|
Practically everybody in New York has half a mind to write a book, and does.
~ Groucho Marx
|
Chapter 4
Hemispheric Specialization
OUTLINE
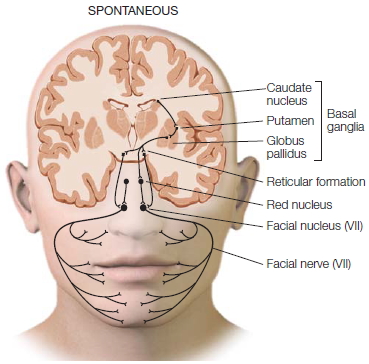
Anatomy of the Hemispheres
Splitting the Brain: Cortical Disconnection
Hemispheric Specialization
The Evolutionary Basis of Hemispheric Specialization
Split-Brain Research as a Window into Conscious Experience
IT WAS 1961, and W.J., a charismatic war veteran, had been suffering two grand mal seizures a week for the previous 10 years. After each seizure subsided, it took him a full day to recover. Although he otherwise appeared perfectly normal, possessed a sharp sense of humor, and charmed all who met him, the seizures were creating havoc in his life. He was willing to try anything that might improve his situation. After critically reviewing the medical literature, a neurosurgery resident, Dr. Joseph Bogen, suggested that W.J. would benefit from a rarely performed surgical procedure that would sever the corpus callosum, the great fiber tract that connects the right and left cerebral hemispheres. A similar procedure had been done successfully 20 years earlier on a series of patients in Rochester, New York. None of these patients reported ill side effects, and all had improvement in seizure control (Akelaitis, 1941). Psychological studies of these patients before and after their surgeries revealed no differences in their brain function or behavior. The concern was that more recent studies of animals that had undergone split-brain procedures told a different story. Cats, monkeys, and chimps with callosal sections had dramatically altered brain function. Nonetheless, W.J. was willing to risk the procedure. He was desperate. In the days following his surgery, it became obvious that the procedure was a great success: W.J. felt no different, and his seizures were completely resolved. His temperament, intellect, and delightful personality remained unchanged. W.J. reported that he felt better than he had in years (Gazzaniga et al., 1962).
Because of the results garnered from the animal experiments, it was puzzling that humans apparently suffered no effects from severing the two hemispheres. Ever the gentleman, W.J. submitted to hours of tests, both before and after the surgery, to help solve this mystery. Using a new method, one of the authors (MSG) devised a way to communicate with each hemisphere separately. This method was based on the anatomy of the optic nerve. The nerve from each eye divides in half. Half of the nerve fibers cross and project to the opposite hemisphere, and the other half projects to the ipsilateral hemisphere (Figure 4.1). The parts of both eyes that view the right visual field are processed in the left hemisphere, and the parts that view the left visual field are processed in the right hemisphere. Thus, if all communication is severed between the two halves of the cerebral cortex, then information presented just to the right visual field would feed into the left side of the brain only, and information presented to the left visual field would be sent to the right side of the brain only, and neither would have access to the other. This type of test had not been tried on the Rochester patients.
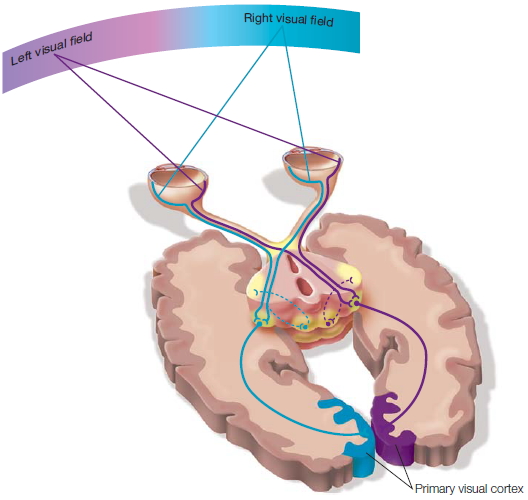
FIGURE 4.1 The optic nerve and its pathway to the primary visual cortex.
Before surgery, W.J. could name objects presented to either visual field or objects placed in either of his hands, just like you can. He could understand a command and carry it out with either hand. Would the results be the same after the surgery? Because our speech center is in the left hemisphere, it was expected that W.J. would be able to name items presented to his right visual field and were sent to his left hemisphere. Earlier testing done in Rochester suggested that the corpus callosum was unnecessary for interhemispheric integration of information. If that were true, then W.J. should also be able to report what was flashed to his left visual field and sent to his right hemisphere. First, a picture of a spoon was flashed to his right visual field; he said “spoon.” Then the moment arrived for the critical test. A picture was flashed to his left visual field, and he was asked, “Did you see anything?” To the amazement of all present he replied, “No, I didn’t see anything.”
At first it appeared that W.J. was blind to stimuli presented to his left visual field, but it soon became clear that this was not the case. Tweaking the experimental technique, the investigators allowed W.J. to respond by using a Morse code key with his left hand (the right hemisphere controls the left hand) rather than with a verbal response. He responded by pressing the key with his left hand when a light was flashed to his left visual field (hence the right hemisphere), but he stated (his left hemisphere talking) that he saw nothing.
The more tests that were done, the more remarkable were the findings: W.J.’s right hemisphere could do things that his left could not do, and vice versa. For example, the two hemispheres were strikingly different in performance on the block design task shown in Figure 4.2. Previously, W.J. had been able to write dictated sentences and carry out any kind of command, such as making a fist or drawing geometric shapes with his right hand. After surgery, though, he could not arrange four red and white blocks in a simple pattern with his right hand. We will see later that the surgery had disconnected specialized systems in the right hemisphere from the motor apparatus in the left hemisphere, which in turn controls the right hand. Even when given as much time as needed, W.J. was unable to perform the task with his right hand, because motor commands specific to the task could not be communicated from the isolated left hemisphere.
W.J.’s right hemisphere, however, was a whiz at this type of test. When blocks were presented to his left hand (controlled by his right hemisphere), he quickly and adeptly arranged them into the correct pattern. This simple observation gave birth to the idea that “Mind Left” and “Mind Right” do different things, supporting the idea that the central nervous system is laterally specialized: Each of the two cerebral hemispheres performs processes that the other does not.

FIGURE 4.2 The block design test.
The pattern in red on the right is the shape that the patient is trying to create with the blocks given to him. (a) With his right hand (left hemisphere), he is unable to duplicate the pattern. (b) With his left hand (right hemisphere), he is able to perform the task correctly.
After the first testing session revealed this separation so clearly, investigators arranged to film W.J. carrying out tasks. The scientists knew a young fashion photographer, Baron Wolman, who dabbled in filmmaking (and would later help found Rolling Stone magazine); he was invited to come to a session during which the whole test was carried out again. Wolman could not believe his eyes. During filming, W.J.’s right hand attempted to arrange the blocks, and his left hand kept trying to intervene. Mind Right saw the problem, knew the solution, and tried to help out just like a good friend. W.J. had to sit on his left hand so that the inadequate but dominant right hand could at least try.
For the film’s final scene, they decided to see what would happen if both hands were allowed to arrange the blocks. Here they witnessed the beginning of the idea that Mind Left can have its view of the world with its own desires and aspirations, and Mind Right can have another view. As soon as Mind Right, working through the left hand, began to arrange the blocks correctly, Mind Left would undo the good work. The hands were in competition! The specializations of each hemisphere were different, and growing out of that difference were the behaviors of each half of the brain. These results raised all sorts of questions. Are there two selves? If not, why not? If so, which one is in charge? Do the two sides of the brain routinely compete? Which half decides what gets done and when? Are consciousness and our sense of self located in one half of the brain? And why do split-brain patients generally feel unified and no different even though their two hemispheres do not communicate? Such questions gave birth to the field of human split-brain research.
The popular press picked up these findings, and the concept that the “right brain” and “left brain” think differently about the world made its way into the mainstream. This led to the boiled-down notion that the left hemisphere is analytical and logical while the right hemisphere is creative, musical, and intuitive. Many general interest books have been written based on this naïve view: that artists, musicians, and poets mostly use their right hemisphere while lawyers, mathematicians, and engineers mostly use their left hemisphere (Figure 4.3). In reality, the science has shown this to be a gross exaggeration of the findings on hemispheric specialization. It turns out that most cognitive processes are redundant and that each hemisphere is capable of carrying out those processes. As we learn in this chapter, however, the hemispheres have some fundamental differences that can help us understand the organization of the cerebral cortex, the evolutionary development and purpose of certain specializations, and the nature of the mind.

FIGURE 4.3 Books perpetuating the common idea that the left brain is analytic and the right brain is creative.
The hemispheres of the brain
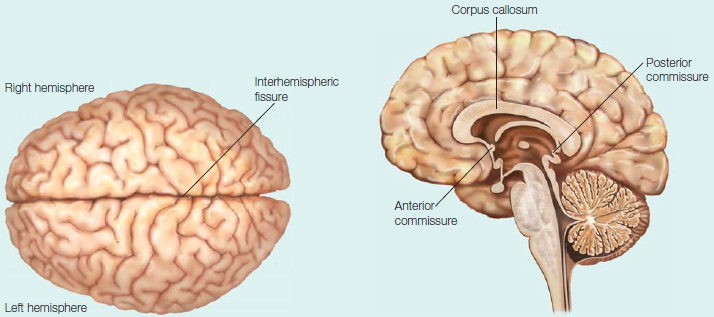
The hemispheres of the brain are distinct yet connected. In the medial view are seen the commissures, the large white matter fiber tracts that connect the hemispheres.
You should keep in mind, however, that despite all we have learned about hemispheric differences and specializations, the fundamental mystery, first discovered in the surgeries of the 1940s, remains today. That is, patients who undergo split-brain surgery report no change in their mental status, even though their “speaking” left hemisphere has been irretrievably isolated from their right hemisphere and all of the special properties that it may include. These two separate but coexisting brains do not result in split personalities, nor do they fight over control of the body. In short, the individual with the split brain does not feel conflicted. At the end of this chapter, we examine why this is the case and revisit what clues it may offer about our general conscious experience (also see Chapter 14, where these ideas are discussed in more detail).
We will find that research on laterality has provided extensive insights into the organization of the human brain, and that the simplistic left-brain/right-brain claims distort the complex mosaic of mental processes that contribute to cognition. Split-brain studies profoundly demonstrate that the two hemispheres do not represent information in an identical manner. Complementary studies on patients with focal brain lesions underscore the crucial role played by lateralized processes in cognition. This research and recent computational investigations of lateralization and specialization have advanced the field far beyond the popular interpretations of left-brain/right-brain processes. They provide the scientific basis for future explorations of many fascinating issues concerning cerebral lateralization and specialization.
In this chapter, we examine the differences between the right and left cerebral hemispheres using data from studies of split-brain patients as well as those with unilateral brain lesions. We also examine the evolutionary reasons for lateralization of functions, and as noted, the chapter ends with some musing about what split-brain research has to say about the conscious experience. We begin, however, at the beginning: the anatomy and physiology of the two halves and their interconnections.
Anatomy of the Hemispheres
Anatomical Correlates of Hemispheric Specialization
For centuries, the effects of unilateral brain damage have revealed major functional differences between the two hemispheres. Most dramatic has been the effect of left-hemisphere damage on language functions. In the late 1950s, the dominant role of the left hemisphere in language was confirmed by employing the Wada test, pioneered by Juhn A. Wada and Theodore Rasmussen. This test is often used before elective surgery for the treatment of disorders such as epilepsy to determine in which hemisphere the speech center is located. A patient is given an injection of amobarbital into the carotid artery, producing a rapid and brief anesthesia of the ipsilateral hemisphere (i.e., the hemisphere on the same side as the injection; Figure 4.4). Then the patient is engaged in a series of tests related to language and memory. The Wada test has consistently revealed a strong bias for language lateralization to the left hemisphere, because when the injection is to the left side, the patient’s ability to speak or comprehend speech is disrupted for several minutes. Functional neuroimaging techniques, such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), have further confirmed that language processing is preferentially biased to the left hemisphere (Binder & Price, 2001). Regions of the right hemisphere, however, are also engaged, especially for language tasks that require higher-level comprehension (Bookheimer, 2002). Since functional lateralization of language processes clearly exists, can we identify anatomical correlates that account for these lateralized functions?
Macroscopic Anatomical Asymmetries The major lobes (occipital, parietal, temporal, and frontal; see Figure 2.00) appear, at least superficially, to be symmetrical, and each half of the cerebral cortex of the human brain is approximately the same size and surface area. The two hemispheres are offset, however. The right protrudes in front, and the left protrudes in back. The right is chubbier (actually has more volume) in the frontal region, and the left is larger posteriorly in the occipital region, frequently nudging the right hemisphere off center and bending the longitudinal fissure between the two hemispheres to the right (Figure 4.5).
Anatomists of the nineteenth century observed that the Sylvian fissure (also called the lateral fissure)—the large sulcus that defines the superior border of the temporal lobe—has a more prominent upward curl in the right hemisphere than it does in the left hemisphere, where it is relatively flat. This difference in the shape of the Sylvian fissure between the two cerebral hemispheres is directly related to subsequent reports of size differences in adjacent cortical regions buried within the fissure. At Harvard Medical School in the 1960s, Norman Geschwind examined brains obtained postmortem from 100 people known to be right-handed (Geschwind & Levitsky, 1968). After slicing through the lateral fissure, Geschwind measured the temporal lobe’s surface area and discovered that the planum temporale, the cortical area at the center of Wernicke’s area (involved with the understanding of written and spoken language), was larger in the left hemisphere—a pattern found in 65 % of the brains. Of the remaining brains, 11 % had a larger surface area in the right hemisphere and 24 % had no asymmetry. The asymmetry in this region of the temporal lobe may extend to subcortical structures connected to these areas. For example, portions of the thalamus (the lateral posterior nucleus) also tend to be larger on the left. Because these temporal lobe asymmetries seem to be a characteristic of the normally lateralized brain, other investigators have explored whether the asymmetry is absent in individuals with developmental language disorders. Interestingly, MRI studies reveal that the area of the planum temporale is approximately symmetrical in children with dyslexia—a clue that their language difficulties may stem from the lack of a specialized left hemisphere. Interestingly, an MRI study on adults with dyslexia found that the typical medial temporal lobe asymmetries were reversed in dyslexic adults (Casanova et al., 2005).

FIGURE 4.4 Methods used in amobarbital (Amytal) testing.
(a) Subsequent to angiography, amobarbital is administered to the left hemisphere, anesthetizing the language and speech systems. A spoon is placed in the left hand, and the right hemisphere takes note. (b) When the left hemisphere regains consciousness, the subject is asked what was placed in his left hand, and he responds, “Nothing.” (c) Showing the patient a board with a variety of objects pinned to it reveals that the patient can easily point to the appropriate object, because the right hemisphere directs the left hand during the match-to-sample task.
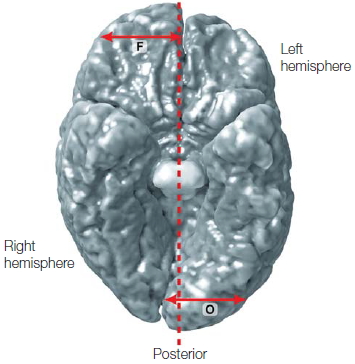
FIGURE 4.5 Anatomical asymmetries between the two cerebral hemispheres.
View looking at the inferior surface of the brain; note that the left hemisphere appears on the right side of the image. In this computer-generated reconstruction, the anatomical asymmetries have been exaggerated.
The asymmetry of the planum temporale is one of the few examples in which an anatomical index is correlated with a well-defined functional asymmetry. The complex functions of language comprehension presumably require more cortical surface. Some questions remain, however, concerning both the validity and the explanatory power of this asymmetry. First, although the left-hemisphere planum temporale is larger in 65 % of right-handers, functional measures indicate that 96 % of right-handers show left-hemisphere language dominance. Second, there is a suggestion that the apparent asymmetries in the planum temporale result from the techniques and criteria used to identify this region. When three-dimensional imaging techniques—techniques that take into account asymmetries in curvature patterns of the lateral fissures—are applied, hemispheric asymmetries become negligible. Whether or not this view is correct, the anatomical basis for left-hemisphere dominance in language may not be fully reflected in gross morphology. We also need to examine the neural circuits within these cortical locations.
Microscopic Anatomical Asymmetries By studying the cellular basis of hemispheric specialization, we seek to understand whether differences in neural circuits between the hemispheres might underlie functional asymmetries in tasks such as language. Perhaps specific organizational characteristics of local neuronal networks—such as the number of synaptic connections—may be responsible for the unique functions of different cortical areas. In addition, regions of the brain with greater volume may contain more minicolumns and their connections (Casanova & Tillquist, 2008; see Chapter 2, p. 53). A promising approach has been to look for specializations in cortical circuitry within homotopic areas (meaning areas in corresponding locations in the two hemispheres) of the cerebral hemispheres that are known to be functionally asymmetrical—and what better place to look than in the language area?
Differences have been found in the cortical microcircuitry between the two hemispheres in both anterior (Broca’s) and posterior (Wernicke’s) language-associated cortex. We leave the discussion of the function of these areas to Chapter 11; here, we are merely concerned about their structural differences.
As we learned in Chapter 2 (p. 38), the cortex is a layered sheet of tightly spaced columns of cells, each comprising a circuit of neurons that is repeated over and over across the cortical surface. From studies of visual cortex, we know that cells in an individual column act together to encode relatively small features of the visual world. Individual columns connect with adjacent and distant columns to form ensembles of neurons that can encode more complex features.
In language-associated regions, several types of micro-level asymmetries between the hemispheres have been identified. Some of these asymmetries occur at the level of the individual neurons that make up a single cortical column. For instance, the left hemisphere has greater high-order dendritic branching than that of their homologs in the right hemisphere, which have more loworder dendritic branching (Scheibel et al., 1985). Other asymmetries are found in the relationships between adjacent neuronal columns: Within Wernicke’s area in the left hemisphere, for example, columns are spaced farther from each other, possibly to accommodate additional connectional fibers between the columns. Asymmetries also are found in larger ensembles of more distant cortical columns (Hutsler & Galuske, 2003). Individual cells within a column of the left primary auditory cortex have a tangential dendritic spread that accommodates the greater distance between cell columns, but secondary auditory areas that show the same increase in distance between the columns do not have longer dendrites in the left hemisphere. The cells in these columns contact fewer adjacent cell columns than do those in the right hemisphere.
Additional structural differences have been documented in both anterior and posterior language cortex. These asymmetries include cell size differences between the hemispheres, such as those shown in Figure 4.6, and may suggest a greater long-range connectivity in the language-associated regions of the left hemisphere. Asymmetries in connectivity between the two hemispheres have been demonstrated directly by tracing the neuronal connections within posterior language-associated regions using dyes that diffuse through postmortem tissue. Such dyes show a patchy pattern of connectivity within these regions of each hemisphere; but within the left hemisphere, these patches are spaced farther apart than those in the right hemisphere (Galuske et al., 2000).
What is the functional significance of these various asymmetries within cortical circuitry, and how might these changes specifically alter information processing in the language-dominant hemisphere? Most interpretations of these findings have focused on the relationship between adjacent neurons and adjacent columns, highlighting the fact that differences in both columnar spacing and dendritic tree size would cause cells in the left hemisphere to connect to fewer neurons. This structural specialization might underlie more elaborate and less redundant patterns of connectivity, which in turn might give rise to better separation between local processing streams. Further refinement of this type could also be driving the larger distance between patches in the left hemisphere, since this larger spacing might also imply more refined connections.
A thorough understanding of the anatomy and physiology of language-associated cortices could shed considerable light on the cortical mechanisms that facilitate linguistic analysis and production, which we will discuss in Chapter 11. Because cortical areas have a basic underlying organization, documenting cortical locations involved in certain functions should distinguish, in terms of form and variety, between the neural structures common to all regions and the structures critical for a region to carry out particular cognitive functions. These questions hold importance not only for the greater understanding of species-specific adaptations such as language, but also for understanding how evolution may build functional specialization into the framework of cortical organization. There are also implications for developmental problems such as dyslexia and autism. For instance, minicolumns in autism are reduced in size and increased in numbers. If changes in these parameters occur early during development, then they would provide for basic alterations in corticocortical connections and information processing (Casanova et al., 2002; 2006).
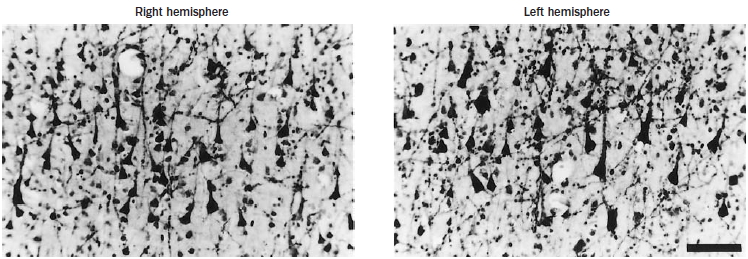
FIGURE 4.6 Layer III pyrimidal cell asymmetry.
Visual examination reveals a subtle difference in the sizes of the largest subgroups of layer III pyramidal cells (stained here with acetylthiocholinesterase): in the left hemisphere they are larger (b) compared to the right (a).
The Anatomy of Communication
The corpus callosum. The left and right cerebral hemispheres are connected by the largest white matter structure in the brain, the corpus callosum. It is made up of approximately 250 million axonal fibers that cross from one side of the brain to the other, facilitating interhemispheric communication. It is located beneath the cortex and runs along the longitudinal fissure. The corpus callosum is divided on a macroscopic level into the anterior portion, called the genu, the middle portion, known as the body, and the posterior portion, called the splenium (Figure 4.7). The neuronal fiber sizes vary across the corpus callosum: Smaller fibers (~0.4 μm) are located anteriorly, fitfully grading to larger fibers (5 μm) located more posteriorly (Aboitiz et al., 1992). The prefrontal and temporoparietal visual areas are connected by the small-diameter, slow-conducting fibers, and the large fibers connect sensorimotor cortices in each hemisphere (Lamantia & Rakic, 1990). As with many parts of the brain, the fiber tracts in the corpus callosum maintain a topographical organization (Zarei et al., 2006).
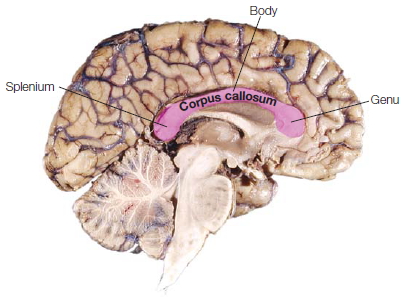
FIGURE 4.7 The corpus callosum.
A sagittal view of the left hemisphere of a postmortem brain. The corpus callosum is the dense fiber tract located below the folds of the cortex. The anterior portion is the genu, the middle portion is the body, and the posterior portion is the splenium.
By using the MRI technique known as diffusion tensor imaging (DTI; see Chapter 3), researchers have traced the white fiber tracks from one hemisphere across the corpus callosum to the other hemisphere. The results indicate that the corpus callosum can be partitioned into vertical segments carrying homotopic and heterotopic connections between specific regions of each hemispheric cortex (Hofer & Frahm, 2006). Heterotopic fibers connect different areas between the hemispheres. Figure 4.8 shows a segmentation of the corpus callosum containing fibers projecting into the prefrontal, premotor, primary motor, primary sensory, parietal, temporal, and occipital areas. As can be clearly seen in the figure, almost all of the visual information processed in the occipital, parietal, and temporal cortices is transferred to the opposite hemisphere via the posterior third of the corpus callosum, whereas premotor and supplementary motor information is transferred across a large section of the middle third of the corpus callosum.
Many of the callosal projections link homotopic areas (Figure 4.9). For example, regions in the left prefrontal cortex project to homotopic regions in the right prefrontal cortex. Although this pattern holds for most areas of the association cortex, it is not always seen in primary cortex. Callosal projections connecting the two halves of the primary visual cortex link only those areas that represent the most eccentric regions of space; and in both the primary motor and the somatosensory cortices, homotopic callosal projections are sparse (Innocenti et al., 1995). Callosal fibers also connect heterotopic areas (regions with different locations in the two hemispheres). These projections generally mirror the ones found within a hemisphere. For instance, a prefrontal area sending projections to premotor areas in the same hemisphere is also likely to send projections to the analogous premotor area in the contralateral hemisphere. Yet, heterotopic projections are usually less extensive than are projections within the same hemisphere.
The commissures. A much smaller band of fibers connecting the two hemispheres is the anterior commissure. It is about one tenth the size of the corpus callosum, is found inferior to the anterior portion of the corpus callosum, and primarily connects certain regions of the temporal lobes, including the two amygdalae (Figure 4.10). It also contains decussating fibers from the olfactory tract and is part of the neospinothalamic tract for pain. Even smaller is the posterior commissure, which also carries some interhemispheric fibers. It is above the cerebral aqueduct at the junction of the third ventricle (Figure 4.10). It contains fibers that contribute to the papillary light reflex.
a
|
b
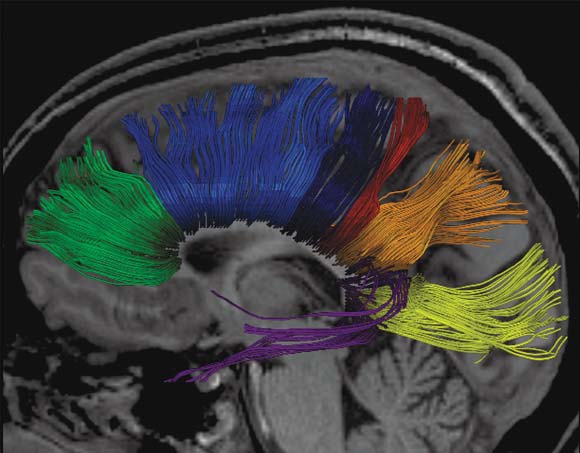
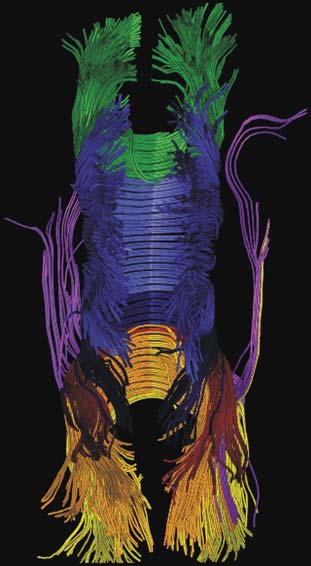
FIGURE 4.8 3-D reconstruction of transcallosal fiber tracts placed on anatomical reference images.
(a) Sagittal view: callosal fiber bundles projecting into the prefrontal lobe (coded in green), premotor and supplementary motor areas (light blue), primary motor cortex (dark blue), primary somatosensory cortex (red), parietal lobe (orange), occipital lobe (yellow), and temporal lobe (violet). (b) Top view. (c) Oblique view.
|

c
|
Function of the Corpus Callosum
The corpus callosum is the primary communication highway between the two cerebral hemispheres. Researchers, of course, are interested in exactly what is being communicated and how. Several functional roles have been proposed for callosal connections. For instance, some researchers point out that in the visual association cortex, receptive fields can span both visual fields. Communication across the callosum enables information from both visual fields to contribute to the activity of these cells. Indeed, the callosal connections could play a role in synchronizing oscillatory activity in cortical neurons as an object passes through these receptive fields (Figure 4.11). In this view, callosal connections facilitate processing by pooling diverse inputs. Other researchers view callosal function as predominantly inhibitory (See the box “How the Brain Works: Interhemispheric Communication”). If the callosal fibers are inhibitory, they would provide a means for each hemisphere to compete for control of current processing. For example, multiple movements might be activated, all geared to a common goal; later processing would select one of these candidate movements (see Chapter 8). Inhibitory connections across the corpus callosum might be one contributor to this selection process.

FIGURE 4.9 Tracing connections between and within the cerebral cortices.
(a) Midsagittal view of the right cerebral hemisphere, with the corpus callosum labeled. (b) The caudal surface of a coronal section of brain roughly through the premotor cortical area. Homotopic callosal fibers (blue) connect corresponding sections of the two hemispheres via the corpus callosum; heterotopic connections (green) link different areas of the two hemispheres of the brain. In primates, both types of contralateral connections (blue and green), as well as ispilateral connections (red), start and finish at the same layer of neocortex.
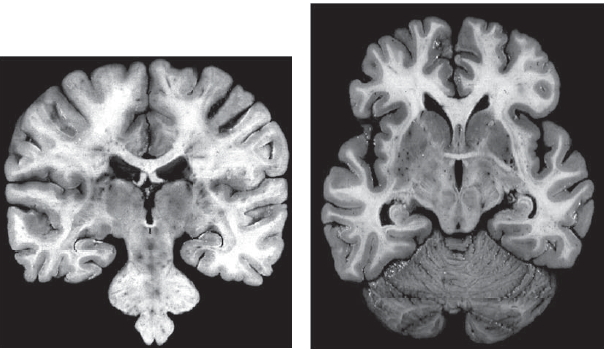
FIGURE 4.10 Coronal sections at (a) the level of the posterior commissure and (b) the anterior commissure.
HOW THE BRAIN WORKS
Interhemispheric Communication: Cooperation or Competition?
Theories of callosal function generally have focused on the idea that this massive bundle of axonal fibers provides the primary pathway for interhemispheric transfer. For example, in Chapter 6 we will discuss Warrington’s model of object recognition. In her view, the right hemisphere performs a specialized operation essential for perceptual categorization. This operation is followed by a left-hemisphere operation for semantic categorization. Interhemispheric communication is essential in this model for shuttling the information through these two processing stages.
On the other hand, interhemispheric communication need not be a cooperative process. Connections across the corpus callosum may underlie a competition between the hemispheres. Indeed, the primary mode of callosal communication may be inhibitory rather than excitatory. By this view, we need not assume that interhemispheric communication is designed to share information processing within the two hemispheres to facilitate concurrent, and roughly identical, activity in homologous regions. Similar to the way in which split-brain behavior is assumed to reflect the independent operation of the two hemispheres, behavior produced by intact brains may also reflect the (fluctuating) dominance of one or the other hemisphere.
One challenge for a cooperative system is that there must be a means to ensure that the two hemispheres are operating on roughly the same information. Such coordination might be difficult, given that both the perceptual input and the focus of our attention are constantly changing. Although computers can perform their operations at lightning speed, neural activity is a relatively slow process. The processing delays inherent in transcallosal communication may limit the extent to which the two hemispheres can cooperate.
A number of factors limit the rate of neural activity. First, to generate an action potential, activity within the receiving dendritic branches must integrate tiny inputs across both space and time in order to reach threshold. Second, the rate at which individual neurons can fire is limited, owing to intrinsic differences in membrane properties, tonic sources of excitation and inhibition, and refractory periods between spike-generating events. Third, and most important, neural signals need to be propagated along axons. These conduction times can be quite substantial, especially for the relatively long fibers of the corpus callosum.
James Ringo and his colleagues (1994) at the University of Rochester provided an interesting analysis of this problem. They began by calculating estimates of transcallosal conduction delays. Two essential numbers were needed: the distance to be traveled, and the speed at which the signal would be transmitted. If the distances were direct, the average distance of the callosal fibers would be short. Most axons follow a circuitous route, however. Taking this point into consideration, a value of 175 mm was used as representative of the average length of a callosal fiber in humans. The speed at which myelinated neural impulses travel is a function of the diameter of the fibers. Using the limited data available from humans, in combination with more thorough measures in the monkey, the average conduction speed was estimated to be about 6.5 m/s. Thus to travel a distance of 175 mm would take almost 30 ms. Single-cell studies in primates have confirmed that interhemispheric processing entails relatively substantial delays.
Ringo used a neural network to demonstrate the consequences of slow interhemispheric conduction times. The network consisted of two identical sets of processing modules, each representing a cerebral hemisphere. It included both intrahemispheric and interhemispheric connections; the latter were much sparser to reflect the known anatomy of the human brain. This network was trained to perform a pattern recognition task. After it had learned to classify all of the patterns correctly, the interhemispheric connections were disconnected. Thus, performance could now be assessed when each hemisphere had to operate in isolation.
The critical comparison was between networks in which the interhemispheric conduction times during learning had been either slow or fast. The results showed that, for the network trained with fast interhemispheric connections, the disconnection procedure led to a substantial deterioration in performance. Thus, object recognition was dependent on cooperative processing for the network with fast interhemispheric connections. In contrast, for the network trained with slow interhemispheric connections, performance was minimally affected by the disconnection procedure. For this network, recognition was essentially dependent only on intrahemispheric processing. These results led Ringo to conclude that a system with slow interhemispheric conduction delays—for example, the human brain—ends up with each hemisphere operating in a relatively independent manner.
Interestingly, these delays could be reduced if the callosal fibers were larger because the larger size would increase conduction speed. Larger fibers, however, would require a corresponding increase in brain volume. For example, reducing the conduction delay by a factor of two would lead to a 50% increase in brain volume. Such an increase would have severe consequences for metabolic demands as well as for childbirth. The brain appears to have evolved such that each hemisphere can have rapid access to information from either side of space, but with limited capability for tasks that would require extensive communication back and forth across the corpus callosum. The delays associated with transcallosal communication not only might limit the degree of cooperation between two hemispheres but also might have provided an impetus for the development of hemispheric specialization. Independent processing systems would be more likely to evolve non-identical computational capabilities.
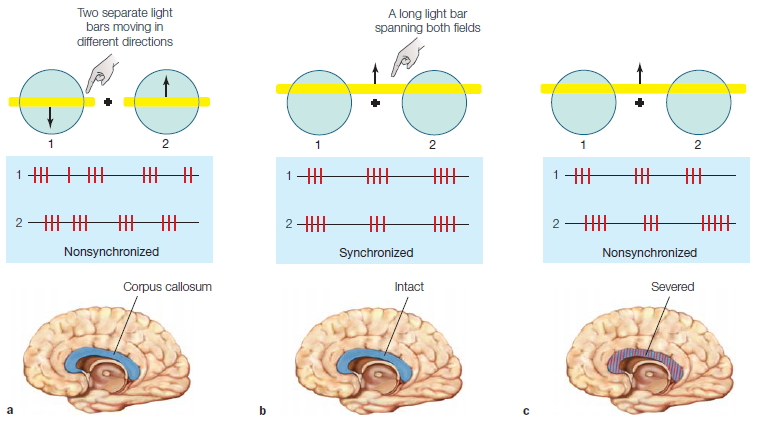
FIGURE 4.11 Synchrony in cortical neurons.
(a) When receptive fields (1 and 2) on either side of fixation are stimulated by two separate light bars moving in different directions (as indicated by the arrows), the firing rates of the two cells are not correlated. (b) In animals with an intact corpus callosum, cells with spatially separate receptive fields fire synchronously when they are stimulated by a common object, such as a long light bar spanning both fields. (c) In animals whose corpus callosum has been severed, synchrony is rarely observed.
Callosal connections in the adult, however, are a scaled-down version of what is found in immature individuals. In developing animals, callosal projections are diffuse and more evenly distributed across the cortical surface. Cats and monkeys lose approximately 70 % of their callosal axons during development; some of these transient projections are between portions of the primary sensory cortex that, in adults, are not connected by the callosum. Yet this loss of axons does not produce cell death in each cortical hemisphere. This is because a single cell body can send out more than one axon terminal: one to cortical areas on the same side of the brain, and one to the other side of the brain. Thus, loss of a callosal axon may well leave its cell body—with its secondary collateral connection to the ipsilateral hemisphere—alive and well, just like pruning a bifurcating peach tree branch leaves the branch thriving. The refinement of connections is a hallmark of callosal development, just as such refinement characterizes intrahemispheric development (see Chapter 2).
In general terms, hemispheric specialization must have been influenced and constrained by callosal evolution. The appearance of new cortical areas might be expected to require more connections across the callosum (i.e., expansion). In contrast, lateralization might have been facilitated by a lack of callosal connections. The resultant isolation would promote divergence among the functional capabilities of homotopic regions, resulting in cerebral specializations.
As with the cerebral hemispheres, researchers have investigated functional correlates of anatomical differences in the corpus callosum. Usually, investigators measure gross aspects like the cross-sectional area or shape of the callosum. Variations in these measures are linked to gender, handedness, mental retardation, autism, and schizophrenia. Interpretation of these data, however, is complicated by methodological disagreements and contradictory results. The underlying logic of measuring the corpus callosum’s cross-sectional area relies on the relation of area to structural organization. Callosal size could be related to the number and diameter of axons, the proportion of myelinated axons, the thickness of myelin sheaths, and measures of nonneural structures such as the size of blood vessels or the volume of extracellular space with resultant functional differences. Among large samples of callosal measurements from age-matched control subjects, sex-based differences are seen in the shape of the midsagittal sections of the callosum but not in its size. More recently, studies looking at the parasagittal size and asymmetry of the corpus callosum have found an increased rightward callosal asymmetry in males compared to females (Lunder et al., 2006). That is, a larger chunk of the callosum bulges off to the right side in males. It may be that what side of the hemispheric fence the major part of the callosum sits on is the important factor. Thus, this sexually dimorphic organization of the corpus callosum (more on the right than the left in males) may involve not just the corpus callosum, but asymmetric hemispheric development also, reflected in the distribution of parasagittal callosal fibers (Chura et al., 2009). This structure could in turn account for the observed patterns of accelerated language development in females, who have more acreage in the left hemisphere, and the enhanced performance in males during visuospatial tasks and increased rate of left-handedness in males thanks to their rightward bulge. Tantalizing research by Linda Chura and her colleagues found that with increasing levels of fetal testosterone, there was a significantly increasing rightward asymmetry (e.g., right . left) of a posterior subsection of the callosum, called the isthmus, that projects mainly to parietal and superior temporal areas.
TAKE-HOME MESSAGES
- The Wada test is used to identify which hemisphere is responsible for language before brain surgery is performed.
- The two halves of the cerebral cortex are connected primarily by the corpus callosum, which is the largest fiber system in the brain. In humans, this bundle of white matter includes more than 250 million axons.
- Two smaller bands of fibers, the anterior and posterior commissures, also connect the two hemispheres.
- The corpus callosum has both homotopic and heterotopic connections. Homotopic fibers connect the corresponding regions of each hemisphere (e.g., V1 on the right to V1 on the left), whereas heterotopic fibers connect different areas (e.g., V1 on the right to V2 on the left).
- Differences in neural connectivity and organization may underlie many of the gross asymmetries between the hemispheres.
- Ninety-six percent of humans, regardless of which hand is dominant, have a left-hemisphere specialization for language.
- The planum temporale encompasses Wernicke’s area and is involved in language. The asymmetry of the planum temporale is one of the few examples in which an anatomical index is correlated with a well-defined functional asymmetry.
- Differences have been found in the specifics of cortical microcircuitry between the two hemispheres in both anterior (Broca’s) and posterior (Wernicke’s) language-associated cortex.
Splitting the Brain: Cortical Disconnection
Because the corpus callosum is the primary means of communication between the two cerebral hemispheres, we learn a great deal when we sever the callosal fibers. This approach was successfully used in the pioneering animal studies of Ronald Myers and Roger Sperry at the California Institute of Technology. They developed a series of animal experiments to assess whether the corpus callosum is crucial for unified cortical function. First, they trained cats to choose a “plus” stimulus versus a “circle” stimulus randomly alternated between two doors. When a cat chose correctly, it was rewarded with food. Myers and Sperry made the startling discovery that when the corpus callosum and anterior commissure were sectioned, such visual discriminations learned by one hemisphere did not transfer to the other hemisphere. Further studies done on monkeys and chimpanzees showed that visual and tactile information lateralized to one hemisphere did not transfer to the opposite hemisphere, thus corroborating the results from cats.
This important research laid the groundwork for comparable human studies initiated by Sperry and one of the authors (MSG; Sperry et al., 1969). Unlike lesion studies, the split-brain operation does not destroy any cortical tissue; instead, it eliminates the connections between the two hemispheres. With split-brain patients, functional inferences are not based on how behavior changes after a cortical area is eliminated. Rather, it becomes possible to see how each hemisphere operates in relative isolation.
The Surgery
Corpus callosotomy, or split-brain surgery, is used to treat intractable epilepsy when other forms of treatment, such as medication, have failed. This procedure was first performed in 1940 by a Rochester, New York, surgeon, William Van Wagenen. One of Van Wagenen’s patients, who had a history of severe epileptic seizures, improved after developing a tumor in his corpus callosum (Van Wagenen & Herren, 1940). Epileptic seizures are the result of abnormal electrical discharges that zip across the brain. The improvement in his patient’s condition gave Van Wagenen the idea that if he were to sever the patient’s corpus callosum, perhaps the electrical impulses causing seizures would be unable to spread from one hemisphere to the other: The epileptogenic activity would be held in check, and a generalized seizure would be prevented. The idea was radical, particularly when so little was really understood about brain function. The surgery itself was also painstaking, especially without today’s microsurgical techniques, because only a thin wall of cells separates the ventricles from the corpus callosum. With the limited treatment options available at the time, however, Van Wagenen had desperate patients; and to twist a phrase, they called for desperate measures. One great fear loomed: What would be the side effect—a split personality with two minds fighting for control over one body? To everyone’s relief, the surgery was a great success. Remarkably, the patients appeared and felt completely normal. The seizures typically subsided immediately, even in patients who, before the operation, experienced up to 15 seizures per day. Eighty percent of the patients enjoyed a 60 % to 70 % decrease in seizure activity, and some were free of seizures altogether (Akelaitis, 1941). Everyone was happy, yet puzzled. Twenty of the surgeries were performed without any discernible psychological side effects: no changes to the psyche, personality, intellect, sensory processing, or motor coordination. Akelaitis concluded:
The observations that some of these patients were able to perform highly complex synchronous bilateral activities as piano-playing, typewriting by means of the touch system and dancing postoperatively suggests strongly that commissural pathways other than the corpus callosum are being utilized. (Akelaitis, 1943, p. 259)
Methodological Considerations in Studying Split-Brain Patients
A number of methodological issues arise in evaluations of the performance of split-brain patients. First, bear in mind that these patients were not neurologically normal before the operation; they were all chronic epileptics, whose many seizures may have caused neurologic damage. Therefore, it is reasonable to ask whether they provide an appropriate barometer of normal hemispheric function after the operation. There is no easy answer to this question. Several patients do display abnormal performance on neuropsychological assessments, and they may even be mentally retarded. In some patients, however, the cognitive impairments are negligible; these are the patients studied in closest detail.
FIGURE 4.12 This MRI shows a sagittal view of a brain in which the corpus callosum has been entirely sectioned.
Second, it is important to consider whether the transcortical connections were completely sectioned, or whether some fibers remained intact. In the original California operations, reviewing surgical notes was the only way to determine the completeness of the surgical sections. In recent years though, MRIs, such as in Figure 4.12, diffusion tensor imaging, and electrical brainmapping techniques have provided a more accurate representation of the extent of surgical sections. Accurate documentation of a callosal section is crucial for learning about the organization of the cerebral commissure.
The main methods of testing the perceptual and cognitive functions of each hemisphere have changed little over the past 30 years. Researchers use primarily visual stimulation, not only because of the preeminent status of this modality for humans but also because the visual system is more strictly lateralized (see Figure 4.1) than are other sensory modalities, such as the auditory and olfactory systems.
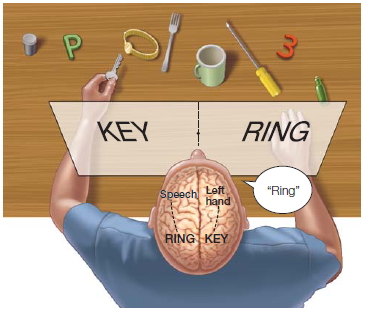
FIGURE 4.13 Restricting visual stimuli to one hemisphere.
The split-brain patient reports through the speaking hemisphere only the items flashed to the right half of the screen and denies seeing left-field stimuli or recognizing objects presented to the left hand. Nevertheless, the left hand correctly retrieves objects presented in the left visual field, about which the patient verbally denies knowing anything.
The visual stimulus is restricted to a single hemisphere by quickly flashing the stimulus in one visual field or the other (Figure 4.13). Before stimulation, the patient is required to fixate on a point in space. The brevity of stimulation is necessary to prevent eye movements, which would redirect the information into the unwanted hemisphere. Eye movements take roughly 200 ms, so if the stimulus is presented for a briefer period of time, the experimenter can be confident that the stimulus was lateralized. More recent image stabilization tools—tools that move in correspondence with the subject’s eye movements—allow a more prolonged, naturalistic form of stimulation. This technological development has opened the way for new discoveries in the neurological and psychological aspects of hemisphere disconnection.
Functional Consequences of the Split-Brain Procedure
The results of testing done on the patient W.J. were contrary to the earlier reports on the effects of the split-brain procedure as reported by A. J. Akelaitis (1941), who had found no significant neurological and psychological effects after the callosum was sectioned. Careful testing with W.J. and other California patients, however, revealed behavioral changes similar to those seen in split-brain primates (see below). Visual information presented to one half of the brain was not available to the other half. The same principle applied to touch. Patients were able to name and describe objects placed in the right hand but not objects presented in the left hand. Sensory information restricted to one hemisphere was also not available to accurately guide movements with the ipsilateral hand. For example, when a picture of a hand portraying the “OK” sign was presented to the left hemisphere, the patient was able to make the gesture with the right hand, which is controlled from the left half of the brain. The patient was unable to make the same gesture with the left hand, however, which is controlled from the disconnected right hemisphere.
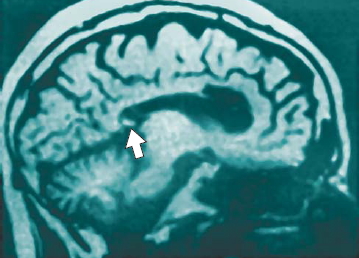
FIGURE 4.14 An incomplete corpus callostomy.
MRI scan showing that the splenium (arrow) was spared in the split-brain procedure performed on this patient. As a result, visual information can still be transferred between the cerebral hemispheres.
From a cognitive point of view, these initial studies confirmed long-standing neurological knowledge about the nature of the two cerebral hemispheres, which had been obtained earlier from patients with unilateral hemispheric lesions: The left hemisphere is dominant for language, speech, and major problem solving. Its verbal IQ and problem-solving capacity (including mathematical tasks, geometric problems, and hypothesis formation) remain intact after callosotomy (Gazzaniga, 1985). Isolating half the brain, cutting its acreage by 50 %, causes no major changes in cognitive function—nor do the patients notice any change in their abilities. The right hemisphere is impoverished in its ability to perform cognitive tasks, but it appears specialized for visuospatial tasks such as drawing cubes and other three-dimensional patterns. The split-brain patients cannot name or describe visual and tactile stimuli presented to the right hemisphere, because the sensory information is disconnected from the dominant left (speech) hemisphere. This does not mean that knowledge about the stimuli is absent in the right hemisphere, however. Nonverbal response techniques are required to demonstrate the competence of the right hemisphere. For example, the left hand can be used to point to named objects or to demonstrate the function of depicted objects presented in the left visual field.
Split-Brain Evidence for Callosal Function Specificity We have seen that when the corpus callosum is fully sectioned, little or no perceptual or cognitive interaction occurs between the hemispheres. Surgical cases in which callosal section is limited or part of the callosum is inadvertently spared have enabled investigators to examine specific functions of the callosum by region. For example, when the splenium, the posterior area of the callosum that interconnects the occipital lobe, is spared, visual information is transferred normally between the two cerebral hemispheres (Figure 4.14). In these instances, pattern, color, and linguistic information presented anywhere in either visual field can be matched with information presented to the other half of the brain. The patients, however, show no evidence of interhemispheric transfer of tactile information from touched objects. Tactile information turns out to be transferred by fibers in a region just anterior to the splenium, still located in the posterior half of the callosum.
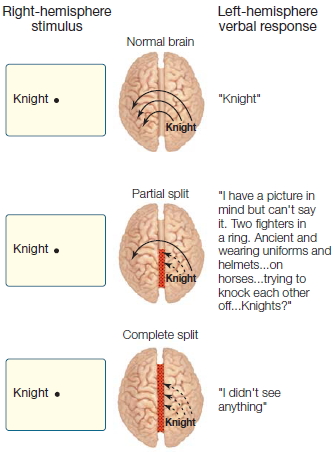
FIGURE 4.15 Schematic representation of split-brain patient J.W.’s naming ability for objects in the left visual field at each operative stage.
Surgeons sometimes perform the split-brain procedure in stages, restricting the initial operation to the front (anterior) or back (posterior) half of the callosum. The remaining fibers are sectioned in a second operation only if the seizures continue to persist. This two-stage procedure offers a unique glimpse into what the anterior and posterior callosal regions transfer between the cerebral hemispheres. When the posterior half of the callosum is sectioned, transfer of visual, tactile, and auditory sensory information is severely disrupted, but the remaining intact anterior region of the callosum is still able to transfer higher order information. For example, one patient (J.W.) was able to name stimuli presented in the left visual field following a resection limited to the posterior callosal region. Close examination revealed that the left hemisphere was receiving higher order cues about the stimulus without having access to the sensory information about the stimulus itself (Figure 4.15). In short, the anterior part of the callosum transfers semantic information about the stimulus but not the stimulus itself. After the anterior callosal region was sectioned in this patient, this capacity was lost.
TAKE-HOME MESSAGES
- In some of the original animal studies on callosotomies, Myers and Sperry demonstrated that visual discrimination learned by one hemisphere did not transfer to the other hemisphere when the hemispheres were disconnected.
- The splenium is the most posterior portion of the corpus callosum. When the posterior half of the callosum is sectioned in humans, transfer of visual, tactile, and auditory sensory information is severely disrupted. The anterior part of the callosum is involved in the higher order transfer of semantic information.
Hemispheric Specialization
Evidence from Split-Brain Patients
As we saw in Chapter 1, the history of cerebral specialization—the notion that different regions of the brain have specific functions—began with Franz Joseph Gall in the early 1800s. Although it fell repeatedly in and out of fashion, this idea could not be discounted, because so many clinical findings, especially in patients who had suffered strokes, provided unassailable evidence that it was so. Over the last 50 years, studies done with split-brain patients have demonstrated that some of the brain’s processing is lateralized. In this section, we review some of these findings. The most prominent lateralized function in the human brain is the left hemisphere’s capacity for language and speech, which we examine first. We also look at the lateralization of visuospatial processing, attention and perception, information processing, and how we interpret the world around us.
Language and Speech When we are trying to understand the neural bases of language, it is useful to distinguish between grammatical and lexical functions. The grammar–lexicon distinction is different from the more traditional syntax–semantics distinction commonly invoked to improve understanding of the differential effects of brain lesions on language processes (see Chapter 11). Grammar is the rule-based system that humans have for ordering words to facilitate communication. For example, in English, the typical order of a sentence is subject (noun)—action (verb)—object (noun). The lexicon is the mind’s dictionary, where words are associated with specific meanings. A “dog” is, well, associated with a dog; but so is a chien and a cane, depending on the language that you speak.
The grammar–lexicon distinction takes into account factors such as memory, because, with memory, word strings as idioms can be learned by rote. For example, “How are you?” or “Comment allez-vous?” is most likely a single lexical entry. Although the lexicon cannot possibly encompass the infinite number of unique phrases and sentences that humans can generate—such as the one you are now reading—memory does play a role in many short phrases. When uttered, such word strings do not reflect an underlying interaction of syntax and semantic systems; they are, instead, essentially an entry from the lexicon. This is more apparent when you are learning a new language. You often learn stock phrases that you speak as a unit, rather than struggle with the grammar. With this in mind, it might be predicted that some brain areas ought to be wholly responsible for grammar, whereas the lexicon’s location ought to be more elusive, since it reflects learned information and thus is part of the brain’s general memory and knowledge systems. The grammar system, then, ought to be discrete and hence localizable, and the lexicon should be distributed and hence more difficult to damage completely.
Language and speech are rarely present in both hemispheres; they are either in one or the other. While it is true that the separated left hemisphere normally comprehends all aspects of language, the linguistic capabilities of the right hemisphere do exist, although they are uncommon. Indeed, out of dozens of split-brain patients who have been carefully examined, only six showed clear evidence of residual linguistic functions in the right hemisphere. And even in these patients, the extent of right-hemisphere language functions is severely limited and restricted to the lexical aspects of comprehension.
Interestingly, the left and right lexicons of these special patients can be nearly equal in their capacity, but they are organized quite differently. For example, both hemispheres show a phenomenon called the word superiority effect (see Chapter 5). Normal English readers are better able to identify letters (e.g., L) in the context of real English words (e.g., belt) than when the same letters appear in pseudowords (e.g., kelt) or nonsense letter strings (e.g., ktle). Because pseudowords and nonwords do not have lexical entries, letters occurring in such strings do not receive the additional processing benefit bestowed on words. Thus, the word superiority effect emerges.
While the patients with right-hemisphere language exhibit a visual lexicon, it may be that each hemisphere accesses this lexicon in a different way. To test this possibility, investigators used a letter-priming task. Participants were asked to indicate whether a briefly flashed uppercase letter was an H or a T. On each trial, the uppercase letter was preceded by a lowercase letter that was either an h or a t. Normally, participants are significantly faster, or primed, when an uppercase H is preceded by a lowercase h than when it is preceded by a lowercase t.

FIGURE 4.16 Letter priming as a function of visual field in splitbrain patients.
The graph shows the response latencies for compatible and incompatible pairs of letters in the left and right visual fields (LVF and RVF, respectively). The latencies for both types of trials are much longer for the left visual field (right hemisphere).
The difference between response latency on compatible (h–H) versus incompatible (t–H) trials is taken to be a measure of letter priming. J.W., a split-brain participant, performed a lateralized version of this task in which the prime was displayed for 100 ms to either the right or the left visual field, and 400 ms later the target letter appeared in either the right or the left visual field. The results, shown in Figure 4.16, provide no evidence of letter priming for left visual field (LVF) trials but clear evidence of priming for trials of the right visual field (RVF). Thus, the lack of a priming phenomenon in the disconnected right hemisphere suggests a deficit in letter recognition, prohibiting access to parallel processing mechanisms. J.W. exhibited a variety of other deficiencies in right-hemisphere function as well. For example, he was unable to judge whether one word was superordinate to another (e.g., furniture and chair), or whether two words were antonyms (e.g., love and hate).
In sum, there appear to be two lexicons, one in each hemisphere. The right hemisphere’s lexicon seems organized differently from the left hemisphere’s lexicon, and these lexicons are accessed in different ways. These observations are consistent with the view that lexicons reflect learning processes and, as such, are more widely distributed in the cerebral cortex. A long-held belief has been that in the general population, the lexicon appears to be in the left hemisphere. Recent evidence from functionalimaging studies, however, suggests a broader role for the right hemisphere in language processing, although the precise nature of that role remains to be defined. Some theorists have suggested that the language ability of the left hemisphere gives it a superior ability to perform higher cognitive functions like making inferences and solving mathematics problems. Split-brain patients who have an extensive right-brain lexicon, however, do not show any attendant increase in their right brain’s ability to perform these tasks (Gazzaniga & Smylie, 1984).
In contrast, generative syntax is present in only one hemisphere. Generative syntax means that by following rules of grammar, we can combine words in an unlimited number of meanings. Although the right hemisphere of some patients clearly has a lexicon, it performs erratically on other aspects of language, such as understanding verbs, pluralizations, the possessive, or active–passive differences. In these patients, the right hemisphere also fails to use word order to disambiguate stimuli for correct meaning. For instance, the meaning of the phrase “The dog chases the cat” cannot be differentiated from the meaning of “The cat chases the dog.” Yet these right hemispheres can indicate when a sentence ends with a semantically odd word. “The dog chases cat the” would be flagged as wrong. What’s more, right hemispheres with language capacities can make grammar judgments. For some peculiar reason, although they cannot use syntax to disambiguate stimuli, they can judge that one set of utterances is grammatical while another set is not. This startling finding suggests that patterns of speech are learned by rote. Yet recognizing the pattern of acceptable utterances does not mean that a neural system can use this information to understand word strings (Figure 4.17).
A hallmark of most split-brain patients is that their speech is produced in the left hemisphere and not the right. This observation, along with amobarbital studies (see Wada and Rasmussen, 1960) and functional imaging studies, confirms that the left hemisphere is the dominant hemisphere for speech production in most (96 %) of us. Nonetheless, there are now a handful of documented cases of split-brain patients who can produce speech from both the left and the right hemispheres. Although speech is restricted to the left hemisphere following callosal bisection, in these rare patients the capacity to make one-word utterances from the disconnected right hemisphere has emerged over time. This intriguing development raises the question of whether information is somehow transferring to the dominant hemisphere for speech output or whether the right hemisphere itself is capable of developing speech production. After extensive testing, it became apparent that the latter hypothesis was correct. For example, the patients were able to name an object presented in the left field, say a spoon, and in the right field, a cow, but were not able to judge whether the two objects were the same. Or, when words like father were presented such that the fixation point fell between the t and the h, the patients said either “fat” or “her,” depending on which hemisphere controlled speech production. These findings illustrate that an extraordinary plasticity lasts sometimes as long as 10 years after callosal surgery. In one patient, in fact, the right hemisphere had no speech production capability for approximately 13 years before it “spoke.”
Finally, note that although most language capabilities are left lateralized, the processing of the emotional content of language appears to be right lateralized. It is well known that patients with damage to certain regions of the left hemisphere have language comprehension difficulties. Speech, however, can communicate emotion information beyond the meanings and structures of the words. A statement, such as “John, come here,” can be interpreted in different ways if it is said in an angry tone, a fearful tone, a seductive tone, or a surprised tone. This nonlinguistic, emotional component of speech is called emotional prosody. One patient with left-hemisphere damage reportedly has difficulty comprehending words but shows little deficit in interpreting the meaning of emotional prosody (Barrett et al., 1999). At the same time, several patients with damage to the temporoparietal lobe in the right hemisphere have been shown to comprehend the meaning of language perfectly but have difficulty interpreting phrases when emotional prosody plays a role (Heilman et al., 1975). This double dissociation between language and emotional prosody in the comprehension of meaning suggests that the right hemisphere is specialized for comprehending emotional expressions of speech.
Visuospatial Processing Early testing of W.J. made it clear that the two hemispheres have different visuospatial capabilities. As Figure 4.2 shows, the isolated right hemisphere is frequently superior on neuropsychological tests such as the block design task, a subtest of the Wechsler Adult Intelligence Scale. In this simple task of arranging red and white blocks to match a given pattern, the left hemisphere of a split-brain patient performs poorly while the right hemisphere easily completes the task. Functional asymmetries like these, however, have proven to be inconsistent. In some patients, performance is impaired with either hand; in others, the left hemisphere is quite adept at this task. Perhaps a component of this task, rather than the whole task, is lateralized. Additional testing has shown that patients who demonstrate a right-hemisphere superiority for the block design task exhibit no asymmetry on the perceptual aspects of the task (contrary to what you may have predicted). If a picture of the block design pattern is lateralized, either hemisphere can easily find the match from a series of pictures. Since each hand is sufficiently dexterous, the crucial link must be in the mapping of the sensory message onto the capable motor system.
The right hemisphere is also specialized for efficiently detecting upright faces and discriminating among similar faces (Gazzaniga & Smylie, 1983). The left hemisphere is not good at distinguishing among similar faces, but it is able to distinguish among dissimilar ones when it can tag the feature differences with words (blond versus brunette, big nose versus button nose). As for the recognition of familiar faces in general, the right hemisphere outperforms the left hemisphere in this task (Turk, 2002).

FIGURE 4.18 Morphed images of J.W. and M.G.
The image on the far left contains 10% M.G. and 90% J.W. and changes in 10% increments from left to right, to 90% M.G. and 10% J.W. on the far right. The two original photographs of M.G. and J.W. pictured above and these nine morphed images were presented to each hemisphere randomly.
What about that most familiar of faces, one’s own? In one study, software was used to morph the face of one split brain patient J.W. in 10 % increments, into that of a familiar other, Mike (Figure 4.18). The faces were flashed randomly to J.W.’s separated hemispheres. Then that hemisphere was asked, in the first condition, “Is that you?” and, in another condition, “Is that Mike?” A double dissociation was found (Figure 4.19). The left hemisphere was biased towards recognizing one’s own face, while the right hemisphere had a recognition bias for familiar others (Turk et al., 2002).
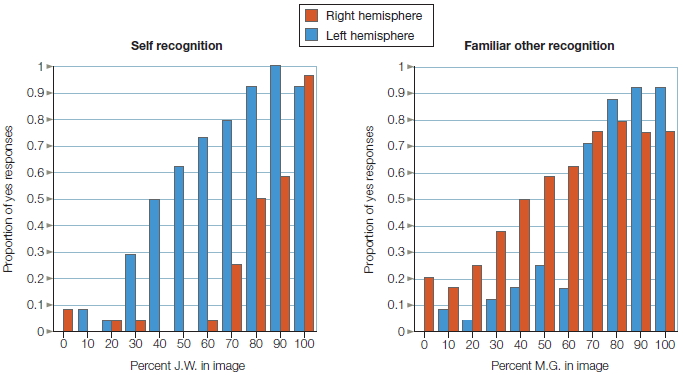
FIGURE 4.19 Left hemisphere is better at recognizing self, and right hemisphere is superior to recognizing familiar other.
The proportion of “yes” responses to recognition judgments are plotted on the y-axis as a function of the percentage of the individual contained in the image and the cerebral hemisphere to which the image was presented.
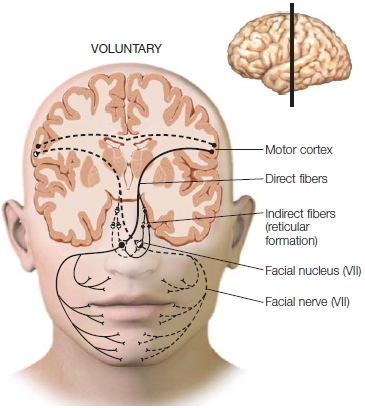
Both hemispheres can generate spontaneous facial expressions, but you need your left hemisphere to produce voluntary facial expressions. Indeed, people appear to have two neural systems for controlling facial expressions (Figure 4.20; Gazzaniga & Smylie, 1990). The left hemisphere sends its messages directly to the contralateral facial nucleus via cranial nerve VII, which in turn innervates the right facial muscles. At the same time, it also sends a command over the corpus callosum to the right half of the brain. The right hemisphere sends the message down to the left facial nucleus, which in turn innervates the left half of the face. The result is that a person can make a symmetrical voluntary facial response, such as a smile or frown. When a split-brain patient’s left hemisphere is given the command to smile, however, the lower right side of the face responds first while the left side responds about 180 msec later. Why does the left side respond at all? Most likely the signal is rerouted through secondary ipsilateral pathways that connect to both facial nuclei, which then eventually send the signal over to the left-side facial muscles.
Unlike voluntary expressions, which only the left hemisphere can trigger, spontaneous expressions can be managed by either half of the brain. When either half triggers a spontaneous response, the pathways that activate the brainstem nuclei are signaled through another pathway—one that does not course through the cortex. Each hemisphere sends signals straight down through the midbrain and out to the brainstem nuclei, which then signal the facial muscles. Clinical neurologists know of the distinction between these two ways of controlling facial muscles. For example, a patient with a lesion in the part of the right hemisphere that participates in voluntary expressions is unable to move the left half of the face when told to smile. But the same patient can easily move the left half of the face when spontaneously smiling, because those pathways are unaffected by right-hemisphere damage. In contrast, patients with Parkinson’s disease, whose midbrain nuclei no longer function, are unable to produce spontaneous facial expressions, whereas the pathways that support voluntary expressions work fine. Such patients can lose their masked-face appearance when asked to smile (Figure 4.21).
|
|
|
a
|
b
|
|
FIGURE 4.20 The neural pathways that control voluntary and spontaneous facial expression are different.
(a) Voluntary expressions that can signal intention have their own cortical networks in humans. (b) The neural networks for spontaneous expressions involve older brain circuits and appear to be the same as those in chimpanzees. (inset) The location of the section that has been overlaid onto each face.
|
The Interactions of Attention and Perception The attentional and perceptual abilities of split-brain patients have been extensively explored. After cortical disconnection, perceptual information is not shared between the two cerebral hemispheres. Sometimes the supporting cognitive processes of attentional mechanisms, however, do interact. Some forms of attention are integrated at the subcortical level, and other forms act independently in the separated hemispheres.
We noted earlier that split-brain patients cannot integrate visual information between the two visual fields. When visual information is lateralized to either the left or the right disconnected hemisphere, the unstimulated hemisphere cannot use the information for perceptual analysis. This is also true for certain types of somatosensory information presented to each hand. Although touching any part of the body is noted by either hemisphere, patterned somatosensory information is lateralized. Thus, when holding an object in the left hand, a split-brain patient is unable to find an identical object with the right hand. Some investigators argue that higher order perceptual information is integrated by way of subcortical structures, but others have not replicated these results.

FIGURE 4.21 Facial expressions of two kinds of patients.
The patient in the upper row suffered brain damage to the right hemisphere. (a) The lesion did not interfere in spontaneous expression but (b) it did interfere with voluntary expression. (c) This Parkinson’s disease patient has a typical masked face. Because Parkinson’s disease involves the part of the brain that controls spontaneous facial expression, the faces of these patients, when they are told to smile (d), light up because the other pathway is still intact.
For example, split-brain patients sometimes drew pictures that combined word information presented to the two hemispheres. When “ten” was flashed to one hemisphere and “clock” was flashed to the other, the patient drew a clock set at 10. This outcome initially seemed to imply that subcortical transfer of higher order information was taking place between the hemispheres. Subsequent observations (Figure 4.22; Kingstone & Gazzaniga, 1995), however, suggested that it actually reflects dual hemispheric control of the drawing hand (with control biased to the left hemisphere). When conceptually ambiguous word pairs, such as hot dog, were presented, they were always depicted literally (e.g., a dog panting in the heat) and never as emergent objects (e.g., a frankfurter). This suggests that no transfer of higher order information occurred. Moreover, right- and left-hand drawings often depicted only the words presented to the left hemisphere. The subcortical transfer of information is more apparent than real.
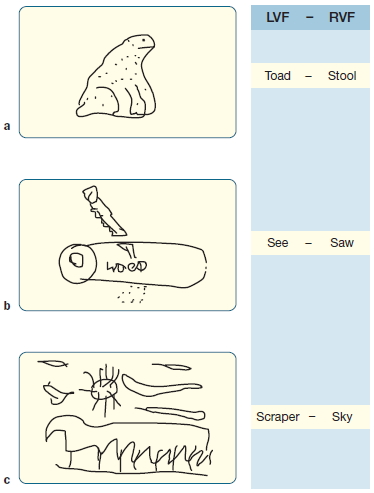
FIGURE 4.22 Pictures drawn by split-brain participant J.W.’s left hand in response to stimuli presented to the left and right visual fields (LVF and RVF).
(a) Drawing of the LVF word Toad (ipsilateral to the drawing hand). (b)Drawing of the RVF Saw (contralateral to the drawing hand). (c)Drawing combining both words: Scraper and Sky (ipsilateral + contralateral).
We have seen that object identification seems to occur in isolation in each hemisphere of split-brain patients. In other studies, evidence suggested that crude information concerning spatial locations can be integrated between the hemispheres. In one set of experiments, the patient fixated on a central point located between two 4-point grids, one in each visual field (Holtzman, 1984). In a given trial, one of the positions on one of the grids was highlighted for 500 msec. Thus information went in to either the left hemisphere or the right hemisphere, depending on which grid was illuminated. For example, in Figure 4.23a, the upper-left point of the grid in the left visual field was highlighted. This information would be registered in the right hemisphere of the subject. After 1 sec, a tone sounded and the subject was asked to move her eyes to the highlighted point within the visual field with the highlighted stimulus. The results were as expected. Information from the left visual field that went to the right hemisphere guided eye movement back to the same location where the light flashed. In the second condition, the subject was required to move her eyes to the relative point in the visual field opposite to the one with the highlighted stimulus (Figure 4.23b). If she could do this, it would mean that information about the location of light stimulus was coming in to the left hemisphere from the right visual field and was guiding her eye movement to the analogous location in the right-brain-controlled left visual field. Split-brain subjects did this task easily. So some type of spatial information is transferred and integrated between the two half brains, enabling attention to be transferred to either visual field. The ability remained intact even when the grid was randomly positioned in the test field.
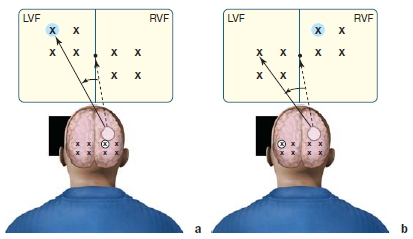
FIGURE 4.23 Cross-integration of spatial information.
(a) On within-field trials, the eye moved to the stimulus that was surrounded by the probe. (b) On between-field trials, the eye moved to the corresponding stimulus in the other hemifield.
These results raised a question: Are the attentional processes associated with spatial information affected by cortical disconnection? As we will see in Chapter 7, surprisingly, split-brain patients can use either hemisphere to direct attention to positions in either the left or the right visual field. This conclusion was based on studies using a modified version of the spatial cuing task (see Figure 7.8 on page 279). In this task, participants respond as quickly as possible upon detecting a target that appears at one of several possible locations. The target is preceded by a cue, either at the target location (a valid cue) or at another location (an invalid cue). Responses are faster on valid trials, indicating spatial orienting to the cued location. In split-brain patients, as with normal participants, a cue to direct attention to a particular point in the visual field was honored no matter which half of the brain was presented with the critical stimulus (Holtzman et al., 1981). These results suggest that the two hemispheres rely on a common orienting system to maintain a single focus of attention.
The discovery that spatial attention can be directed with ease to either visual field raised the question of whether each separate cognitive system in the split-brain patient, if instructed to do so, could independently and simultaneously direct attention to a part of its own visual field. Can the right hemisphere direct attention to a point in the left visual field while the left hemisphere attends to a point in the right visual field? Normal subjects cannot divide their attention that way, but perhaps the split-brain operation frees the two hemispheres from this constraint. As it turns out, the answer is no. The integrated spatial attention system remains intact following cortical disconnection (Reuter-Lorenz & Fendrich, 1990). Thus, as in neurologically intact observers, the attentional system of split-brain patients is unifocal. They, like us, are unable to prepare simultaneously for events taking place in two spatially disparate locations.
The dramatic effects on perception and cognition of disconnecting the cerebral hemispheres initially suggested that each hemisphere has its own attentional resources (Kinsbourne, 1982). If that model were true, then the cognitive operations of one hemisphere, no matter what the difficulty, would have little influence on the cognitive activities of the other. The left brain could be solving a differential equation while the right brain was planning for next weekend. The alternative view is that the brain has limited resources to manage such processes: If most of our resources are being applied to solving our math problems, then fewer resources are available for planning the weekend’s activities. This phenomenon has been studied extensively, and all of the results have confirmed that the latter model is correct: Our central resources are limited.

FIGURE 4.24 Division of cognitive resources in split brain patients improved visual search performance.
As more items are added to a set, for split brain patients the increase in reaction time for bilateral arrays is only half as fast as when all objects are confined to one side.
Attentional resources are shared. The concept that attentional resources are limited should be distinguished from limitations in processing that are a result of other properties of the sensory systems. Even though the overall resources that a brain commits to a task appear constant, the method of deploying them can vary depending on the task. For example, the time needed to detect a complex object increases as more items are added to the display. Normal control subjects require an additional 70 ms to detect the target when two extra items are added to the display, and another 70 ms for each additional pair of items. In split-brain patients, when the items are distributed across the midline of the visual field (so that objects are in both visual fields—that is, a bilateral array), as opposed to all being in one visual field, the increase in reaction time to added stimuli is cut almost in half (Figure 4.24) (Luck et al., 1989). Two half brains working separately can do the job in half the time that one whole brain can. Division of cognitive resources improved performance. Separation of the hemispheres seems to have turned a unified perceptual system into two simpler perceptual systems that, because they are unable to communicate, don’t “interfere” with each other. The large perceptual problem, which the normal brain faces, is broken down into smaller problems that a half brain can solve when each hemisphere perceives only half the problem. It appears as if the patient’s total information processing capacity has increased so that it is superior to that of normal participants. How can this be, if resources remain constant? This conundrum forces us to consider where resources are applied in a perceptual–motor task.
It appears that each hemisphere employs a different strategy to examine the contents of its visual field. The left hemisphere adopts a helpful cognitive strategy in solving the problem, whereas the right hemisphere does not possess those extra cognitive skills. This phenomenon was shown in a different experiment. Here, the task was to find a black circle in a field of equally numbered black squares and gray circles. Randomly interspersed through the trials were “guided” trials, where the search for the black circle had a guide—that is, a clue: There were fewer black squares in a ratio of about 2:5. A cognitive or “smart” approach would be to use the clue: concentrating on the black colored figures should enable a subject to complete the task faster than concentrating on the circular shaped figures. In two out of three split-brain patients, the left, dominant hemisphere used the clue, which decreased its reaction time in the guided trials, but the right hemisphere did not (Kingstone et al., 1995). In control groups, 70 % of people have a faster reaction time to guided trials and use the “smart” strategy. This result indicates that not all people use guided search; but when they do, their left hemisphere is using it. This apparent discrepancy supports other evidence that multiple mechanisms of attention operate at different stages of visual search processing from early to late, some of which might be shared across the disconnected hemispheres and others of which might be independent. Thus, each hemisphere uses the available resources but at different stages of processing.
What’s more, using a “smart strategy” does not mean the left hemisphere is always better at orienting attention. It depends on the job. For instance, the right hemisphere, superior in processing upright faces, automatically shifts attention to where a face is looking; but the left hemisphere does not have the same response to gaze direction (Kingstone et al., 2000).

FIGURE 4.25 Global and local representations.
We represent information at multiple scales. At its most global scale, this drawing is of a house. We can also recognize and focus on the component parts of the house.
When thinking about neural resources and their limitations, people often consider the mechanisms that are being engaged while performing voluntary processing. For example, what is happening as we try to rub our stomach, pat our head, and do a calculus problem at the same time? Searching a visual scene, however, calls upon processes that may well be automatic, built-in properties of the visual system itself. Indeed, the hemispheres interact quite differently in how they control reflex and voluntary attention processes. It appears that reflexive automatic attention orienting is independent in the two hemispheres, as the right hemisphere’s automatic shifting of attention to gaze direction indicates. Voluntary attention orienting, however, is a horse of a different color. Here, it appears, the hemispheres are competing, and the left has more say (Kingstone et al., 1995). That these systems are distinct is reflected in the discovery that splitting brains has a different effect on the processes.
Global and local processing. What is the picture in Figure 4.25? A house, right? Now describe it more fully. You might note its architectural style, and you might point out the detailing on the front door, the double hung windows running across the front façade, and the shingled roof. In recounting the picture, you would have provided a hierarchical description. The house can be described on multiple levels: Its shape and attributes indicate it is a house. But it is also a specific house, with a specific configuration of doors, windows, and materials. This description is hierarchical in that the finer levels of description are embedded in the higher levels. The shape of the house evolves from the configuration of its component parts—an idea that will be developed in Chapter 6. David Navon (1977) of the University of Haifa introduced a model task for studying hierarchical structure. He created stimuli that could be identified on two different levels (e.g., Figure 4.26). At each level, the stimulus contains an identifiable letter. The critical feature is that the letter defined by the global shape is composed of smaller letters (the local shape). In Figure 4.26a, for example, the global H is composed of local Fs.
FIGURE 4.26 Local and global stimuli used to investigate hierarchical representation.
Each stimulus is composed of a series of identical letters whose global arrangement forms a larger letter. The participants’ task is to indicate whether the stimulus contained an H or an L. When the stimulus set included competing targets at both levels (b), the participants were instructed to respond either to local targets only or to global targets only. Neither target is present in (e).
Navon was interested in how we perceive hierarchical stimuli. He initially found that the perceptual system first extracted the global shape. The time required to identify the global letter was independent of the identity of the constituent elements, but when it came to identifying the small letters, reaction time was slowed if the global shape was incongruent with the local shapes. Subsequent research qualified these conclusions. Global precedence does depend on object size and the number of local elements. Perhaps different processing systems are used for representing local and global information. Lynn Robertson and her colleagues (1988) found evidence that supports this hypothesis. Patients who have a lesion in either the left or right hemisphere were presented with local and global stimuli in the center of view (the critical laterality factor was whether the lesion was in the left or right hemisphere). Patients with left-side lesions were slow to identify local targets, and patients with right-side lesions were slow with global targets, demonstrating that the left hemisphere is more adept at representing local information and the right hemisphere is better with global information.
Keep in mind that both hemispheres can abstract either level of representation, but they differ in how efficiently local and global information are represented. The right is better at the big picture, and the left is more detail oriented. Thus, patients with left-hemisphere lesions are able to analyze the local structure of a hierarchical stimulus, but they must rely on an intact right hemisphere, which is less efficient at abstracting local information. Further support for this idea comes from studies of local and global stimuli with split-brain patients (Robertson et al., 1993). Here, too, patients generally identify targets at either level, regardless of the side of presentation. As with normal participants and patients with unilateral lesions, however, split-brain patients are faster at identifying local targets presented to the right visual field (i.e., the left hemisphere) and global targets presented to the left visual field (i.e., the right hemisphere).
Theory of Mind
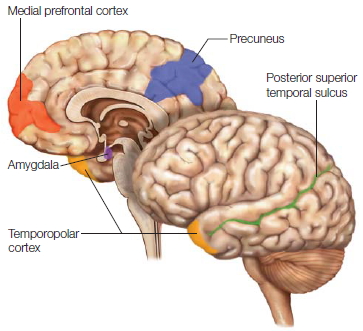
FIGURE 4.27 Theory of mind tasks activate a network of regions bilaterally.
These include the medial prefrontal cortex, posterior superior temporal sulcus, precuneus (hidden in the medial longitudinal fissure in the parietal lobe), and the amygdala-temporopolar cortex. The attribution of beliefs is located in the right hemisphere’s temporal parietal junction.
Theory of mind refers to our ability to understand that other individuals have thoughts, beliefs, and desires. In terms of laterality, theory of mind is an interesting case. You might expect theory of mind to be another hemispheric specialization, lateralized to the left hemisphere like language is, given its dependency on reasoning. Much of the prevailing research on theory of mind, however, suggests that if it is lateralized at all, it is lateralized to the right hemisphere. Many neuroimaging studies show a network of regions in both hemispheres engaged in theory of mind tasks, including the medial prefrontal cortex (PFC), posterior superior temporal sulcus (STS), precuneus, and the amygdala–temporopolar cortex (Figure 4.27). Rebecca Saxe and her colleagues (2009), however, have demonstrated in several fMRI studies, using a version of the false belief task (see Chapter 13), that the critical component of theory of mind, the attribution of beliefs to another person, is localized to the temporal parietal junction in the right hemisphere. This finding may sound merely interesting to you, but to split-brain researchers it was shocking. Think about it for a second. If this information about the beliefs of others is housed in the right hemisphere, and if, in split-brain patients, it isn’t transferred to the speaking, left hemisphere, wouldn’t you expect that these patients would suffer a disruption in social and moral reasoning? Yet they don’t. Split-brain patients act like everyone else. Do these findings also suggest that the recursive nature of thinking about the beliefs of another person is lateralized to the right hemisphere?
A split-brain study by Michael Miller and colleagues at UCSB may provide some insight into these questions (M. Miller et al., 2010). They tested three full-callosotomy patients and three partial-callosotomy patients on a moral reasoning task that depended on the ability to attribute beliefs to another person (the same task, used above by Saxe and colleagues, that produced activations in the right hemisphere). The task involved hearing a scenario in which the actions of an agent conflicted with the beliefs of the agent. For example: Grace works in a chemical plant, and she is fixing coffee for her friend. She adds a white powder to her friend’s coffee, believing that the white powder is sugar. The white powder was mislabeled, however, and was actually quite toxic. Her friend drinks the coffee and dies. After hearing the scenario, the subject is asked this question: Was it morally acceptable for Grace to give the coffee to her friend? Participants with an intact corpus callosum would typically say that it was morally acceptable to give her friend the coffee, because they think Grace believed that the white powder was sugar and intended no harm. That is, they realize that Grace had a false belief.
If the special mechanisms that attribute belief are lateralized to the right hemisphere, then the speaking left hemisphere of the split-brain patients should be cut off from those mechanisms. Split-brain patients would thus respond in a way that relies on the outcome of the actions (i.e., her friend died) and is not based on the beliefs of the actors. Children younger than age 4 typically respond in this way (because they do not yet have a fully developed theory of mind). Indeed, Miller and colleagues found that all of the split-brain patients responded that Grace’s action was morally unacceptable.
This intriguing result leaves open a question: If splitbrain patients are cut off from this important theory-of-mind mechanism, then why don’t they act like severely autistic patients, who are unable to comprehend the thinking and beliefs of other people? Some scientists have suggested that the specialized mechanism observed in the right hemisphere may be used for the fast, automatic processing of belief attributions, and that slower, more deliberate reasoning mechanisms of the left hemisphere could perform the same function given time for deliberation. In fact, Miller and colleagues observed that patients in the moral reasoning study were often uncomfortable with their initial judgments. They would offer spontaneous rationalizations for responding in a particular way. For example, in another scenario, a waitress knowingly served sesame seeds to somebody who she believed was highly allergic to them. The outcome, however, was harmless, because the person was not allergic. The patient judged the waitress’s action to be morally acceptable. Some moments later, however, he appeared to rationalize his response by saying, “Sesame seeds are tiny little things. They don’t hurt nobody.” This patient had to square his automatic response, which did not benefit from information about the belief state of the waitress, with what he rationally and consciously knew is permissible in the world. This brings us to a discussion of the left brain interpreter mechanism.
The Interpreter
A hallmark of human intelligence is our ability to make causal interpretations about the world around us, to formulate hypotheses and predictions about the events and actions in our lives, and to create a continuous sensible narrative about our place in the world. This ability allows us to adapt to a constantly changing world and easily solve problems that may arise. We make the causal interpretations almost on a moment-to-moment basis without realizing it. Imagine going to a movie on a sunny afternoon. Before entering the theater, you notice that the street and parking lot are dry, and only a few clouds are in the sky. Once the movie is over, however, and you walk back outside, the sky is gray and the ground is very wet. What do you instantly assume? You would probably assume that it rained while you were watching the movie. Even though you did not witness the rain and nobody told you that it had rained, you make that interpretation based on the evidence of the wet ground and gray skies. This ability to make interpretations is a critical component of our intellect.
After a callosotomy surgery, the verbal intelligence and problem-solving skills of a split-brain patient remain relatively intact. There may be minor deficits, including free recall ability, but for the most part intelligence remains unchanged. An intact intelligence, however, is true only for the speaking left hemisphere, not for the right hemisphere. The intellectual abilities and problem-solving skills of the right hemisphere are seriously impoverished. A large part of the right hemisphere’s impoverishment can be attributed to the finding that causal inferences and interpretations appear to be a specialized ability of the left hemisphere. One of the authors (MSG) has referred to this unique specialization as the interpreter.
The interpreter has revealed itself in many classic experiments over the years. A typical observation is when the speaking left hemisphere offers up some kind of rationalization to explain the actions that were initiated by the right hemisphere, but whose motivation for the actions are unknown to the left hemisphere. For example, when the split-brain patient P.S. was given the command to stand up in a way that only the right hemisphere could view, P.S. stood up. When the experimenter asked him why he was standing, P.S.’s speaking left hemisphere immediately came up with a plausible explanation: “Oh, I felt like getting a Coke.” If his corpus callosum were intact, then P.S. would have responded that he stood up because that was the instruction he had received.
The effects of the interpreter manifest itself in a number of ways. Sometimes it interprets the actions initiated by the right hemisphere, as in the example just described, but sometimes it interprets the moods caused by the experiences of the right hemisphere. Emotional states appear to transfer between the hemispheres subcortically, so severing the corpus callosum does not prevent the emotional state of the right hemisphere from being transferred to the left hemisphere, even though all of the perceptions and experiences leading up to that emotional state are still isolated. One of the authors (MSG) reported on a case in which he showed some negatively arousing stimuli to the right hemisphere alone. The patient denied seeing anything; but at the same time, she was visibly upset. Her left hemisphere felt the autonomic response to the emotional stimulus, but had no idea what had caused it. When asked what was upsetting, her left brain responded that the experimenter was upsetting her. In this case, the left hemisphere felt the valence of the emotion but was unable to explain the actual cause of it, so the interpreter constructed a theory from the available information.
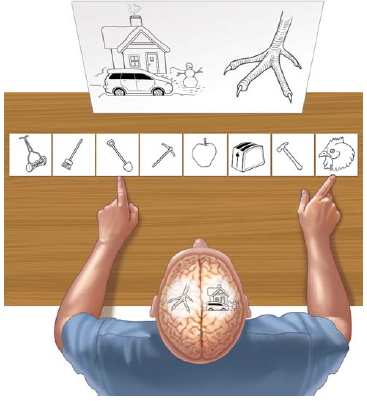
FIGURE 4.28 The Interpreter at work.
Split brain patient P.S. His left hemisphere had seen a chicken claw and his right hemisphere had seen a snow scene. When asked to point to a picture associated with the image he had just seen, his right hand (guided by his left hemisphere) pointed to the chicken (to go with the claw), and his left hand pointed to the shovel (“to clean out the chicken shed”).
Probably the most notable example of the interpreter at work is an experiment done by Gazzaniga and Joseph LeDoux (1978) using a simultaneous concept task. A split-brain patient was shown two pictures, one exclusively to the left hemisphere and one exclusively to the right. Then he was asked to choose, from an array of pictures placed in full view in front of him, those that were associated with the pictures lateralized to the left and right sides of the brain (Figure 4.28). In one example of this kind of test, a picture of a chicken claw was flashed to the left hemisphere and a picture of a snow scene to the right hemisphere. Of the array of pictures placed in front of the subject, the obviously correct association is a chicken for the chicken claw and a shovel for the snow scene. Patient P.S. responded by choosing the shovel with the left hand and the chicken with the right. When asked why he chose these items, he (his left hemisphere) replied, “Oh, that’s simple. The chicken claw goes with the chicken, and you need a shovel to clean out the chicken shed.” Remember that the left brain has no knowledge about the snow scene or why he picked the shovel. The left brain, having seen the left hand’s response, has to interpret that response in a context consistent with what it knows. What it knows is that there is a chicken, and his left hand is pointing to a shovel. It does not have a clue about a snow scene. What is the first sensible explanation it can come up with? Ahh—the chicken shed is full of chicken manure that must be cleaned out.
The interpreter can affect a variety of cognitive processes. For example, it may be a major contributor to the distortion of memories. In a study by Elizabeth Phelps and one of the authors (MSG), split-brain patients were asked to examine a series of pictures that depicted an everyday storyline, such as a man getting out of bed and getting ready for work (Phelps & Gazzaniga, 1992). During a recognition test, the patients were shown an intermingled series of photos that included the previously studied pictures, new pictures unrelated to the storyline, and new pictures that were closely related to the storyline (Figure 4.29). The left hemisphere falsely recognized the new pictures related to the story, while the right hemisphere rarely made that mistake. Both hemispheres were equally good at recognizing the previously studied pictures and rejecting new unrelated pictures. The right hemisphere, however, was more accurate at weeding out the new related pictures. Because of the left hemisphere’s tendency to make an inference that something must have occurred since it fit with its general schema of the event, it falsely recognized new related photos.

|

|
FIGURE 4.29 Split-brain patients first examined a series of pictures that told the story of a man getting up in the morning and getting ready to go to work.
A recognition test was done a while later testing each hemisphere separately. In this test the patients were shown a stack of pictures that included the original pictures and other pictures, some that had no relation to the story and others that could have been part of the story but weren’t.
|
George Wolford and colleagues at Dartmouth College also demonstrated this phenomenon using a probability-guessing paradigm (Wolford et al., 2000). Participants were presented with a simple task of trying to guess which of two events would happen next. Each event had a different probability of occurrence (e.g., a red stimulus might appear 75 % of the time and a green one 25 % of the time), but the order of occurrence of the events was entirely random. There are two possible strategies for responding in this task: matching and maximizing. In the red–green example, frequency matching would involve guessing red 75 % of the time and guessing green 25 % of the time. Because the order of occurrence was random, this strategy potentially would result in a great number of errors. The second strategy, maximizing, involves simply guessing red every time. That approach ensures an accuracy rate of 75 % because red appeared 75 % of the time. Animals such as rats and goldfish maximize. Humans match. The result is that nonhuman animals perform better than humans in this task. The humans’ use of this suboptimal strategy has been attributed to a propensity to try to find patterns in sequences of events, even when we are told that the sequences are random. In Las Vegas casinos, the house maximizes; you don’t. We all know how that ends up.
Wolford and colleagues tested each hemisphere of splitbrain patients using the probability-guessing paradigm. They found that the left hemisphere used the frequencymatching strategy, whereas the right hemisphere maximized. When patients with unilateral damage to the left or right hemisphere were tested on the probability-guessing paradigm, the findings indicated that damage to the left hemisphere resulted in use of the maximizing strategy, whereas damage to the right hemisphere resulted in use of the suboptimal frequency-matching strategy.
Together, these findings suggest that the right hemisphere outperforms the left hemisphere because the right hemisphere approaches the task in the simplest possible manner, with no attempt to form complicated hypotheses about the task. The left hemisphere, on the other hand, engages in the human tendency to find order in chaos. The left hemisphere persists in forming hypotheses about the sequence of events, even in the face of evidence that no pattern exists. Although this tendency to search for causal relationships has many potential benefits, it can lead to suboptimal behavior when there is no simple causal relationship. Some common errors in decision making are consistent with the notion that we are prone to search for and posit causal relationships, even when the evidence is insufficient or random. This search for causal explanations appears to be a left-hemisphere activity and is the hallmark of the interpreter.
Note, however, that the right hemisphere is not devoid of causal reasoning. Matt Roser and colleagues (2005) discovered that while judgments of causal inference are best when the information is presented in the right visual field to the left hemisphere, judgments of causal perception are better when the information is presented in the left visual field. In one experiment, Roser had both control and splitbrain participants watch a scenario in which two switches are pressed, either alone or together. When switch A is pressed, a light goes on; when B is pressed, it does not go on; when both are pressed, it does come on. When asked what caused the light to come on, only the left brain could make the inference that it was switch A. In a separate test, Roser had the same participants look at films of two balls interacting. Either one ball hits the second and it moves; one hits the second and there is a time gap before it moves; or one comes close, but there is a space gap, and the second one moves. The subject is asked if one ball caused the other to move. In this case, the right brain could determine the causal nature of the collision. These results suggest that the right hemisphere is more adept at detecting that one object is influencing another object in both time and space—computations essential for causal perception.
FIGURE 4.30 The human right hemisphere can process some things better than the left.
While either hemisphere can decide whether the illusory shapes in the left column are “fat” or “thin,” if outlines are added then only the right hemisphere can still tell the difference. The right hemisphere is able to perceive the whole when only a part is visible, known as amodal completion.
To perceive objects in the environment as unified, the visual system must often extrapolate from incomplete information about contours and boundaries. Paul Corballis and colleagues (1999) used stimuli containing illusory contours to reveal that the right hemisphere can perceptually process some things better than the left can. As can be seen in Figure 4.30, both the left and right hemispheres perceived a fat shape in the top left figure and a skinny shape in the lower left figure, but only the right hemisphere could perceive the same shapes in the figures of amodal completion on the right side. Corballis termed this ability by the right hemisphere as the “right hemisphere interpreter.”
The unique specialization of the left hemisphere—the interpreter—allows our mind to seek explanations for internal and external events in order to produce appropriate response behaviors. It is a powerful mechanism that, once glimpsed, makes investigators wonder how often our brains make spurious correlations. As we noted earlier and will see in Chapter 9, the interpreter also attempts to explain our emotional states and moods. Finally, as we discuss at the end of the chapter, this specialization offers us unique insight into the nature of our conscious awareness.
Evidence From Patients With Unilateral Cortical Lesions
Research on hemispheric specialization has not been limited to split-brain studies. Many researchers have examined the performance of patients with unilateral, focal brain lesions, which we present in this section. We then close this portion of the chapter with some clever experimental designs that test the differential processing of the two hemispheres in people with intact brains.
When testing patients having unilateral brain lesions, the basic idea has been to compare the performance of patients with right-hemisphere lesions against those with left-hemisphere lesions. An appealing feature of this approach is that there is no need to lateralize the stimuli to one side or the other. Laterality effects are assumed to arise because of the unilateral lesions. If lesions to the left hemisphere result in more disruption in reading tasks, for example, then the deficit is attributed to the hemisphere’s specialization in reading processes. To properly interpret these types of studies, it is necessary to carry out double dissociations (see Chapter 3) to determine whether similar lesions to the opposite hemisphere produce a similar deficit. For instance, it has been demonstrated consistently that lesions in the left hemisphere can produce deficits in language functions (such as speaking and reading) that are not seen in patients with comparable lesions to the right hemisphere. Similarly, lesions to the right hemisphere can disrupt spatial orientation, such as the ability to accurately locate visually presented items. Comparable lesions to the left hemisphere do not cause corresponding spatial deficits.
FIGURE 4.31 Interhemispheric communication in split-brain monkeys.
Experimental setup; details are in the text. (b) Spatial error was measured by the difference between the end of the second eye movement and the target location. Accuracy was near perfect when the second eye movement was in the same direction as the first (red curve). During the initial test sessions, the monkey failed to move its eyes to the second location in the across-hemifield condition (blue curve) and generally moved its eyes straight above the end of the first eye movement. The increase in error starting around the fifth session occurred when the animal generated large eye movements in attempting to locate the second target. With subsequent sessions, performance quickly improved, and eventually the monkey was equally accurate in both conditions, suggesting that interhemispheric transfer could be accomplished by intact subcortical pathways.
Because information can travel along multiple pathways through the brain, it is important to study lateralization by comparing results of experiments using a number of independent methods. Are interhemispheric connections between the two halves of the cerebral cortex always necessary for spatial processing? Carol Colby and her colleagues at the University of Pittsburgh (Berman et al., 2005) used a clever method to ask if updating of spatial information can occur without a corpus callosum. They based their experiment on the understanding that our brain constructs a dynamic map of space as our eyes move about collecting visual information. Further, this information—stored as retinotopic coordinates—can be updated as we “scan” our memory to reconstruct where something was previously located. First, split-brain monkeys (including the anterior commissure), while focusing on a fixation point (FP in Figure 4.31), were shown two targets in succession: T1 remained on the screen, and T2 was rapidly extinguished. Next, the monkeys had to turn their gaze to T1 (first eye movement) and then, from memory, they were to look toward the location of T2 (second eye movement). Neurophysiological studies have shown that when the eyes move to the first target, the retinotopic coordinates of the second target are updated in our memory. Interestingly, when T2 was located between FP and T1, the memory trace of T2’s location shifts between the monkey’s hemispheres (see the left-hand panel in Figure 4.31a). This happens because T2 was seen in the right visual field when the monkey was staring at FP; but when its gaze shifts to T1, then the relative position of T2 is now left of the location of T1, so it is now considered to be in the left visual field, which is mapped onto the right hemisphere. (Recall that our visual system is highly contralateralized.) If this shift requires the corpus callosum, animals that have undergone the callosotomy procedure should fail miserably. And they do, for a while. Surprisingly, though, the animals quickly mastered the task (blue curve in Figure 4.31b). One hypothesis is that, in the absence of transcallosal connections, subcortical pathways may be sufficient to support the transfer of visuospatial information.
In extreme cases in humans, however, the hemispheric biases for one level of representation can completely override other levels. In the case study at the beginning of this chapter, W.J. was unable to manipulate blocks into their global configuration when he was restricted to using his right hand. Similar dramatic things happen with stroke victims. Figure 4.32 displays drawings made by patients who recently had a stroke in either the right or the left hemisphere. They were shown a hierarchical stimulus and asked to reproduce it from memory. Drawings from patients with left-hemisphere lesions faithfully followed the contour, but without any hint of local elements. In contrast, patients with right-hemisphere lesions produced only local elements. Note that this pattern was consistent whether the stimuli were linguistic or nonlinguistic; hence, the representational deficits were not restricted to certain stimuli. Note also that, because of the plasticity of the brain, such stark differences might dissipate and not be seen months after the stroke.
Evidence From the Normal Brain

FIGURE 4.32 Extreme failures of hierarchical processing following brain damage.
Two patients were asked to draw the two figures shown in the left column of each panel. The patient with right-hemisphere damage was quite accurate in producing the local element—the Z in (a) or the square in (b)—but failed to arrange these elements into the correct global configuration. The patient with left-hemisphere damage drew the overall shapes but left out all of the local elements. Note that for each patient, the drawings were quite consistent for both the linguistic (a) and the nonlinguistic (b) stimuli, suggesting a task-independent representational deficit.
Researchers have also designed clever experiments to test the differential processing of the two hemispheres in people with intact brains. In the visual domain, comparisons are made between presentations of stimuli to the left or right visual field. Although this procedure ensures that information will be projected initially to the contralateral hemisphere, the potential for rapid transcortical transfer is high. Even so, consistent differences are observed depending on which visual hemifield is stimulated. For example, participants are more adept at recognizing whether a briefly presented string of letters forms a word when the stimulus is shown in the right visual field than they are when it is presented in the left visual field. Such results led to the hypotheses that transfer of information between the hemispheres is of limited functional utility, or that the information becomes degraded during transfer. Thus, we conclude that performance is dominated by the contralateral hemisphere with peripheral visual input.
Studies of auditory perception similarly attempt to isolate the input to one hemisphere. As in vision work, the stimuli can be presented monaurally—that is, restricted to one ear. Because auditory pathways are not as strictly lateralized as visual pathways (see Figure 5.3 on p. 168), however, an alternative methodology for isolating the input is the dichotic listening task shown in Figure 4.33a. In this task, introduced in the early 1970s by Doreen Kimura (1973), two competing messages are presented simultaneously, one to each ear, and the subject tries to report both messages. The ipsilateral projections from each ear presumably are suppressed when a message comes over the contralateral pathway from the other ear.
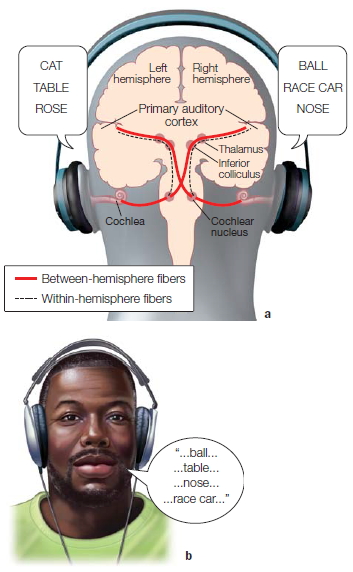
FIGURE 4.33 The dichotic listening task is used to compare hemispheric specialization in auditory perception.
(a) Competing messages are presented, one to the left ear and one to the right ear. Auditory information is projected bilaterally. Although most of the ascending fibers from the cochlear nucleus project to the contralateral thalamus, some fibers ascend on the ipsilateral side. (b) Participants are asked either to report the stimuli or to judge whether a probe stimulus was part of the dichotic message. Comparisons focus on whether they heard the reported information in the right or left ear, with the assumption that the predominant processing occurred in the contralateral hemisphere. With linguistic stimuli, participants are more accurate in reporting the information presented to the right ear.
In a typical study, participants heard a series of dichotically presented words. When asked to repeat as many words as possible, participants consistently produced words that had been presented to the right ear—an effect dubbed the right-ear advantage (Figure 4.33b). Results like these mesh well with expectations that the left hemisphere is dominant for language.
The demonstration of visual and auditory performance asymmetries with lateralized stimuli generated great excitement among psychologists. Here at last were simple methods for learning about hemispheric specialization in neurologically healthy people. It is not surprising that thousands of laterality studies on healthy participants have been conducted using almost every imaginable stimulus manipulation.
The limitations of this kind of laterality research should be kept in mind (Efron, 1990), however.
- The effects are small and inconsistent, perhaps because healthy people have two functioning hemispheres connected by an intact corpus callosum that transfers information quite rapidly.
- There is a bias in the scientific review process to publish papers that find significant differences over papers that report no differences. It is much more exciting to report asymmetries in the way we remember lateralized pictures of faces than to report that effects are similar for right- and left-visual-field presentations.
- Interpretation is problematic. What can be inferred from an observed asymmetry in performance with lateralized stimuli? In the preceding examples, the advantages of the right visual field and the right ear were assumed to reflect that these inputs had better access to the language processes of the left hemisphere. Perhaps, however, people are just better at identifying information in the right visual field or in the right ear.
To rule out this last possibility, investigators must identify tasks that produce an advantage for the left ear or left visual field. For example, shortly after Kimura’s initial work, scientists discovered that people are better at recognizing the left-ear member of dichotic melody pairs; indeed, a double dissociation happens when participants are presented with dichotic pairs of sung melodies (Bartholomeus, 1974). We find a right-ear advantage for the song’s words but a left-ear advantage for its melodies (Figure 4.34).

FIGURE 4.34 A right-ear advantage is not found on all tasks. Participants listened to a dichotic message in which each ear was presented with a series of letters sung to short melodies. When given a recognition memory test, participants were more accurate on the letters task for stimuli heard in the right ear. In contrast, a left-ear advantage was observed when the participants were tested on the melodies.
TAKE-HOME MESSAGES
- The left hemisphere is dominant for language, speech, and major problem solving; the right hemisphere appears specialized for visuospatial tasks such as drawing cubes and other three-dimensional patterns. Thus, split-brain patients cannot name or describe visual and tactile stimuli presented to the right hemisphere, because the sensory information is disconnected from the dominant left (speech) hemisphere.
- There may be two lexicons (associations of words with specific meanings), one in each hemisphere. The right hemisphere’s lexicon seems organized differently from the left hemisphere’s lexicon, and these lexicons are accessed in different ways.
- The right hemisphere has been linked to one aspect of speech perception, prosody, which is the connotative aspect of oral language—the way we vary articulation to convey affect or intonation.
- Some studies show that the right hemisphere is specialized for visuospatial processing.
- The right hemisphere has special processes devoted to the efficient detection of upright faces. The left hemisphere outperforms the right hemisphere when the faces are dissimilar, and the right hemisphere outperforms the left when the faces are similar.
- Although touching any part of the body is noted by either hemisphere, patterned somatosensory information is lateralized. Thus, a split-brain patient who is holding an object in the left hand is unable to find an identical object with the right hand.
- Surprisingly, split-brain patients can use either hemisphere to direct attention to positions in either the left or the right visual field.
- The right hemisphere appears to be specialized for causal perception (the ability to detect that one object is influencing another object in both time and space), and the left hemisphere is more capable with tasks that require causal inference.
- Using Navon’s stimuli, investigators showed that patients with left-sided lesions were slow to identify local targets, and patients with right-sided lesions were slow with global targets, thus demonstrating that the left hemisphere is more adept at representing local information and the right hemisphere is better with global information.
- The left hemisphere contains what Michael Gazzaniga and Joseph LeDoux have called the interpreter, a system that seeks explanations for internal and external events in order to produce appropriate response behaviors.
The Evolutionary Basis of Hemispheric Specialization
So far in this chapter, we have reviewed general principles of hemispheric specialization in humans. Humans, of course, have evolutionary ancestors, so we might expect to find evidence of lateralized functions in other animals. Indeed, this is the case.
Hemispheric Specialization in Nonhumans
Due to the central role of language in hemispheric specialization, laterality research has focused primarily on humans. But the evolutionary pressures that underlie hemispheric specialization—the need for unified action, rapid communication, and reduced costs associated with interhemispheric processing—would also be potentially advantageous to other species. It is now clear that hemispheric specialization is not a uniquely human feature (Bradshaw & Rogers, 1993).
FIGURE 4.35 Visual pathways in birds are completely crossed.
This organization indicates that there is little overlap in the regions of space seen by each eye, and thus the visual input to the left hemisphere is independent of the visual input to the right hemisphere. This anatomical segregation would be expected to favor the emergence of hemispheric asymmetries.
In birds, almost all of the optic fibers cross at the optic chiasm, ensuring that all of the visual input from each eye projects solely to the contralateral hemisphere. The lack of crossed and uncrossed fibers probably reflects the fact that there is little overlap in the visual fields of birds, owing to the lateral placement of the eyes (Figure 4.35). Moreover, birds lack a corpus callosum, so communication between the visual systems within each hemisphere is limited, and functional asymmetries might result. Several asymmetries are known in birds. Chickens and pigeons are better at categorizing stimuli viewed by the right eye and left hemisphere than by the left eye and right hemisphere. You may wonder what is meant when a chicken categorizes stimuli. Here is one such category: Edible or not? Chickens are more proficient in discriminating food from nonfood items when stimuli are presented to the right eye, whereas the right hemisphere (left eye) is more adept when they are trained to respond to unique properties like color, size, and shape, or when the task requires them to learn the exact location of a food source.
Almost all birds have a communication system: They caw, tweet, and chirp to scare away enemies, mark territory, and lure mates. In many species, the mechanisms of song production depend on structures in the left hemisphere. Fernando Nottebohm of Rockefeller University discovered that sectioning the canary’s hypoglossal nerve in its left hemisphere severely disrupted song production (Nottebohm, 1980). In contrast, right hemisphere lesions had little effect. A similar effect can be found in other bird species, although in some species lesions to either hemisphere can interfere with song production.
Nonhuman primates also show differences in hemispheric structure and perhaps function. Old World monkeys and apes have lateral fissures that slope upward in the right hemisphere, similar to the asymmetry found in humans. Whether these anatomical asymmetries are associated with behavioral specializations remains unclear. Unlike humans, however, nonhuman primates do not commonly show a predominance of right-handedness. Individual animals may show a preference for one hand or the other, but there is no consistent trend for the right hand to be favored over the left hand, either when making manual gestures or when using tools, except in one case. The great apes appear to use the right hand and arm when making communicative gestures (Meguerditchian et al., 2010). We will discuss this more in Chapter 11, as it suggests the possibility that gestural communication was a forerunner of language.
Perceptual studies, however, have provided more provocative indications of asymmetrical functions in nonhuman primates. Like humans, rhesus monkeys are better at tactile discriminations of shape when using the left hand. Even more impressive is that splitbrain monkeys and split-brain humans have similar hemispheric interactions in visual perception tasks. For example, in a face recognition task, the monkeys, like humans, have a right-hemisphere advantage; in a line orientation task, the monkeys share a left-hemisphere advantage. The visual system of monkeys, however, transfers visual information across an intact anterior commissure, but there is no transfer of visual information across the human anterior commissure. In addition, left-hemisphere lesions in the Japanese macaque can impair the animal’s ability to comprehend the vocalizations of conspecifics. Unlike the effects on some aphasic patients, however, this deficit is mild and transient. There is also evidence from split-brain monkeys that unlike humans, their left brain is better at spatial judgments. This observation is tantalizing, because it is consistent with the idea that the evolution of language in the left hemisphere has resulted in the loss of some visuospatial abilities.
In summary, like humans, nonhuman species exhibit differences in the function of the two hemispheres. The question remains, how should we interpret these findings? Does the left hemisphere, which specializes in birdsong and human language, reflect a common evolutionary antecedent? If so, this adaptation has an ancient history, because humans and birds have not shared a common ancestor since before the dinosaurs. The hemispheric specialization that occurs in many species may instead reflect a general design principle of the brain.
Modularity
In this chapter, we have reviewed general principles of hemispheric specialization in humans. A first step in understanding why these specializations exist is to look at what is known about the structure of the brain and its organizing principles. In Chapter 2 (see the box “How the Brain Works: Billions and Billions”), we briefly touched on the idea that certain “wiring laws” apply to the evolutionary development of the large human brain (Striedter, 2005). We saw that as the brain grew larger, the proportional connectivity decreases, thus changing the internal structure and resulting in a decrease in overall connectivity.
The wiring plan that evolved, which has a high degree of local efficiency and fast communication with the global network, is known as “small-world” architecture (Watts & Strogatz, 1998). This structure is common to many complex systems, that is, systems whose overall behavior can be characterized as more than the sum of their parts. This mode of organization is characterized by many short connections between components, resulting in faster signaling and lower energy requirements. It also has a high level of clustering, which gives the overall system greater tolerance to the failure of individual components or connections. The local networks in the brain are made up of elements (neurons) that are more highly connected to one another than to elements in other networks. This division of circuits into numerous networks both reduces the interdependence of networks and increases their robustness. What’s more, it facilitates behavioral adaptation (Kirschner & Gerhart, 1998), because each network can both function and change its function without affecting the rest of the system. These local specialized networks, which can perform unique functions and can adapt or evolve to external demands, are known as modules. The general concept of modularity is that the components of a system can be categorized according to their functions (Bassett & Gazzaniga, 2011).
By reducing constraints on change, the principle of modularity forms the structural basis on which subsystems can evolve and adapt (Wagner et al., 2007) in a highly variable environment. Hemispheric specialization takes that idea a step further and says that cerebral asymmetries in this modular organization must also have adaptive value. Therefore, cerebral asymmetries should not be proposed lightly, and investigators must be sure they are real. For instance, during the 1990s, a popular model of the organization of memory in the brain based on early neuroimaging studies suggested that episodic encoding was predominantly a left hemisphere function and that episodic retrieval was predominantly a right hemisphere function (the model was called HERA, for hemispheric encoding/ retrieval asymmetry). When this model was tested directly with split-brain patients, however, it turned out that each hemisphere was equally efficient at encoding and retrieval (M. Miller et al., 2002). This study showed that apparent asymmetries in memory encoding could be produced by varying the stimuli being encoded. Verbal material was preferentially processed in the participants’ left hemisphere, and facial material was preferentially processed in the right—a pattern somewhat reminiscent of the chicken’s and pigeon’s lateralized object discrimination.
Hemispheric Specialization: A Dichotomy in Function or Stylishly Different?
Laterality researchers continually grapple with appropriate ways to describe asymmetries in the function of the two hemispheres (Allen, 1983; Bradshaw & Nettleton, 1981; Bryden, 1982). While early hypotheses fixed on the stimuli’s properties and the tasks employed, a more recent approach is to look for differences in processing style. This concept suggests that the two hemispheres process information in complementary ways, dividing the workload of processing a stimulus by tackling it differently. From this perspective, the left hemisphere has been described as analytic and sequential, and the right hemisphere is viewed as holistic and parallel.
Hemispheric specializations may emerge because certain tasks benefit from one processing style or another. Language, for example, is seen as sequential: We hear speech as a continuous stream that requires rapid dissection and analysis of its component parts. Spatial representations, in contrast, call for not just perceiving the component parts, but seeing them as a coherent whole. The finding that the right hemisphere is more efficient at global processing is consistent with this idea.
Although this analytic–holistic dichotomy has intuitive appeal, it is difficult to know whether a particular cognitive task would benefit more from analytic or holistic processing. In many cases, the theoretical interpretation disintegrates into a circular re-description of results. For example, a right-ear advantage exists in the perception of consonants, but no asymmetry is found for vowels; consonants require the sequential, analytic processors of the left hemisphere, and vowel perception entails a more holistic form of processing. Here we have redefined the requirements of processing vowels and consonants according to our theoretical framework, rather than using the data to establish and modify that theoretical framework.
With verbal–spatial and analytic–holistic hypotheses, we assume that a single fundamental dichotomy can characterize the differences in function between the two hemispheres. The appeal of “dichotomania” is one of parsimony: The simplest account of hemispheric specialization rests on a single difference. Current dichotomies, however, all have their limitations.
It is also reasonable to suppose that a fundamental dichotomy between the two hemispheres is a fiction. Hemispheric asymmetries have been observed in many task domains: language, motor control, attention, and object recognition. Perhaps specializations are specific to particular task domains and are the consequences of more primitive hemispheric specializations. There need not be a causal connection between hemispheric specialization in motor control (e.g., why people are right or left-handed) and hemispheric differences in representing language or visuospatial information. Maybe the commonality across task domains is their evolution: As the two hemispheres became segregated, they shared an impetus for the evolution of systems that were non-identical.
Asymmetry in how information is processed, represented, and used may be a more efficient and flexible design principle than redundancy across the hemispheres. With a growing demand for cortical space, perhaps the forces of natural selection began to modify one hemisphere but not the other. Because the corpus callosum exchanges information between the hemispheres, mutational events could occur in one lateralized cortical area while leaving the contralateral hemisphere intact, thus continuing to provide the previous cortical function to the entire cognitive system. In short, asymmetrical development allowed for no-cost extensions; cortical capacity could expand by reducing redundancy and extending its space for new cortical zones. Support for this idea is provided by the fascinating work of Galuske and colleagues, which has revealed that differences in the neuronal organization of the left and right Brodmann area 22 are related to the processing of auditory signals associated with human speech (Galuske et al., 2000; Gazzaniga, 2000). The left is specialized for word detection and generation; the right is specialized for melody, pitch, and intensity, which are properties of all auditory communication from bird tweets to monkey calls.
The idea of asymmetrical processing also underscores an important point in modern conceptualizations of hemispheric specialization—namely, that the two hemispheres may work in concert to perform a task, even though their contributions may vary widely. There is no need to suppose that some sort of master director decides which hemisphere is needed for a task. While language is predominantly the domain of the left hemisphere, the right hemisphere also might contribute, although the types of representations it derives may not be efficient or capable of certain tasks. In addition, the left hemisphere does not defer to the right hemisphere on visuospatial tasks, but processes this information in a different way. By seeing the brain organized in this way, we begin to realize that much of what we learn from clinical tests of hemispheric specialization tells us more about our tasks rather than the computations performed by each hemisphere. This point is also evident in splitbrain research. With the notable exception of speech production, each hemisphere has some competence in every cognitive domain.
Is There a Connection Between Handedness and Left-Hemisphere Language Dominance?
With all this talk of laterality, your left hemisphere no doubt is searching for a causal relationship between the predominance of right-handedness and the left hemisphere’s specialization for language. Join the club. Many researchers have tried to establish a causal relationship between the two by pointing out that the dominant role of the left hemisphere in language strongly correlates with handedness. About 96 % of right-handers are left-hemisphere dominant for speech. Most left-handers (60 %), however, are also left-hemisphere dominant for speech (Risse et al., 1997). Because left-handers constitute only 7 % to 8 % of the total population, this means that 96 % of humans, regardless of which hand is dominant, have a left-hemisphere specialization for language.
Some theorists point to the need for a single motor center as the critical factor. Although there may be benefits to perceiving information in parallel, that is, it is okay for the input to be asymmetric, our response to these stimuli—the output—must be unified. Imagine what it would be like if your left hemisphere could choose one course of action while your right hemisphere opted for another. What happens when one hemisphere is commanding half your body to sit, and the other hemisphere is telling the other half to vacuum? Our brains may have two halves, but we have only one body. By localizing action planning in a single hemisphere, the brain achieves unification.
One hypothesis is that the left hemisphere is specialized for the planning and production of sequential movements. Speech certainly depends on such movements. Our ability to produce speech is the result of many evolutionary changes that include the shape of the vocal tract and articulatory apparatus. These adaptations make it possible for us to communicate, and to do so at phenomenally high rates (think of auctioneers); the official record is 637 words per minute, set on the late-1980s British television show Motormouth. Such competence requires exquisite control of the sequential gestures of the vocal cords, jaw, tongue, and other articulators.
The left hemisphere has also been linked to sequential movements in domains that are not involved with speech. For example, left-hemisphere lesions are more likely to cause apraxia—a deficit in motor planning, in which the ability to produce coherent actions is lost, even though the muscles work properly and the person understands and wants to perform an action (see Chapter 8). In addition, oral movements have left-hemisphere dominance, whether the movements create speech or nonverbal facial gestures. Evidence suggests that facial gestures are more pronounced on the right side of the face, and activation of the right facial muscles occurs more quickly than activation of the corresponding muscles on the left. Time-lapse photography reveals that smiles light up the right side of the face first. Hence, the left hemisphere may have a specialized role in the control of sequential actions, and this role may underlie hemispheric asymmetries in both language and motor functions.
Some have theorized that the recursive processing capabilities used by the speech center are available to other left-hemisphere functions, including control of the right hand. With bipedalism, the hands became free to operate independently. This ability is unlike that of our quadruped friends, whose forelimbs and hind limbs are used primarily for locomotion. Here, symmetry is vital for the animal to move in a linear trajectory. If the limbs on one side of the body were longer or stronger than the other, an animal would move in a circle. As our ancestors adopted an upright posture, however, they no longer had to use their hands to move symmetrically.
The generative and recursive aspects of an emerging communication system also could have been applied to the way hands manipulated objects, and the lateralization of these properties would have favored the right hand. The favoring of one hand over another would be most evident in tool use. Although nonhuman primates and birds can fashion primitive tools to gain access to foods that are out of reach or encased in hard shells, humans manufacture tools generatively: We design tools to solve an immediate problem, and we also can recombine the parts to create new tools. The wheel, an efficient component of devices for transportation, can be used to extract energy from a flowing river or record information in a compact, easily accessible format. Handedness, then, is most apparent in our use of tools. As an example, right-handers differ only slightly in their ability to use either hand to block balls thrown at them. But when they are asked to catch or throw the balls, the dominant hand has a clear advantage.
Or, the situation could have been reversed. The left hemisphere’s dominance in language may be a consequence of an existing specialization in motor control. The asymmetrical use of hands to perform complex actions, including those associated with tool use, may have promoted the development of language. From comparative studies of language, we believe that most sentence forms convey actions; infants issue commands such as “come” or “eat” before they start using adjectives (e.g., “hungry”). If the right hand was being used for many of these actions, there may have been a selective pressure for the left hemisphere to be more proficient in establishing these symbolic representations.
HOW THE BRAIN WORKS
To Approach or Withdraw: The Cerebral Tug-of-War
It is Friday night, and you are heading to a party at the apartment of a friend of a friend. You arrive and look around: Loud music and swirling bodies move about the living room, and a throng has gathered in the kitchen around a counter laid out with chips and dips. Unfortunately, your friend is nowhere to be seen, and you have yet to recognize a single person among the crowd.
Your reaction will depend on a number of factors: how comfortable you feel mingling with strangers, how lively you are feeling tonight, whether a host approaches and introduces you to a few of the guests. Unless you have a flair for flamboyance, you are unlikely to head straight to the dance floor. A more likely response is that you will head for the kitchen and find yourself something to drink.
Richard Davidson (1995) of the University of Wisconsin proposed that the fundamental tension for any mobile organism is between approach and withdrawal. Is a stimulus a potential food source to be approached and gobbled up, or a potential predator that must be avoided? Even the most primitive organisms display at least a rudimentary distinction between approach and withdrawal behaviors. The evolution of more complex nervous systems has provided mechanisms to modulate the tension between these two behavioral poles: We might overcome our initial reaction to flee the party, knowing that if we stay we are likely to make a few new friends and have a few good laughs.
According to Davidson, this tension involves a delicate interplay between processing within the medial regions of the prefrontal cortex in the right and left cerebral hemispheres. The prefrontal cortex is a major point of convergence in the central nervous system, processing information not only from other cortical regions but also from subcortical regions, especially those involved in emotional processing (see Chapter 10). In Davidson’s theory, these inputs are processed asymmetrically. Left-hemisphere processing is biased to promote approach behaviors; in contrast, right-hemisphere processing is biased to promote withdrawal behaviors.
This theory has provided an organizing principle to evaluate the changes in behavior that follow neurological damage. For example, damage to the left frontal lobe can result in severe depression, a state in which the primary symptom is withdrawal and inactivity. Although we might expect depression to be a normal response to brain injury, the opposite profile has been reported in patients with right frontal damage. These patients may appear manic. Damage to the right-hemisphere “withdrawal” system biases the patient to be socially engaging, even when such behaviors are no longer appropriate.
More compelling evidence comes from physiological studies that have looked at the brain’s response to affective, or emotional, stimuli (Gur et al., 1994). By their very nature, positive stimuli are likely to elicit approach, and negative stimuli will elicit withdrawal or avoidance. Thus, depending on its valence, an affective stimulus is likely to engage the two hemispheres differentially.
Davidson (1995) tested this idea by taking electroencephalographic (EEG) measurements while participants viewed short video clips that were chosen to evoke either positive (e.g., a puppy playing with flowers) or negative (e.g., a leg being amputated) emotional reactions. The EEG activity during these stimuli was compared to that during a baseline condition in which the participants watched a neutral video segment. As predicted, more neural activity was observed over the left frontal lobe when the participants watched the positive videos in comparison to the negative videos. In contrast, a huge increase in activity over the right frontal lobe was recorded while participants viewed the disturbing video.
There are, of course, individual differences in this cerebral tug-of-war between approach and withdrawal. Depression has been linked to an abnormal imbalance favoring neural activity in the right hemisphere. Whether the imbalance preceded or followed the depression remains unclear. More provocative, EEG asymmetries in 3-year-old children are correlated with how well the kids tolerate being separated from their mothers. Children showing higher basal EEG activity in the right hemisphere are more inhibited, staying next to their mother even when surrounded by an array of new toys. Children with higher basal EEG activity in the left hemisphere are quite content to leave their mother to play with the toys.
The study of hemispheric asymmetries in emotion is in its infancy. Before the 1990s, physiological studies of emotion generally focused on interactions between the subcortical limbic system and the cortex. In developing his account of cortical differences, Davidson started from a consideration of a marked behavioral dichotomy. What remains to be explored are the computations that might lead to one type of behavior over another, and whether these computations are related to those uncovered in the study of hemispheric specialization in other cognitive domains.
In the interim, however, we might cull from this work one strategy to test the next time we find ourselves alone at a party: Start talking to someone, just to get the left hemisphere active. Perhaps the reason why the left hemisphere appears specialized to promote approach behavior is its dominance in language, that most social of all behaviors.
But remember, correlation is not causation. It is also possible (and your left brain is just going to have to get over it) that the mechanisms producing hemispheric specialization in language and motor performance are unrelated. The correlation between these two cardinal signs of hemispheric asymmetry is not perfect. Not only do a small percentage of right-handers exhibit either right hemisphere language or bilateral language, but in at least half of the left-handed population, the left hemisphere is dominant for language.
These differences may reflect the fact that handedness is affected at least partly by environmental factors. Children may be encouraged to use one hand over the other, perhaps owing to cultural biases or to parental pressure. Or handedness and language dominance may simply reflect different factors. Fred Previc (1991), a researcher with the U.S. Air Force, proposed an intriguing hypothesis along these lines. According to Previc, the left-hemisphere dominance for language is related primarily to a subtle asymmetry in the skull’s structure. In most individuals, the orofacial bones on the left side of the face are slightly larger—an enlargement that encroaches on middle-ear function and could limit the sensitivity to certain sound frequencies. Previc maintained that this enlargement has a deleterious effect on the projection of auditory information to the right hemisphere, especially in the frequency region that carries most of the critical information for speech. As such, the left hemisphere is favored for phonemic analysis and develops a specialization for language.

FIGURE 4.36 The womb may affect postnatal manual coordination.
According to Fred Previc, functional asymmetries in manual coordination are sometimes attributed to the prenatal environment of the fetus. The position of the fetus in the uterus is thought to influence prenatal vestibular experience. Most fetuses are oriented with the right ear facing outward, resulting in a larger vestibular signal in the right hemisphere. At birth, the left side of the body is more stable, freeing the right hand for exploration.
In contrast to this explanation of hemispheric specialization, Previc (1991) argued that handedness is determined by the position of the fetus during gestation (Figure 4.36). Two thirds of fetuses are oriented with the head downward and the right ear facing the mother’s front. This orientation leads to greater in vitro stimulation of the left utricle, part of the vestibular apparatus in the inner ear that is critical for balance. This asymmetrical stimulation will lead to a more developed vestibular system in the right side of the brain, causing babies to be born with a bias to use the left side of the body for balance and the maintenance of posture. Thus the right side of the body is freed for more exploratory movement, resulting in right-handedness. This still leaves 33 % with reversed symmetry, but only 7 % to 8 % of the population actually is reversed. So other factors, either environmental or biological, likely play a role. According to Previc’s theories, different factors determine language asymmetries and handedness. Current data are too scant for evaluating either mechanism, but they do raise the interesting possibility that many unrelated factors determine patterns of hemispheric specialization.
Several genetic models attempt to explain the distribution of handedness among humans. One model states that one gene has two alleles: The D (as in the Latin dextro) allele specifies right-handedness, and the C allele leaves the handedness to chance. In this model, 100 % of DD individuals are right-handed, 75 % of the heterozygotes (CD) are right-handed, and 50 % of CC individuals are right-handed (McManus, 1999). Marian Annett proposed a different model that could also fit with Previc’s theory, in which handedness exists on a spectrum and the alleles are for cerebral dominance rather than for handedness (Annett, 2002). In her model, right-handedness implies left-hemisphere dominance. Her two alleles are the “right shift” allele (RS+) and an ambivalent allele that has no directional shift (RS-). Homozygous individuals, designated RS++, would be strongly right-handed; heterozygous individuals (RS+-) would be less strongly right-handed; and the handedness of homozygous (RS--) individuals would be up to chance, but still on a spectrum from right- to left-handed, where some would be ambidextrous. Although genes may play a role in handedness or other asymmetries, no genes for handedness have been identified.
TAKE-HOME MESSAGES
- Hemispheric specialization is not a unique human feature, though it is most extensive in humans. The evolutionary pressures underlying hemispheric specialization—the need for unified action, rapid communication, and reduced costs associated with interhemispheric processing—exist across species.
- In general, many tasks can be performed successfully by either hemisphere, although the two hemispheres may differ in efficiency.
- The two hemispheres may work in concert to perform a task, even though their contributions may vary.
Split-Brain Research as a Window into Conscious Experience
As we mentioned at the beginning of the chapter, the fundamental mystery presented by split-brain patients remains unsolved; that is, these patients feel no difference in their conscious experience before and after surgery that disconnects their two hemispheres. This essential finding, along with the discovery of the interpreter, specialized to the left hemisphere, may provide a unique window into the true nature of our conscious experience.
One astonishing quality of split-brain patients is that they are utterly unaware of their special status. Although they have lost the ability to transfer most information between their cerebral hemispheres, it has no impact on their overall psychological state. For example, it doesn’t bother them that following the callosotomy, they have lost the ability to verbalize what is in their left visual field. It is not because they have been warned that it will occur; they do not even comment that it is occurring. The left hemisphere in these patients doesn’t seem to miss the right hemisphere at all. More than that, the left brain acts as if the right brain had never been there. This finding has major implications for understanding the role of the brain in conscious experience.
Perhaps consciousness is not a single, generalized process. Rather, consciousness may be an emergent property, arising out of hundreds or thousands of specialized systems—that is, modules (Gazzaniga, 2011). These specialized neural circuits enable the processing and mental representation of specific aspects of conscious experience. Many of these modules may be connected to some of the other modules, but not to most of them. And these components compete for attention. For instance, the neural circuits responsible for the itch on your back, the memory of Friday night’s date, the rumblings of your stomach, the feeling of the sun on your cheek, and the paper that you are working on are fighting for attention. From moment to moment, different modules win the competition, and its neural representation is what you are conscious of in that moment. This dynamic, moment-to-moment cacophony of systems comprises our consciousness. Yet, the weird thing is that we don’t experience the chatter going on up there as the battle rages. What emerges is a unified experience in which our consciousness flows smoothly from one thought to the next, comprising a single unified narrative. The interpreter is crafting this narrative. This specialized neural system continually interprets and rationalizes our behavior, emotions, and thoughts after they occur.
Remarkably, this view of consciousness is completely dependent on the existence of the specialized modules. If a particular module is impaired or loses its inputs, it alerts the whole system that something is wrong. For example, if the optic nerve is severed, the patient immediately notices that he is blinded. But if the module itself is removed, as in the case of cortical blindness (see Chapter 5), then no warning signal is sent and the specific information processed by that specialized system is no longer acknowledged (out of sight, out of mind—so to speak).
This view explains the phenomenon known as anosognosia, in which patients with certain brain lesions are unaware of and deny that they have clearly observable deficits. For instance, one whole side of their body may be paralyzed, yet they deny they have any problems.
This model of the physical basis of conscious experience can also explain the behavior of split-brain patients. When the left hemisphere’s interpreter does not receive input from any of the right hemisphere’s modules, then the right hemisphere and any knowledge of the right hemisphere cease to consciously exist. Thus, the splitbrain patient’s speaking left brain never complains about the shortcomings it may be experiencing due to its disconnection from the right brain. It doesn’t know there are any. Some may argue that this is because the right hemisphere contributes little to cognition, but we have seen in this chapter that the right brain is clearly superior at a number of tasks, including part–whole relations, spatial relationships, spatial matching, veridical memory recollections, amodal completion, causal perception, and processing faces. The right hemisphere must contribute to conscious experience when the corpus callosum is intact; yet when severed, the right hemisphere is not missed. This observation is in synch with the idea that our entire conscious experience arises out of the moment-to-moment tussle as an untold number of specialized modules in the brain are vying for attention, while the left hemisphere’s interpreter strings them together in a coherent narrative.
Summary
Research on laterality has provided extensive insights into the organization of the human brain. Surgical disconnection of the cerebral hemispheres has produced an extraordinary opportunity to study how perceptual and cognitive processes are distributed and coordinated within the cerebral cortex. We have seen how visual perceptual information, for example, remains strictly lateralized to one hemisphere following callosal section. Tactile-patterned information also remains lateralized, but attentional mechanisms are not divided by separation of the two hemispheres. Taken together, cortical disconnection produces two independent sensory information-processing systems that call upon a common attentional resource system in the carrying out of perceptual tasks.
Split-brain studies also have revealed the complex mosaic of mental processes that contribute to human cognition. The two hemispheres do not represent information in an identical manner, as evidenced by the fact that each hemisphere has developed its own set of specialized capacities. In the vast majority of individuals, the left hemisphere is clearly dominant for language and speech and seems to possess a uniquely human capacity to interpret behavior and to construct theories about the relationship between perceived events and feelings. Right-hemisphere superiority, on the other hand, can be seen in tasks such as facial recognition and attentional monitoring. Both hemispheres are likely to be involved in the performance of any complex task, but each contributes in its specialized manner.
Complementary studies on patients with focal brain lesions and on normal participants tested with lateralized stimuli have underscored not only the presence, but the importance, of lateralized processes for cognitive and perceptual tasks. Recent work has moved laterality research toward a more computational account of hemispheric specialization, seeking to explicate the mechanisms underlying many lateralized perceptual phenomena. These theoretical advances have moved the field away from the popular interpretations of cognitive style and have refocused researchers on understanding the computational differences and specializations of cortical regions in the two hemispheres.
Key Terms
anterior commissure (p. 128)
corpus callosum (p. 128)
dichotic listening task (p. 151)
functional asymmetry (p. 126)
heterotopic areas (p. 128)
hierarchical structure (p. 144)
homotopic areas (p. 126)
interpreter (p. 146)
posterior commissure (p. 128)
splenium (p. 128)
Sylvian fissure (p. 125)
transcortical (p. 134)
amobarbital (p. 125)
cerebral specialization (p. 132)
handedness (p. 156)
module (p. 154)
planum temporale (p. 126)
split-brain research (p. 123)
Wada test (p. 125)
Thought Questions
- What have we learned from over 50 years of split-brain research? What are some of the questions that remain to be answered?
- What are the strengths of testing patients who have suffered brain lesions? Are there any shortcomings to this research approach? If so, what are they? What are some of the ethical considerations?
- Why are double dissociations diagnostic of cerebral specializations? What pitfalls exist if a conclusion is based on a single dissociation?
- Why do you think the human brain evolved cognitive systems that are represented asymmetrically between the cerebral hemispheres? What are the advantages of asymmetrical processing? What are some possible disadvantages?
Suggested Reading
Brown, H., & Kosslyn, S. (1993). Cerebral lateralization. Current Opinion in Neurobiology, 3, 183–186.
Gazzaniga, M. S. (2000). Cerebral specialization and interhemispheric communication: Does the corpus callosum enable the human condition? Brain, 123, 1293–1326.
Gazzaniga, M. S. (2005). Forty-five years of split brain research and still going strong. Nature Reviews Neuroscience, 6, 653–659.
Gazzaniga, M. S. (2011). Who’s in charge: Free will and the science of the brain. New York: (Ecco) Harper Row.
Hellige, J. B. (1993). Hemispheric asymmetry: What’s right and what’s left. Cambridge, MA: Harvard University Press.
Hutsler, J., & Galuske, R. A. (2003). Hemispheric asymmetries in cerebral cortical networks. Trends in Neuroscience, 26, 429–435.