas of research in the cognition of action. This research group was conducting a study of premotor cortex, recording from neurons that were involved in the control of hand and mouth actions. The story goes that a graduate student walked into the lab holding a cone of gelato. As he moved the cone to his mouth to lick it, a surge in cellular activity was observed in the monkey’s neuron that would be activated were the monkey to grasp and move something to his mouth, even though, in this instance, the animal was not moving. In fact, the animal seemed distracted, having shifted its focus to the grad student.
Rizzolatti and his colleagues had previously demonstrated that premotor cells show an increase in activity when the monkey performs goal-based actions, such as grasping or tearing an object, independent of the specific context for that action. As for the gelato incident, years later Rizzolatti commented, “It took us several years to believe what we were seeing” (Blakeslee, 2006). What they were seeing was that simply observing or imagining the action was all it took to activate some of the same premotor cells. For instance, they had monkeys view different objects. On some trials, the monkey produced an action such as reaching for or grasping the object (e.g., a peanut). On other trials, the monkey observed the experimenter performing similar actions. Although some premotor neurons were active only during production trials, other neurons were also active during action perception. Exactly the same neuron fired when an individual monkey observed the action of reaching for a peanut and when it performed the same action itself (Figure 8.28a–c). Perception and action were linked. These latter neurons were appropriately named mirror neurons.
You might suppose that the activity in mirror neurons reflects the similar visual properties of the action and perception conditions. A hand moving toward a peanut looks much the same whether it is your hand or someone else’s. Additional experiments, however, ruled out this hypothesis. First, the same mirror neuron is activated by the sound of a peanut being cracked (Figure 8.28d). Second, mirror neurons are also active when a monkey watches someone reach behind a screen for a peanut but cannot see the grasping of the peanut. In fact, there doesn’t even need to be a peanut behind the screen, as long as the monkey thinks that there is. If the monkey knows that there is no hidden peanut behind the screen, however, the mirror neurons remain silent (Umilta et al., 2001). Thus, the activity of the mirror cell is correlated with a goal-oriented action—retrieving a peanut—independent of how this information is received—by the monkey’s own action, by viewing another person’s action, by hearing another person’s action, or by viewing only a portion of another person’s action but believing that the action is taking place.
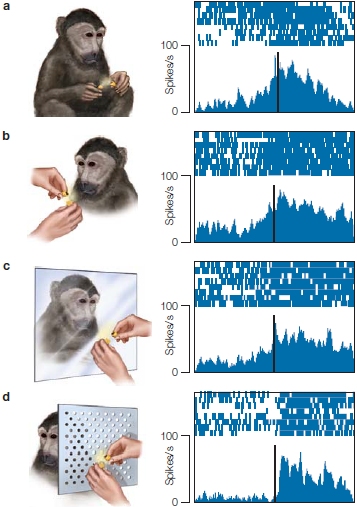
FIGURE 8.28 Identification of a mirror neuron.
Responses of a single neuron in a monkey’s ventral premotor cortex during the performance or perception of different actions: (a) when the monkey itself breaks a peanut and views and hears the breaking of the peanut, (b) when the monkey watches someone else breaking a peanut and views and hears the breaking of the peanut, (c) when the monkey sees someone else breaking a peanut but cannot hear the peanut breaking, and (d) when the monkey hears but does not see someone else breaking a peanut. This neuron is considered a mirror neuron because it responds to actions that are undertaken by the monkey, as well as to actions that are viewed or heard by the monkey.
The intimate link between perception and action is underscored by the finding that our comprehension of the actions of others appears to depend on the activation of the neural structures that would be engaged if we were to produce the action ourselves. In recognition of this codependency, neuroscientists speak of a mirror system to describe a distributed network of neural regions involved in action production and comprehension. The term mirror here is intended to capture the idea that understanding the actions of another person involves referring to our knowledge of how that action would be produced. The perceptual system is not divorced from the action system. The brain does not form abstract representations of visual patterns that conform to actions such as grasping, throwing, or dancing. Rather, our comprehension of such actions involves referring to our own ability to grasp an object or to dance with another individual. This notion of self-reference is sometimes referred to as embodied cognition: Our conceptual knowledge is grounded in our body knowledge.
Mirror neurons are not limited to the premotor cortex. Neurons in parietal and temporal lobes also show similar activity patterns during action production and comprehension, suggesting a distributed mirror system rather than a dedicated local region for linking perception and action. This point is supported by many neuroimaging studies in humans. In Chapter 6, we saw that the dorsal pathway, including parietal lobe and premotor cortex, was activated when people were asked to make judgments about the use of an object. These regions are also activated during movement execution. Interestingly, the extent and intensity of the activation pattern reflect the individual’s own particular motor repertoire. Skilled dancers show stronger activation in the mirror network when watching videos of familiar dance routines as compared to unfamiliar dances (Figure 8.29).
Imaging studies fail to show activation of the primary motor cortex during the observation of action. Even so, the excitability of neurons in motor cortex is modulated when people observe actions produced by another individual. Indeed, this modulation shows a high degree of effector specificity. When motor evoked potentials (MEPs) were recorded from muscles following transcranial stimulation of the motor cortex, their amplitude correlated with motor excitability. For example, TMS-elicited MEPs in hand muscles are larger when people observe video clips of gestures being made with the same hand as compared to videos of the same gestures by the opposite hand. Similar effects are elicited with relatively abstract presentations of the actions, such as the sounds of hands clapping.
The excitability changes within motor cortex also reflect the participants’ expertise. One study of action comprehension compared three groups of people: elite basketball players, sports journalists (selected because they watched basketball 7–8 hours a week), and a control group who knew nothing about basketball (Aglioti et al., 2008). The participants were shown short video clips, either of a person about to shoot a basketball free throw or initiate a free kick in soccer (Figure 8.30). The basketball players and the journalists both showed an increase in motor cortex excitability while watching the basketball shots, but not while watching soccer kicks. In contrast, the novices showed a nonspecific effect—an increase in hand MEPs for both basketball and soccer videos. Even more interesting, only the skilled players showed a differential response to whether the video clip depicted a free throw that was either going to be successful or inaccurate even before the outcome was known. This response suggests that, with expertise, the motor system has a fine sensitivity to discriminate good and poor performance during action observation, a form of action comprehension. It also suggests that the well-practiced motor system is anticipatory in nature, giving it the ability to predict others’ actions in the arena of their expertise.
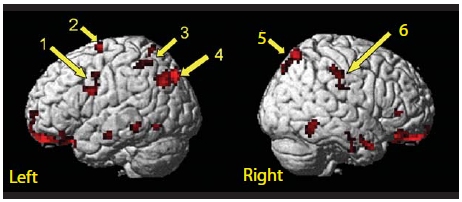
|
FIGURE 8.29 Activation of mirror neurons is affected by level of expertise.
When skilled dancers observe a dance they are experts in (versus a dance they have no expertise in), an increase in the BOLD response is observed in the premotor cortex (1, 2), intraparietal sulcus (3, 6), posterior superior temporal sulcus (4), and superior parietal lobe (5). These areas make up the neural network of action observation and include regions that are also activated when the person produces skilled movements, constituting what is considered the human mirror neuron system.
|
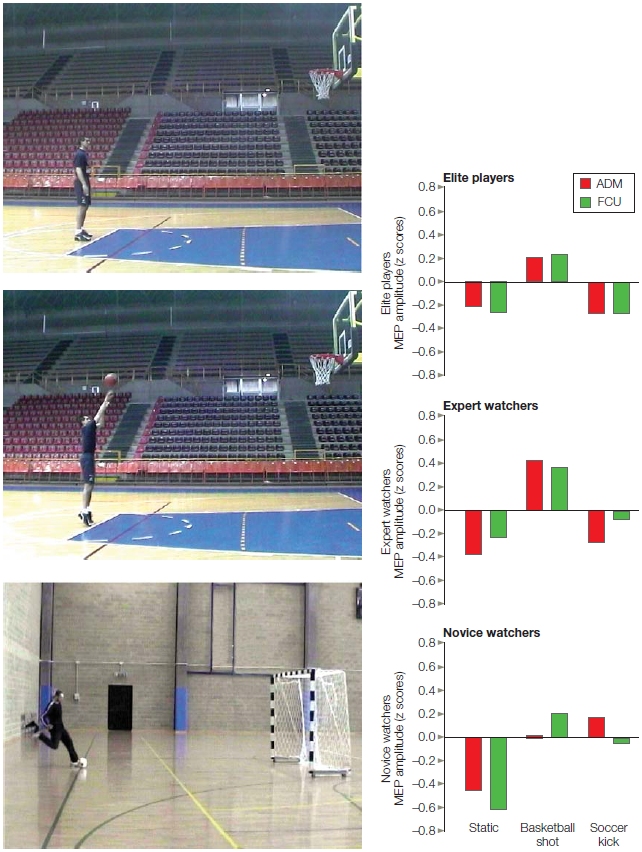
FIGURE 8.30 Increased excitation of motor cortex during action observation by skilled performers. Examples of photographs shown to elite basketball players, expert observers and novices while MEPs were recorded from hand muscles (ADM=abductor digiti minimi; red) and forearm (FCU=flexor carpi ulnaris; green) muscles. Relative to the static condition (top photo), the basketball players and expert observers showed an increase in hand and arm muscle MEPs when observing the player shooting a basketball, but not when shooting a soccer ball. The novices show a more inconsistent pattern, with an increase in excitability in one of the muscles when viewing the active images.
Mirror systems have been implicated in more than motor action understanding. Many neuroscientists argue that they are important for imitation and learning new skills and for simulating the actions of others, leading to understanding their intentions. What’s more, by simulating the emotions of others, mirror systems provide the neural basis for empathy. We will discuss these ideas in Chapter 13.
Is the activation that is seen in motor areas during observation of action essential for comprehending action? Does the modulation of excitability in motor cortex indicate that understanding the actions of another requires representations in motor cortex? Or are these activation patterns some sort of priming effect, reflecting the subtle and automatic planning of the action when presented with a familiar stimulus? These are difficult questions to answer (see Hickok, 2009). Nonetheless, fMRI and TMS studies are important in demonstrating the degree of overlap between neural systems involved in perception and action. They remind us that dividing the brain into perception and motor regions may be useful for pedagogical reasons (say, for defining chapters in a textbook), but that the brain does not honor such divisions.
TAKE-HOME MESSAGES
- Mirror neurons are neurons in premotor cortex and other areas (like the parietal lobe) that respond to an action, both when that action is produced by an animal and when the animal observes a similar action produced by another animal.
- The mirror system has been hypothesized to be essential for comprehending the actions produced by other individuals.
- The engagement of the mirror system is modulated by motor expertise.
Learning and Performing New Skills
Dick Fosbury was a revolutionary figure in the world of sports. In high school, he was a very good high jumper, though not quite good enough to get the scholarship he desired to go to college and study engineering. One day, however, he had an idea. His school had recently replaced the woodchip landing pad in the high-jump pit with soft foam rubber. Fosbury realized that he no longer had to land on his feet to avoid injury. Instead of taking off on the inside foot and “scissoring” his legs over the bar, he could rotate his body to go over the bar backward, raising his feet toward the sky, and then land on his back. With this conceptual breakthrough, Fosbury went on to reach new heights, culminating in the gold medal at the 1968 Olympics in Mexico City. High jumpers all over the world adopted the “Fosbury flop.” And yes, Fosbury did get his scholarship and became an engineer.
Shift in Cortical Control with Learning
People frequently attribute motor learning to low levels of the hierarchy. We speak of “muscle memory,” or our muscles having learned how to respond—for example, how to maintain balance on a bike, or how our fingers type away at the keyboard. The fact that we have great difficulty verbalizing how to perform these skills reinforces the notion that the learning is noncognitive. The Olympic gymnast Peter Vidman expressed this sentiment when he said, “As I approach the apparatus... the only thing I am thinking about is... the first trick.... Then, my body takes over and hopefully everything becomes automatic” (Schmidt, 1987, p. 85).
On closer study, however, we find that some aspects of motor learning are independent of the muscular system used to perform the actions. Demonstrate this independence to yourself by taking a piece of paper and signing your name. Having done this, repeat the action but use your nondominant hand. Now do it again, holding the pen between your teeth. If you feel especially adventurous, you can take off your shoes and socks and hold the pen between your toes.

FIGURE 8.31 Motor representations are not linked to particular effector systems.
These five productions of the words Cognitive Neuroscience were produced by the same person moving a pen with the right hand (a), the right wrist (b), the left hand (c), the mouth (d), and the right foot (e). The productions show a degree of similarity, despite the vast differences in practice writing with these five body parts.
Although the atypical productions will not be as smooth as your standard signature, the more dramatic result of this demonstration is the high degree of similarity across all of the productions. Figure 8.31 shows the results of one such demonstration. This high-level representation of the action is independent of any particular muscle group. The differences in the final product show that some muscle groups simply have more experience in translating an abstract representation into a concrete action.
When people are acquiring a new action, the first effects of learning likely will be at a more abstract level. Fosbury’s learning started in the abstract realm with a simple insight: The new landing material could allow for a different landing. From this point, he was able to adopt a radically new style of jumping. As Fosbury describes it, “I adapted an antiquated style and modernized it to something that was efficient” (Zarkos, 2004). These cognitive abilities no doubt apply to all types of learning, not just learning motor skills. For instance, the same abilities would contribute to the makings of a great jazz improvisationist. She is great not because of the technical motor expertise of her fingers (though that is important), but because she sees new possibilities for a riff, a new pattern.
Once Fosbury had settled on what to do, he had to learn to do it. Our motor system has some basic movement patterns down. Learning to perform a new action builds on these basic patterns. Learning the skill takes practice—what we typically mean when we talk about motor learning. Motor learning can involve linking a series of gestures in a completely new way. Or it may involve a more subtle retuning, repeating a learned sequence over and over to get the coordination pattern exactly right. The latter is frequently referred to as motor adaptation. Gradually the motor system learns to execute the movement in what feels like an automatic manner, requiring little conscious thought.
Learning how to produce the action in an optimal manner—becoming an expert—takes us to a different level of skill. Becoming an expert fine-tunes the system to make the movement in the most efficient and skillful manner. This result requires other cognitive abilities, such as persistence, attention, and self-control. Motor skill also involves honing perceptual skills. LeBron James’s skill on the basketball court is due not only to his extraordinary motor skills but also to his ability to rapidly recognize the position of his teammates and opponents. His pattern recognition abilities allow him to quickly determine if he should drive to the basket or pull up and pass to one of his open teammates. Becoming skillful at any task can be acquired only through practice, and a lot of it. In fact, the rule of thumb is that expertise in any domain requires at least 10,000 hours of practice. Ready to become an expert at something? Got 3 hours a day to devote to that activity for the next 10 years? That’s what you’ll need.
Adaptive Learning Through Sensory Feedback
Imagine climbing aboard a boat that is rocking in the waves. At first you feel clumsy, unwilling to let go of the gunwales, but soon you adapt, learning to remain steady despite the roll of the boat. Next, you’re even willing to venture a few steps across the deck. After a few hours at sea, you’re an old salt, not giving a thought to the pitch and roll of the boat. When you come back to shore, you are surprised to find your first few steps are wobbly again. It takes a moment or two to become acclimated to the stability of the dock, and to abandon your rolling gait.
This example is a form of sensorimotor adaptation. Researchers have devised all sorts of novel environments to challenge the motor system and explore the neural mechanisms essential for this form of motor learning. One of the first and most radical tests was performed by George Stratton, the founder of the psychology department at the University of California, Berkeley. He devised a set of eyeglasses that inverted the visual input. After initially donning his new spectacles, Stratton was at a loss, afraid to take a step for fear he would fall over. Reaching was impossible. He would reach for a glass and observe his arm moving in the wrong direction. But with time, Stratton’s motor system adapted (just as the monkeys in BMI studies did when the decoder algorithm was shuffled). By the fourth day, he was walking about at a nearly normal speed and his movements were coordinated. With time, observers were hard-pressed to realize from watching Stratton that his world was topsy-turvy. His sensorimotor system had adapted to the new environment.
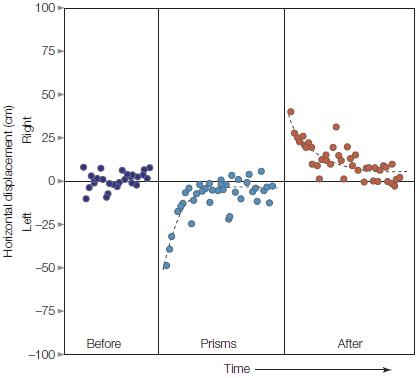
|
FIGURE 8.32 Prism adaptation.
Participants throw a ball, attempting to hit a visual target. At baseline (“Before”), the responses are scattered about the target. After putting on the prism glasses, the throws are shifted to the left. After about 20 throws, the person becomes adapted to the glasses and is again successful in landing near the target. When the glasses are removed, the person makes large errors in the opposite direction. This aftereffect eventually disappears as the person “de-adapts.”
|
More modern studies of sensorimotor adaption use less dramatic environmental distortions. In some, visuomotor rotations are imposed when people perform the center-out task such that the visual feedback of the limb is displaced by 30 degrees, introducing a mismatch between the visual and proprioceptive (felt position of the limb) information (Figure 8.32). In others, force fields are imposed that displace the moving limb to the side when a person attempts to reach directly to a target. The motor system is amazingly adept at modifying itself in response to these perturbations. Within a hundred movements or so, people have modified their behavior and make straight movements to the targets. Although they were aware that the environment had been altered with the introduction of the perturbation, the system quickly adapts, and the person is soon unaware of the change. This becomes obvious when the perturbation is removed, and the person has to repeat the adaptation process (or what is called de-adaptation, just as when you step from a boat back onto the dock). We cannot simply “switch back” to the normal state, but rather must relearn how to control our limbs in the absence of a visual or force distortion.
Neural Mechanisms of Adaptation
Cognitive neuroscientists have employed many tools to explore the neural systems of sensorimotor learning. Imaging studies show that with the introduction of a perturbation, such as a visuomotor rotation, there is a large increase in activity in many cortical areas, including prefrontal, premotor, and motor cortex in the frontal lobes, as well as changes in parietal, temporal, and even visual cortex (Seidler, 2006). Increases are also seen subcortically in the cerebellum and basal ganglia. With practice, the activation in these areas is reduced, returning back toward that observed when you move without a perturbation.
Knowing exactly how to interpret these activation patterns is difficult: Do they reflect the formation and storage of new motor patterns? Or are the activations indicative of other processes that are engaged when a perturbation is introduced? For example, a visuomotor rotation introduces a violation of a visual expectancy—you expect the cursor to move up, but it moves to the side: the activations could be the result of this prediction error, or they may reflect the increased attention needed to adjust to the visual feedback (see Chapter 7). Motor cortex changes could be the result of adaptation, or they could result because people tend to make corrective movements when the feedback indicates an error. Other activations may be triggered by the participants’ awareness that the environment has been distorted.
To gain more insight into the functional contribution of the different areas identified in the imaging studies, researchers have conducted neuropsychological and brain stimulation studies. For instance, patients who have cerebellar damage due to either degenerative processes or stroke have severe impairments in learning to move in novel environments, such as when a visuomotor perturbation is introduced (Figure 8.33; T. Martin et al., 1996). Similar problems can be observed in patients with prefrontal or parietal lesions.
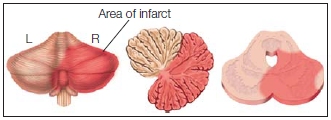
|
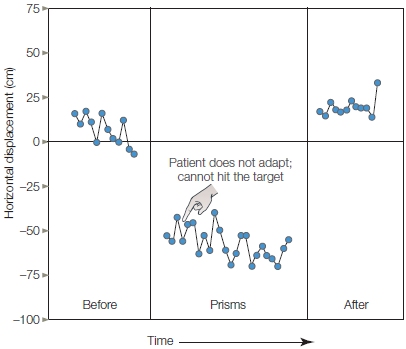
|
|
FIGURE 8.33 Impaired prism adaptation in a patient with a large cerebellar lesion.
The shaded regions in the top figures show the extent of damage in the inferior cerebellum. The damage is mostly in the right cerebellum, although it extends across the midline. There is no evidence of adaptation when wearing the prism glasses. The patient shows a bias to throw the ball slightly to the right before and after adaptation.
|
Can we identify differential contributions of these neural regions? Joseph Galea and his colleagues (2011) applied transcranial direct current stimulation (tDCS) during a visuomotor adaptation task, targeting either primary motor cortex or the cerebellum. As discussed in Chapter 3, this procedure is thought to increase the excitability of the area under the anodal electrode. Assuming that more excitable neurons are also better for learning (e.g., more “plastic” as described in Chapter 9), the researchers considered two hypotheses. First, if an area is involved in using the error information to modify the sensorimotor system, then learning to compensate for the visuomotor perturbation should occur more quickly. Second, if an area is involved in retaining the new behavior, the effects of learning should persist for a longer period of time, even when the perturbation is removed. To look at retention in this study, the feedback was removed and the experimenters measured how long it took for the person to show normal reaching movements.
The results point to a striking functional dissociation between the cerebellum and motor cortex (Figure 8.34). Cerebellar tDCS led to faster learning. Participants receiving stimulation over this region learned to compensate for the visuomotor perturbation faster than those receiving tDCS over M1 or sham stimulation over the cerebellum in which the stimulator is turned on for only a few seconds. When the rotation was removed, however, the effects of learning decayed (or were implicitly “forgotten”) at the same rate as for the sham group. The opposite pattern was observed for the group receiving M1 tDCS. For these participants, learning occurred at the same rate as those given sham stimulation, but the retention interval was extended. In sum, results indicate that the cerebellum is essential for learning the new mapping, but M1 is important for consolidating the new mapping (long-term retention).
|
FIGURE 8.34 Double dissociation in sensorimotor adaptation following tDCS of the cerebellum and motor cortex.
Anodal tDCS was applied during a baseline phase (Pre2) and throughout the adaptation phase in which the visual feedback was rotated. Learning was faster when the tDCS was applied over the cerebellum, compared to the sham and M1 conditions, although all three groups eventually reached comparable levels of adaptation. When the rotation was removed, the aftereffect persisted for a longer time when tDCS was applied over M1, suggesting stronger consolidation in this condition.
|
Earlier in the chapter, we discussed the role of dopamine in reinforcement learning, focusing on the projections from the substantia nigra to the striatum. Dopamine terminals are also scattered across the cerebral cortex, including in M1. The origin of these fibers, however, is not in the SNr; it is in a different brainstem nucleus, the ventral tegmental area (VTA; Chapter 12). To determine if these dopamine neurons are important for motor learning, one study placed rats into a specialized apparatus in which they could retrieve food pellets by making a reaching movement with their forelimb (Hosp et al., 2011). This task was challenging for the rats—reaching is not a typical part of their motor repertoire. They typically use their forelimbs for locomotion (being quadrupeds) or to hold pellets of food. Nonetheless, when motivated by extra tasty food pellets, the animals were able to maneuver their forelimbs to grasp a pellet and bring the morsel to their mouth. Animals with lesions of the VTA were unable to learn the task. If L-DOPA was then directly applied to M1, however, the animals recovered their ability to learn the novel reaching movements (Figure 8.35). Thus, the dopaminergic pathway from the VTA to M1 is necessary for acquiring a novel motor skill through repeated training.
FIGURE 8.35 Destroying VTA dopaminergic neurons prevents learning a motor skill.
Rats were trained to retrieve a food reward by reaching with their forepaws, a difficult task for a rodent. Two groups of animals received lesions of the VTA, eliminating a primary source of dopamine to the motor cortex. Whereas animals with sham lesions became relatively proficient with practice, the VTAlesioned animals failed to improve. Starting with the ninth day of training, the sham animals and one of the lesioned group received L-dopa injections into M1. The lesioned animals now improved, consistent with the idea that dopamine release in the cortex is important for motor skill learning. Performance in lesioned animals remained stable when the injections were discontinued (blue background).
Forward Models: Using Sensorimotor Predictions for Motor Control and Learning
You may have had the experience of walking down a set of stairs in the complete dark and thinking that you had stepped off the last stair when, in fact, there was another to go. Your body already had automatically adjusted its balance in preparation for stepping across level ground, but lo and behold, you sank another 8 inches. If you were fortunate, you quickly adjusted your balance and corrected your movement. If not so fortunate, you ended up falling or twisting an ankle. This example captures how the brain operates in a predictive mode: Your motor system is issuing commands for movement, and it is also generating predictions of the anticipated sensory consequences of those movements. Errors occur when the actual feedback doesn’t match this prediction. The brain uses this information to make adjustments to an ongoing movement as well as for learning.
Prediction is especially important because the brain is working with a system in which the motor commands to the muscles and sensory signals from the limbs take time to travel back and forth. It can take 50 to 150 ms for a motor command to be generated in the cortex and for the sensory consequences of that action to return to the cortex. By then, things in the periphery will have changed, especially if the signals involve moving parts. For skilled motor behavior, that time lag is enough to throw off smooth, coordinated movement. To compensate for these delays, we have a system that generates an expectancy of the sensory consequences of our action, or what is referred to as a forward model.
The cerebellum is a key part of the neural network for the generation of forward models (Wolpert et al., 1998). It receives a copy of motor signals being sent to the muscles from the cortex, information that can be used to generate sensory predictions. It also receives massive input from the various receptors of the somatosensory system. By comparing these sources of information, the cerebellum can help ensure that an ongoing movement is produced in a coordinated manner. It can also use a mismatch to aid in sensorimotor learning. For example, when we put on prism glasses, the visual information is shifted to one side. If we reach to a target, a mismatch will occur between where the hand was directed and our visual (and tactile) feedback of the outcome of the movement. Given sufficient time, we use that error to correct the movement to reach the target. The error is also used to correct future predictions, thus, adapting learning such that we make more predictions that are suited for this novel environment. Consider again the tDCS results discussed in the previous section. Cerebellar stimulation led to faster learning, presumably because the error signals were amplified. Imaging studies of motor learning support a similar conclusion. In general, activation in the cerebellum decreases with practice, a finding interpreted as reflecting a reduction in error as skill improves.
As noted earlier, forward models are also important for online control of movements. People with ataxia are capable of making movements; they can select the right muscles and activate them in the right sequence. Their movements are far from smooth, however. The concept of the forward model can be useful for understanding this loss of coordination. Consider what would happen if motor commands were based on outdated sensory signals; say, for example, the system had to work with the actual sensory signals instead of the expected sensory signals. If you were to reach rapidly for a target, you would overshoot the goal because you failed to slow your hand in an anticipatory manner. Damage to the spinocerebellum frequently results in hypermetric movements, those that extend beyond the intended target or that oscillate around the target location (Hore et al., 1991). Prediction is especially important when producing complex actions that require coordination across multiple joints. Ataxia is especially pronounced in such situations, underscoring why this deficit is frequently described as a loss of skilled movement.
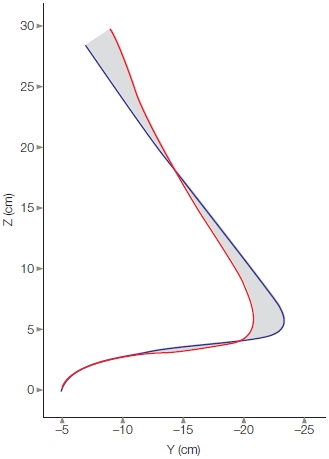
FIGURE 8.36 Predictive function of the cerebellum.
Participants performed a two-step task, first moving their arm laterally (y-direction) and following the onset of a tone, to reach towards an unseen target positioned in front of them (z-direction). Because the arm is moving, the participant must estimate where the arm will be at the start of the forward component of the movement. On control trials (blue), the final position of the hand was slightly displaced to the right of the target. This error was much larger when TMS was applied over the lateral cerebellum, suggesting that the participants failed to fully anticipate the lateral displacement of their arm.
Chris Miall and his colleagues (2007) provided an elegant demonstration of the role of the cerebellum in the utilization of a forward model. They designed a task in which participants were shown a visual target located in front of them. Each participant was then required to move her right arm to the side until she heard a tone. The tone signaled that she should now move as quickly as possible to the target. To accomplish this task, the participant’s motor system must anticipate that, due to momentum, her arm actually would be displaced a bit farther sideways before she initiated the forward reach. In short, she has to predict where her arm actually will be when she hears the reach command. In a normal context, the participants had no difficulty reaching the target, even when visual feedback of the reach was eliminated. When transcranial magnetic stimulation (TMS) was applied over the cerebellum, however, the participants’ reaches missed the target. Their hands landed at a location that indicated they were using outdated sensory information to plan the movement (Figure 8.36). In combination with the work on motor learning, we now see how the cerebellum uses forward models for coordinating ongoing movements as well as for motor learning.
Prediction is a feature of all brain areas (everything is doing pattern matching of some sort). In addition, it has also been hypothesized that the cerebellum is also critical for sensorimotor learning, because it generates predictions that are temporally precise. We need to know more than what is coming in the future; we also need to predict exactly when it is coming. When going down the stairs, we anticipate the contact of our foot with a surface at a specific moment in time. Though cortical areas primarily select the effectors needed to perform a task, the cerebellum supplies the precise timing needed for activating these effectors.
The timing hypothesis offers another way to think about the role of the cerebellum in motor learning. Cerebellar lesions are most disruptive to highly practiced movements, which present the greatest need for precise timing. The novice tennis player may be pleased if he can simply get the ball over the net, but the expert requires exquisite timing to make the perfect shot.
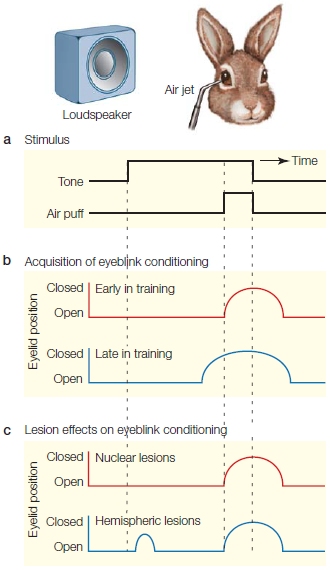
FIGURE 8.37 Lesions of the cerebellum disrupt the learned response in eyeblink conditioning.
(a) A neutral tone precedes and co-terminates with an aversive air puff to the eye. (b) Early in training, the air puff causes the animal to blink. Late in training, the animal blinks in response to the tone, thus reducing the impact of the air puff. (c) Lesions of the deep cerebellar nuclei abolish the learned response. The animal continues to blink reflexively in response to the air puff; this behavior indicates that the lesion has produced a learning deficit and not a motor deficit. The anticipatory, learned responses are still present following lesions of the cerebellar cortex. However, they are timed inappropriately and thus are no longer adaptive.
This point is highlighted in an experiment involving a simple model of motor learning: eyeblink conditioning. When a puff of air is directed at the eye, a reflexive blink is produced—an evolved response to minimize potential eye damage. If a neutral stimulus, such as a tone, is presented in advance of the air puff on a consistently timed basis, the animal learns to blink in response to the tone (Figure 8.37). What’s more, the timing of the acquired response is perfectly adaptive: The eye closure reaches the highest amplitude exactly at the onset of the air puff. As Figure 8.37c shows, rabbits with cerebellar lesions have no motor problem and continue to blink to the tone, but the response is no longer appropriately timed: The eye is exposed at the time of the air puff and, thus, the blink is no longer adaptive in avoiding the air puff (S. Perrett et al., 1993). At a computational level, the timing hypothesis helps specify how the cerebellum contributes to motor learning. It is important for the animal to learn that the tone and air puff co-occur, but the response is adaptive only if the animal learns that the tone predicts exactly when the air puff will occur. The animal must be able to represent the temporal relationship between the two stimuli.
Experts
How do experts differ from nonexperts (Figure 8.38)? The multitalented Francis Galton, Charles Darwin’s cousin, opined that it required innate ability, zeal, and laborious work (remember the 10,000 hours of practice?) to become eminent in a field (see Ericsson et al., 1993). Do experts have brains that differ in both structure and function? Are these differences innate, the result of extensive practice, or some combination of nature and nurture?
Neuroanatomists have identified some realms of skilled performance that are associated with structural differences. Studies using diffusion-weighted MRI have found evidence that the connectivity in a specific region of the corpus callosum between the left and right supplementary motor areas varies between individuals. The degree of bimanual coordination that a person exhibits correlates positively with the connectivity between the two regions (Figure 8.39; Johansen-Berg et al., 2007). Certainly an interesting observation, but it tells us nothing about causality. Did the person become more coordinated because of the stronger connectivity, or has this difference in connectivity emerged because she engages in more bimanual activities, perhaps because she finds them more rewarding?

FIGURE 8.38 Humans show an extraordinary ability to develop motor skills.
|
FIGURE 8.39 Relating motor skill to brain anatomy.
Participants performed a bimanual coordination task, producing alternating taps with the two fingers. The tapping rate was varied such that, at high frequencies, the participants had trouble maintaining the pattern. Measures of FA in voxels from the body of the corpus callosum (left) correlated with bimanual coordination (high ratio indicates better performance). Red circles indicate female participants and the blue circles are male.
|
|
To get at causality, researchers have looked at changes that occur in the brain after extensive practice. Consider juggling, a skill that requires the coordination of the two hands, not to mention the ability to integrate complex spatial patterns created by the motions of the hands and balls. To the novice, juggling may seem impossible; but with just a modest amount of daily practice, most people can become quite skilled after a few months. This level of practice in one sample was sufficient to produce measurable increases in gray matter in areas V5 and IP—temporal and parietal regions associated with motion processing and movement planning and control (Draganski et al., 2004). When the jugglers stopped practicing, the gray matter volume in these regions of interest shrank, although it remained above the baseline level. Findings like these indicate that practice can readily shape the macroscopic landscape of the brain.
Our parents and teachers may often remind us that practice makes perfect, but it is also hard not to argue that other factors are at play in determining expertise. Some individuals just seem to be more adept at certain skills. Some differences may reflect genetic differences, or gene–environment interactions. Genetic polymorphisms have been associated with physiological differences that affect oxygen uptake and consumption, cardiac output, and muscle type and strength. How much these factors contribute to an individual becoming an elite athlete is yet to be determined, but it looks like Galton’s intuition was on the right track.
One factor we tend to ignore when thinking about skilled performance is the importance of motivation. We consider how genetics might influence muscle size (and height if we are thinking about a sport like basketball), but there are also large individual differences in motivation: Some people are more willing to put in hours of practice than others. Although Galton defined motivation as “zeal,” a more modern notion is that motivation is about the importance we place on action outcomes and their utilities (Niv, 2007). In other words, is it worth the effort? How much do we value the goal and its predicted reward relative to the cost we have to expend? Worth is subjective and has many variables, and in Chapter 12 we will consider this issue in detail. An interesting study of musical performers revealed that the most elite performers actually found practice less pleasurable than nonelite, but skilled, performers (Ericsson et al., 1993). One inference drawn from this work is that expertise requires not just hours of practice, but effortful practice in which the performer is constantly pushing him or herself to explore new methods or endlessly repeating the selected routine.
It is clear that experts, amateurs, and novices have different brains. Researchers find it easier to identify structural differences in experts in a physical activity—compared to, say, experts in theoretical physics—perhaps because we have a good idea of where we might expect to observe such differences. We can look in the hand area of the right motor cortex to see structural differences between violin players and musicians who play instruments that do not place such emphasis on left-hand fingering skills. Even so, we should be cautious in assuming such differences are at the heart of expertise. Across domains as diverse as motor skills, mathematics, and the arts, many commonalities are found among the most elite performers. A good explanation of the neural correlates of these commonalities has yet to be articulated.
TAKE-HOME MESSAGES
- Sensorimotor learning is improvement, through practice, in the performance of motor behavior.
- The cerebellum is critical for error-based learning. Errors are derived from a comparison of the predicted and observed sensory information. The errors are used to update a forward model, a representation that can be used to generate the sensory expectancies for a movement.
- The predictive capacity of the cerebellum is also important for online control. By anticipating the sensory consequences of movement, it helps compensate for delays introduced by sensorimotor processing.
- The primary motor cortex is critical for the long-term retention of skills. Consolidation is enhanced by dopaminergic input to the motor cortex from the ventral tegmental area of the brainstem.
- Skill requires extensive hours of practice. Expertise is skill specific, but it may be more closely related to domain-independent factors such as motivation rather than a propensity, or inclination, for particular types of performance.
FIGURE 8.40 Functional architecture of the motor system.
(a) Major neural structures, partitioned into areas associated with the planning and execution of movement. (b) Functional hypotheses for how these different structures contribute to actions.
Summary
Cognitive neuroscience has had a major impact on our conceptualization of how the brain produces skilled action. Consider the two halves of Figure 8.40. The diagram in Figure 8.40a, first introduced in 1974, shows the critical circuits of the motor pathway, emphasizing patterns of anatomical connectivity with a crude partitioning of function into motor planning, movement preparation, and movement execution. Figure 8.40b retains the basic circuitry but offers a functional decomposition of the processes involved in planning and programming.
As Figure 8.40 shows, the control of action involves several distributed systems. Nonetheless, this distributed pattern does not necessarily suggest that all of the systems operate in a similar way. As with other processing domains, like attention and memory, the different motor structures have their unique specializations. The cortical pathways for movement selection are biased to provide particular sources of information. The subcortical loops through the basal ganglia and cerebellum are essential for movement preparation, but in quite different ways.
By specifying a functional role for these structures, we can appreciate the limitations of brain theories that focus on the task rather than on the internal computations. For example, focusing on the fact that skilled movements are especially disrupted in patients with cerebellar lesions might lead us to conclude that the representation of an action shifts from one neural locus to another with practice. When the cerebellum is viewed as a structure that is specialized to represent the temporal properties of a movement, however, we can see that the loss of skilled movements is a consequence of a breakdown in the fine timing. This computation would not be as important during the early phases of skill acquisition, when the person builds the representations that underlie the skill. Moreover, this functional analysis makes it clear that the boundaries between perception and action are murky. In the same way that the parietal lobe is essential for both perceiving and acting in space, the timing functions of the cerebellum or shifting functions of the basal ganglia are not restricted to motor control. And don’t forget the motor chauvinists’ taunt that perceptual information is useful only to the extent that it facilitates behavior.
Key Terms
alpha motor neuron (p. 330)
apraxia (p. 336)
ataxia (p. 333)
basal ganglia (p. 333)
bradykinesia (p. 358)
brain–machine interface (BMI) (p. 352)
central pattern generator (p. 338)
cerebellum (p. 332)
corticospinal tract (CST) (p. 334)
deep-brain stimulation (DBS) (p. 362)
effector (p. 330)
endpoint control (p. 340)
extrapyramidal tract (p. 332)
forward model (p. 371)
hemiplegia (p. 335)
Huntington’s disease (p. 358)
hypokinesia (p. 358)
ideational apraxia (p. 336)
ideomotor apraxia (p. 336)
mirror neuron (p. 363)
mirror system (p. 363)
Parkinson’s disease (p. 358)
population vector (p. 342)
preferred direction (p. 342)
premotor cortex (p. 336)
primary motor cortex (M1) (p. 335)
sensorimotor adaptation (p. 367)
sensorimotor learning (p. 368)
spinal interneurons (p. 331)
substantia nigra (p. 328)
supplementary motor area (SMA) (p. 336)
visuomotor adaptation (p. 369)
Thought Questions
- Viewed both from a functional perspective and from a neuroanatomical/neurophysiological perspective, motor control is organized hierarchically. Outline this hierarchy, starting with the most basic or primitive aspects of motor behavior and progressing to the highest level or most sophisticated aspects of motor behavior.
- What is the difference between the pyramidal and extrapyramidal motor pathways? What type of movement disorder would you expect to see if the pyramidal tract were damaged? How would extrapyramidal damage differ?
- Explain the concept of the population vector. How could it be used to control a prosthetic (artificial) limb?
- Why do people with Parkinson’s disease have difficulty moving? Provide an explanation based on the physiological properties of the basal ganglia. How does dopamine replacement therapy or deep brain stimulation improve their condition?
- When we first learn a skill such as skiing, it helps to listen to the instructor provide step-by-step guidance to make a turn. With practice, the action becomes effortless. What changes, both psychologically and neurally, do you expect take place as you move from beginner to expert?
Suggested Reading
Chase, V. D. (2006). Shattered nerves: How science is solving modern medicine’s most perplexing problem. Baltimore, MD: Johns Hopkins University Press.
Cisek, P., & Kalaska, J. F. (2010). Neural mechanisms for interacting with a world full of action choices. Annual Review of Neuroscience, 33, 269–298.
Lebedev, M. A., & Nicolelis, M. A. (2006). Brain–machine interfaces: Past, present, and future. Trends in neurosciences, 29, 536–546.
Rizzolatti, G., Fogassi, L., & Gallese, V. (2000). Cortical mechanisms subserving object grasping and action recognition: A new view on the cortical motor functions. In M. Gazzaniga (Ed.), The new cognitive neurosciences (2nd ed., pp. 539–552). Cambridge, MA: MIT Press.
Shadmehr, R., & Wise, S. P. (2005). The computational neurobiology of reaching and pointing. Cambridge, MA: MIT Press.
Yarrow, K., Brown, P., & Krakauer, J. W. (2009). Inside the brain of an elite athlete: The neural processes that support high achievement in sports. Nature Reviews Neuroscience, 10, 585–596.