
FIGURE 2.1 Jose Delgado halting a charging bull by remote control.

|
You shake my nerves and you rattle my brain. ~ Jerry Lee Lewis |
Chapter 2
Structure and Function of the Nervous System
OUTLINE
The Structure of Neurons
Neuronal Signaling
Synaptic Transmission
The Role of Glial Cells
The Bigger Picture
Overview of Nervous System Structure
A Guided Tour of the Brain
The Cerebral Cortex
Development of the Nervous System
ONE DAY IN 1963, neuroscientist Jose Delgado coolly stood in a bullring in Cordoba, Spain, facing a charging bull. He did not sport the Spanish matador’s typical gear of toreador pants, jacket, and sword, however. No theoretical scientist he, Delgado stepped into the ring in slacks and a pullover sweater while holding a small device in his hand (and a cape, for good effect). He was about to see if it worked. As the bull came charging toward him, Delgado stood his ground, trigger finger itchy on the device’s button. And then he calmly pushed it. The bull slammed on the brakes and skidded to a stop, standing a few feet before the scientist (Figure 2.1). The bull placidly looked at the smiling Delgado. Seemingly, this was no ordinary bull; but yet it was. One odd thing about this bull, however, gave Delgado his confidence: An electric stimulator had been surgically implanted in its caudate nucleus. The device in Delgado’s hand was a transmitter he had built to activate the stimulator. By stimulating the bull’s caudate nucleus, Delgado had turned off its aggression. Years before, Delgado had been horrified by the increasingly popular frontal lobotomy surgical procedure that destroyed brain tissue and function. He was interested in finding a more conservative approach to treating mental disorders through electrical stimulation. Using his knowledge of the electrical nature of neurons, neuroanatomy, and brain function, he designed his devices, the first neural implants ever to be used. Exceedingly controversial at the time, his devices were the forerunners of the now common intracranial devices used for stimulating the brain to treat disorders like Parkinson’s disease, chronic pain, and other maladies.

FIGURE 2.1 Jose Delgado halting a charging bull by remote control.
Delgado understood that our nervous system uses electrochemical energy for communication and that nerves can be thought of as glorified electrical cables running to and from our brains. He also understood that inside our brains, neurons form an intricate wiring pattern: An electrical signal initiated at one location could travel to another location to trigger a muscle to contract or initiate a behavior, such as aggression, to arise or cease. Delgado was banking on the hope that he had figured out the correct circuit involved in aggressive behavior. Delgado’s device was built with the knowledge that neurons use electrochemical signals to communicate. This knowledge is the foundation on which all theories of neuronal signaling are built. Thus, for us, it is important to understand the basic physiology of neurons and the anatomy of the nervous system, which is what this chapter discusses. In many of the following chapters, we will look at what results from the activity within and among specific circuits (i.e., perception, cognition, emotion, action).
Since all theories of how the brain enables the mind must ultimately mesh with the actual nuts and bolts of the nervous system, we need to understand the basics of its organizational structure, function, and modes of communication. In this chapter, we begin with the anatomy of the neuron and an overview of how information is transferred both within a neuron, and from one neuron to the next. Then, we turn to the bigger picture. Our neurons are strung together into circuits that form the brain and extend out to form the entire nervous system. We survey the anatomy and functions of the brain and the nervous system. Finally, we look at the development of the nervous system—prenatally, in the years following birth, and in adults.
The Structure of Neurons
The nervous system is composed of two main classes of cells: neurons and glial cells. Neurons are the basic signaling units that transmit information throughout the nervous system. As Ramón y Cajal and others of his time deduced, neurons take in information, make a “decision” about it following some relatively simple rules, and then, by changes in their activity levels, pass it along to other neurons. Neurons vary in their form, location, and interconnectivity within the nervous system (Figure 2.2), and these variations are closely related to their functions.
Glial cells are nonneural cells that serve various functions in the nervous system, some of which are only now being elucidated. These include providing structural support and electrical insulation to neurons, and modulating neuronal activity. We begin with a look at neuronal structure and function, and then we return to glial cells.
The standard cellular components found in almost all eukaryotic cells are found in neurons as well. A cell membrane encases the cell body (in neurons, it is sometimes called the soma; Greek for “body”), which contains the metabolic machinery that maintains the neuron: a nucleus, endoplasmic reticulum, a cytoskeleton, mitochondria, Golgi apparatus, and other common intracellular organelles (Figure 2.3). These structures are suspended in cytoplasm, the salty intracellular fluid that is made up of a combination of ions, predominantly ions of potassium, sodium, chloride, and calcium, as well as molecules such as proteins. The neuron, like any other cell, sits in a bath of salty extracellular fluid, which is also made up of a mixture of the same types of ions.

|

|

|

|

|

|
|
FIGURE 2.2 Mammalian neurons show enormous anatomical variety. (Clockwise from upper left) Neuron from the vestibular area of the brain—glial cells are the thin white structures (confocal light micrograph); Hippocampal neuron (fluorescent micrograph); Mouse neuron and spinal cord ganglia (transmission electron micrograph); Multipolar neuron cell body from human cerebral cortex (scanning electron micrograph); Neuron from the brain; Nerve culture from dorsal root ganglia of an embryonic rat (fluorescent micrograph). |
||

FIGURE 2.3 Idealized mammalian neuron.
A neuron is composed of three main parts: a cell body, dendrites, and an axon. The cell body contains the cellular machinery for the production of proteins and other cellular macromolecules.
Like other cells, the neuron contains a nucleus, endoplasmic reticulum, ribosomes, mitochondria, Golgi apparatus, and other intracellular organelles (inset). The dendrites and axon are extensions of the cell membrane and contain cytoplasm continuous with that in the cell body.
Neurons, unlike other cells, possess unique cytological features and physiological properties that enable them to transmit and process information rapidly. The two predominant cellular components unique to neurons are the dendrites and axon. Dendrites are branching extensions of the neuron that receive inputs from other neurons. They take many varied and complex forms, depending on the type and location of the neuron. The arborizations may look like the branches and twigs of an old oak tree, as seen in the complex dendritic structures of the cerebellar Purkinje cells (Figure 2.4), or they may be much simpler, such as the dendrites in spinal motor neurons (Figure 2.5). Many dendrites also have specialized processes called spines, little knobs attached by small necks to the surface of the dendrites, where the dendrites receive inputs from other neurons (Figure 2.6).

|

|
| a | b |
|
FIGURE 2.4 Soma and dendritic tree of a Purkinje cell from the cerebellum. |
|

|

|
| a | b |
|
FIGURE 2.5 Spinal motor neuron. |
|

FIGURE 2.6 Dendritic spines on cultured rat hippocampal neurons.
Neuron has been triple stained to reveal the cell body (blue), dendrites (green), and the spines (red).
The axon is a single process that extends from the cell body. This structure represents the output side of the neuron. Electrical signals travel along the length of the axon to its end, the axon terminals, where the neuron transmits the signal to other neurons or other cell types. Transmission occurs at the synapse, a specialized structure where two neurons come into close contact so that chemical or electrical signals can be passed from one cell to the next. Some axons branch to form axon collaterals that can transmit signals to more than one cell (Figure 2.7). Many axons are wrapped in layers of a fatty substance called myelin. Along the length of the axons, there are evenly spaced gaps in the myelin. These gaps are commonly referred to as the nodes of Ranvier (see Figure 2.11), named after the French histologist and anatomist Louis-Antoine Ranvier, who first described them. Later, when we look at how signals move down an axon, we will explore the role of myelin and the nodes of Ranvier in accelerating signal transmission.
TAKE-HOME MESSAGES

|
|
FIGURE 2.7 Axons can take different forms. |
Neuronal Signaling
Neurons receive, evaluate, and transmit information. This process is referred to as neuronal signaling. Information is transferred across synapses from one neuron to the next, or from a neuron to a non-neuronal cell such as those in muscles or glands. It is also conveyed within a neuron, being received at synapses on dendrites, conducted within the neuron, transmitted down the axon, and passed along at synapses on the axon terminals. These two types of transport, within and between neurons, are typically handled in different ways. Within a neuron, transferring information involves changes in the electrical state of the neuron as electrical currents flow through the volume of the neuron. Between neurons, information transfer occurs at synapses, typically mediated by chemical signaling molecules (neurotransmitters) but, in some cases, also by electrical signals. Regarding information flow, neurons are referred to as either presynaptic or postsynaptic in relation to any particular synapse. Most neurons are both presynaptic and postsynaptic: They are presynaptic when their axon makes a connection onto other neurons, and postsynaptic when other neurons make a connection onto their dendrites.
The Membrane Potential
The process of signaling has several stages. Let’s return to Delgado’s bull, because his neurons process information in the same way ours do. The bull may have been snorting about in the dirt, his head down, when suddenly a sound wave—produced by Delgado entering the ring—courses down his auditory canal and hits his tympanic membrane (eardrum). The resultant stimulation of the auditory receptor cells (auditory hair cells) generates neural signals that are transmitted via the auditory pathways to the brain. At each stage of this ascending auditory pathway, neurons receive inputs on their dendrites that typically cause them to generate signals that are transmitted to the next neuron in the pathway.
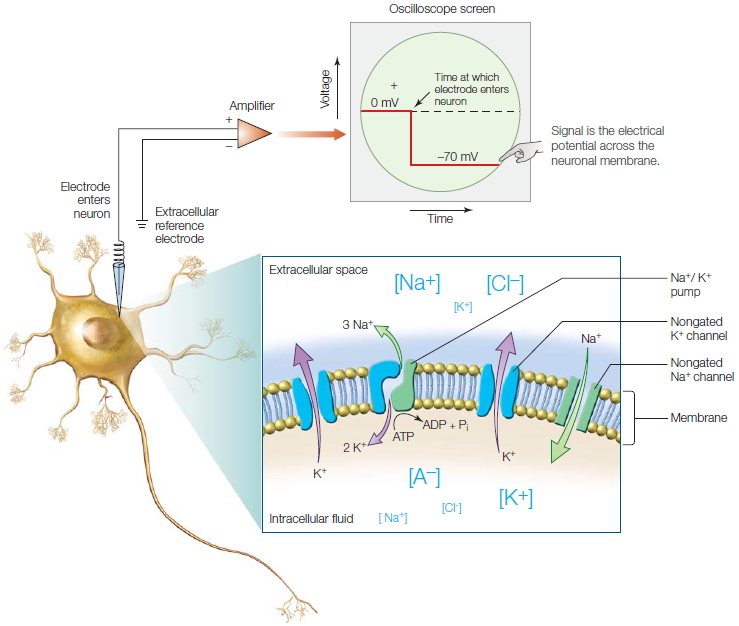
How does the neuron generate these signals, and what are these signals? To answer these questions, we have to understand several things about neurons. First, energy is needed to generate the signals; second, this energy is in the form of an electrical potential across the neuronal membrane. This electrical potential is defined as the difference in the voltage across the neuronal membrane, or put simply, the voltage inside the neuron versus outside the neuron. Third, these two voltages depend on the concentrations of potassium, sodium, and chloride ions as well as on charged protein molecules both inside and outside of the cell. Fourth, when a neuron is not actively signaling—what we call its resting state—the inside of a neuron is more negatively charged than the outside. The voltage difference across the neuronal membrane in the resting state is typically −70 millivolts (mV) inside, which is known as the resting potential or resting membrane potential. This electrical potential difference means that the neuron has at its disposal a kind of battery; and like a battery, the stored energy can be used to do work—signaling work (Figure 2.8).
How does the neuron generate and maintain this resting potential, and how does it use it for signaling? To answer these questions about function, we first need to examine the structures in the neuron that are involved in signaling. The bulk of the neuronal membrane is a bilayer of fatty lipid molecules that separates the cytoplasm from the extracellular milieu. Because the membrane is composed of lipids, it does not dissolve in the watery environments found inside and outside of the neuron. The lipid membrane blocks the flow of watersoluble substances between the inside and the outside of the neuron. It also prevents ions (molecules or atoms that have either a positive or negative electrical charge), proteins, and other water-soluble molecules from moving across it. To understand neuronal signaling, we must focus on ions. This point is important: The lipid membrane maintains the separation of intracellular and extracellular ions and electrical charge that ultimately permits neuronal communication.
|
|
FIGURE 2.8 Ion channels in a segment of neuronal membrane and measuring resting membrane potential. |
The neuronal membrane, though, is not merely a lipid bilayer. The membrane is peppered with transmembrane proteins that serve as conduits for ions to move across the neuronal membrane (Figure 2.8, inset). There are two main types of these proteins: ion channels and ion pumps. Ion channels, as we shall see, are proteins with a pore through their centers, and they allow certain ions to flow down their concentration gradients. Ion pumps use energy to actively transport ions across the membrane against their concentration gradients, that is, from regions of low concentration to regions of higher concentration.
Ion Channels The transmembrane passageways created by ion channels are formed from the three-dimensional structure of these proteins. These hydrophilic channels selectively permit one type of ion to pass through the membrane. The ion channels of concern to us—the ones found in neurons—are selective for either sodium, potassium, calcium, or chloride ions (Na+, K+, Ca2+, and Cl−, respectively; Figure 2.8, inset). The extent to which a particular ion can cross the membrane through a given ion channel is referred to as its permeability. This characteristic of ion channels gives the neuronal membrane the attribute of selective permeability. (Selective permeability is actually a property of all cells in the body; as part of cellular homeostasis, it enables cells to maintain internal chemical stability.) The neuronal membrane is more permeable to K+ than to Na+ (or other) ions, a property that contributes to the resting membrane potential, as we shall learn shortly. The membrane permeability to K+ is larger because there are many more K+-selective channels than any other type of ion channel.

FIGURE 2.9 Ion channels pump ions across the membrane.
The Na+–K+ pump preserves the cell’s resting potential by maintaining a larger concentration of K+ inside the cell and Na+ outside the cell. The pump uses ATP as energy.
Unlike most cells in the body, neurons are excitable, meaning that they can change the permeability of their membranes. This is brought about by ion channels that are capable of changing their permeability for a particular ion. Such proteins are called gated ion channels. They open or close based on changes in nearby transmembrane voltage, or as a response to chemical or physical stimuli. In contrast, ion channels that are unregulated, and hence always allow the associated ion to pass through, are known as nongated ion channels.
Ion Pumps Under normal conditions, there are concentration gradients of different ions across the neuronal membrane. Specifically, Na+ and Cl− concentrations are greater outside of the cell, and K+ concentrations are greater inside the cell. Given that the neuronal membrane contains ion channels that permit the different ions inside and outside of the cell to flow across the neuronal membrane, how does the neuron maintain different concentrations of ions inside compared with outside of the cell? Put another way, why don’t K+ ions flow out of the neuron—down their concentration gradient—until the K+ ion concentrations inside and outside the cell are equal? We can ask the same questions for all other ions. To combat this drive toward equilibrium, neurons use active transport proteins, known as ion pumps. In particular, neurons use a Na+/K+ pump that pumps Na+ ions out of the cell and K+ ions into the cell. Because this process is transporting ions up their concentration gradients, the mechanism requires energy. Each pump is an enzyme that hydrolyzes adenosine triphosphate (ATP). For each molecule of ATP that is hydrolyzed, the resulting energy is used to move three Na+ ions out of the cell and two K+ ions into the cell (Figures 2.8, inset and 2.9). The concentration gradients create forces—the forces of the unequal distribution of ions. The force of the Na+ concentration gradient wants to push Na+ from an area of high concentration to one of low concentration (from outside to inside), while the K+ concentration gradient acts to push K+ from an area of high concentration to an area of low concentration (from inside to outside)—the very thing the pump is working against. Since there are both positively and negatively charged ions inside and outside the cell, why is there a difference in voltage inside versus outside the neuron?

FIGURE 2.10 Selective permeability of the membrane.
The membrane’s selective permeability to some ions, and the concentration gradients formed by active pumping, lead to a difference in electrical potential across the membrane; this is the resting membrane potential. The membrane potential, represented here by the positive charges outside the neuron along the membrane and the negative charges inside along the membrane, is the basis for the transmembrane voltage difference shown in Figure 2.8. Because the concentration gradient for K+ forces K+ out of the cell, a net negative charge develops inside the neuron.
The inside and outside voltages are different because the membrane is more permeable to K+ than to Na+. The force of the K+ concentration gradient pushes some K+ out of the cell, leaving the inside of the neuron slightly more negative than the outside. This creates another force, an electrical gradient, because each K+ ion carries one unit of positive charge out of the neuron as it moves across the membrane. These two gradients (electrical and ionic concentration) are in opposition to one another with respect to K+ (Figure 2.10). As negative charge builds up along the inside of the membrane (and an equivalent positive charge forms along the extracellular side), the positively charged K+ ions outside of the cell are drawn electrically back into the neuron through the same ion channels that are allowing K+ ions to leave the cell by diffusion. Eventually, the force of the concentration gradient pushing K+ out through the K+ channels is equal to the force of the electrical gradient driving K+ in. When that happens, the opposing forces are said to reach electrochemical equilibrium. The difference in charge thus produced across the membrane is the resting membrane potential, that −70 mV difference. The value for the resting membrane potential of any cell can be calculated by using knowledge from electrochemistry, provided that the concentrations of the ions inside and outside the neuron are known.
The Action Potential
We now understand the basis of the energy source that neurons can use for signaling. Next we want to learn how this energy can be used to transmit information within a neuron, from its dendrites that receive inputs from other neurons, to its axon terminals where it makes synapses on the next neurons in the chain. The process begins when excitatory postsynaptic potentials (EPSPs) at synapses on the neuron’s dendrites cause ionic currents to flow in the volume of the cell body. If these currents are strong enough to reach the axon terminals, then the processes of neuronal signaling could be completed. Unfortunately, in the vast majority of cases, this distance is too great for the EPSP to have any effect. Why is this the case?
The small electrical current produced by the EPSP is passively conducted through the cytoplasm of the dendrite, cell body, and axon. Passive current conduction is called electrotonic conduction or decremental conduction. Decremental, because it diminishes with distance from its origin—the synapse, in this case. The maximum distance a passive current will flow is only about 1 millimeter. In most cases, a millimeter is too short to be effective for conducting electrical signals, but in a structure like the retina, a millimeter is enough to permit neuron-to-neuron communication. Most of the time, however, the reduction in signal intensity makes it unlikely that a single EPSP will be enough to trigger the firing of its own cell, much less transmit the signal to another cell (your toes would be in trouble, for example, because they are 1 meter from the spinal cord and close to 2 meters from the brain). How does the neuron solve this problem of decremental conduction and the need to conduct over long distances?
Neurons evolved a clever mechanism to regenerate and pass along the signal initiated in the synapse. It works something like 19th-century firefighters in a bucket brigade, who handed buckets of water from one person to the next along a distance from the source of water to where it was needed at the fire. This regenerative process is an active membrane mechanism known as the action potential. An action potential is a rapid depolarization and repolarization of a small region of the membrane caused by the opening and closing of ion channels.
An action potential is an entirely different animal from the EPSP. Unlike a postsynaptic potential, it doesn’t decrement after only 1 millimeter. Action potentials can travel for meters with no loss in signal strength, because they continuously regenerate the signal. This is one reason there can be giraffes and blue whales. It is, however, metabolically expensive, and it contributes to the inordinate amount of the body’s energy used by the brain.
The action potential is able to regenerate itself due to the presence of voltage-gated ion channels located in the neuronal membrane (Figure 2.11a, inset). These are found at the spike-triggering zone in the axon hillock and along the axon. In myelinated axons, these voltage-gated ion channels are confined to the axon hillock and the nodes of Ranvier (Figure 2.11a). As its name denotes, the spike-triggering zone initiates the action potential. (The term spike is shorthand for an action potential, because when viewed as a recording displayed on an oscilloscope screen, the action potential looks like a little spike in the recorded signal.) How does the spike-triggering zone initiate an action potential?
The passive electrical currents that are generated following EPSPs on multiple distant dendrites sum together at the axon hillock. This current flows across the neuronal membrane in the spike-triggering zone, depolarizing the membrane. If the depolarization is strong enough, meaning the membrane moves from its resting potential of about −70 mV to a less negative value of approximately −55 mV, an action potential is triggered. We refer to this depolarized membrane potential value as the threshold for initiating an action potential. Figure 2.11b illustrates an idealized action potential. The numbered boxes in the figure correspond to the numbered events in the next paragraph. Each event alters a small region of the membrane’s permeability for Na+ and K+ due to the opening and closing of voltage-gated ion channels.

|

|
| a | b |
|
FIGURE 2.11 The neuronal action potential, voltage-gated ion channels, and changes in channel conductance. |
|
When the threshold (Figure 2.11 b, label 1) is reached, voltage-gated Na+ channels open and Na+ flows rapidly into the neuron. This influx of positive ions further depolarizes the neuron, opening additional voltage-gated Na+ channels; thus, the neuron becomes more depolarized (2), continuing the cycle by causing even more Na+ channels to open. This process is called the Hodgkin–Huxley cycle. This rapid, self-reinforcing cycle, lasting only about 1 millisecond, generates the large depolarization that is the first portion of the action potential. Next, the voltagegated K+ channels open, allowing K+ to flow out of the neuron down its concentration gradient. This outward flow of positive ions begins to shift the membrane potential back toward its resting potential (3). The opening of the K+ channels outlasts the closing of the Na+ channels, causing a second repolarizing phase of the action potential; and this drives the membrane potential toward the equilibrium potential of K+, which is even more negative than the resting potential. The equilibrium potential is the particular voltage at which there is no net flux of ions. As a result, (4) the membrane is temporarily hyperpolarized, meaning that the membrane potential is even farther from the threshold required for triggering an action potential (e.g., around −80 mV). Hyperpolarization causes the K+ channels to close, resulting in (5) the membrane potential gradually returning to its resting state. During this transient hyperpolarization state, the voltage-gated Na+ channels are unable to open, and another action potential cannot be generated. This is known as the absolute refractory period. It is followed by the relative refractory period, during which the neuron can generate action potentials, but only with larger-than-normal depolarizing currents. The refractory period lasts only a couple of milliseconds and has two consequences. One is that the neuron’s speed for generating action potentials is limited to about 200 action potentials per second. The other is that the passive current that flows from the action potential cannot reopen the ion-gated channels that generated it. The passive current, however, does flow down the axon with enough strength to depolarize the membrane a bit farther on, opening voltage-gated channels in this next portion of the membrane. The result is that the action potential is propagated down the axon in one direction only—from the axon hillock toward the axon terminal.
So that is the story of the self-regenerating action potential as it propagates itself down an axon (sometimes traveling several meters). But traveling far is not the end of the story. Action potentials must also travel quickly if a person wants to run, or a bull wants to charge, or a very large animal (think blue whale) simply wants to react in a reasonable amount of time. Accelerated transmission of the action potential is accomplished in myelinated axons. The thick lipid sheath of myelin (Figure 2.11a) surrounding the membrane of myelinated axons makes the axon superresistant to voltage loss. The high electrical resistance allows passive currents generated by the action potential to be shunted farther down the axon. The result is that action potentials do not have to be generated as often, and they can be spread out along the axon at wider intervals. Indeed, action potentials in myelinated axons need occur only at the nodes of Ranvier, where myelination is interrupted. This creates the appearance that the action potential is jumping down the axon at great speed, from one node of Ranvier to the next. We call this saltatory conduction. (Saltatory conduction is derived from the Latin word saltare, to jump or leap.) The importance of myelin for efficient neuronal conduction is notable when it is lost, which is what happens when a person is afflicted with multiple sclerosis (MS).
There is one interesting tidbit left concerning action potentials. Action potentials are always the same amplitude; therefore, they are said to be all or none phenomena. Since one action potential is the same amplitude as any other, the strength of the action potential does not communicate anything about the strength of the stimulus. The intensity of a stimulus (e.g., a sensory signal) is communicated by the rate of firing of the action potentials: More intense stimuli elicit higher action potential firing rates.
So, we see how the neuron has solved the problem of long-distance communication as well as communication speed. When the action potential reaches the axon terminal, the signal is now strong enough to cause depolarization of the presynaptic membrane and to trigger neurotransmitter release. The signal is ready to be transferred to the next neuron across the synaptic cleft, the gap between neurons at the synapse.
TAKE-HOME MESSAGES
Synaptic Transmission
A neuron communicates with other neurons, muscles, or glands at a synapse, and the transfer of a signal from the axon terminal to the next cell is called synaptic transmission. There are two major kinds of synapses—chemical and electrical—each using very different mechanisms for synaptic transmission.
Chemical Transmission
Most neurons send a signal to the cell across the synapse by releasing neurotransmitters into the synaptic cleft. The general mechanism is as follows. The arrival of the action potential at the axon terminal leads to the depolarization of the terminal membrane, causing voltage-gated Ca2+ channels to open. The opening of these channels triggers small vesicles containing neurotransmitter to fuse with the membrane at the synapse and release the transmitter into the synaptic cleft. The transmitter diffuses across the cleft and, on reaching the postsynaptic membrane, binds with specific receptors embedded in the postsynaptic membrane (Figure 2.12). Neurotransmitter binding induces a change in the receptor, which opens specific ion channels and results in an influx of ions leading to either depolarization (excitation) or hyperpolarization (inhibition) of the postsynaptic cell (Figure 2.13). Hyperpolarization of the postsynaptic neuron produces an inhibitory postsynaptic potential (IPSP).
Neurotransmitters
The process just described brings us to a hot topic of the popular press: neurotransmitters. While you may have heard of a few of them, more than 100 neurotransmitters have been identified. What makes a molecule a neurotransmitter?
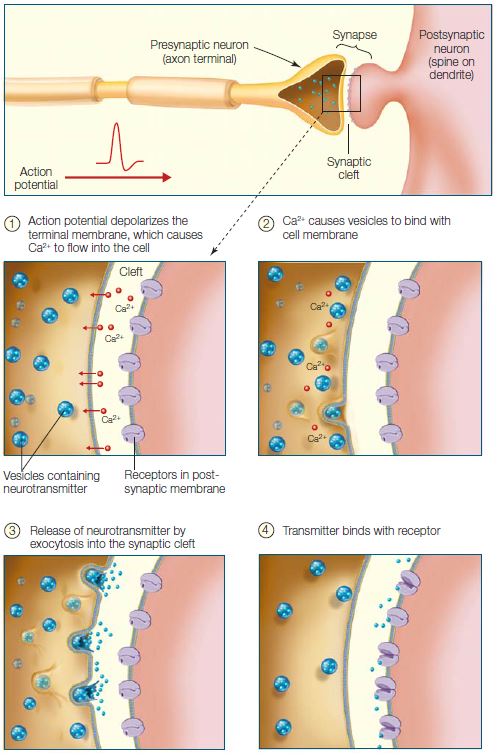
FIGURE 2.12 Neurotransmitter release at the synapse, into synaptic cleft.
The synapse consists of various specializations where the presynaptic and postsynaptic membranes are in close apposition. When the action potential invades the axon terminals, it causes voltage-gated Ca2+ channels to open (1), which triggers vesicles to bind to the presynaptic membrane (2). Neurotransmitter is released into the synaptic cleft by exocytosis and diffuses across the cleft (3). Binding of the neurotransmitter to receptor molecules in the postsynaptic membrane completes the process of transmission (4).
Biochemical Classification of Neurotransmitters Some neurotransmitters are amino acids: aspartate, gamma-aminobutyric acid (GABA), glutamate, and glycine. Another category of neurotransmitters, called biogenic amines, includes dopamine, norepinephrine, and epinephrine (these three are known as the catecholamines), serotonin (5-hydroxytryptamine), and histamine. Acetylcholine (ACh) is a well-studied neurotransmitter that is in its own biochemical class. Another large group of neurotransmitters consists of slightly larger molecules, the neuropeptides (made up of strings of amino acids). More than 100 neuropeptides are active in the mammalian brain, and they are divided into five groups:

FIGURE 2.13 Neurotransmitter leading to postsynaptic potential.
The binding of neurotransmitter to the postsynaptic membrane receptors changes the membrane potential (Vm). These postsynaptic potentials can be either excitatory (depolarizing the membrane), as shown here, or inhibitory (hyperpolarizing the membrane).
Some neurons produce only one type of neurotransmitter, but others produce multiple kinds of neurotransmitters. In the latter case, the neurotransmitters may be released together or separately, depending on the conditions of stimulation. For example, the rate of stimulation by the action potential can induce the release of a specific neurotransmitter.
Functional Classification of Neurotransmitters As mentioned earlier, the effect of a neurotransmitter on the postsynaptic neuron is determined by the postsynaptic receptor rather than by the transmitter itself. That is, the same neurotransmitter released from the same presynaptic neuron onto two different postsynaptic cells might cause one to increase firing and the other to decrease firing, depending on the receptors that the transmitter binds to. The effects of a neurotransmitter also depend on the connections of the neurons that use the transmitter. Nevertheless, neurotransmitters can be classified not only biochemically but also by the typical effect that they induce in the postsynaptic neuron.
Neurotransmitters that usually have an excitatory effect include ACh, the catecholamines, glutamate, histamine, serotonin, and some of the neuropeptides. Usually inhibitory neurotransmitters include GABA, glycine, and some of the peptides. Some neurotransmitters act directly to excite or inhibit a postsynaptic neuron, but other neurotransmitters act only in concert with other factors. These are sometimes referred to as conditional neurotransmitters; that is, their action is conditioned on the presence of another transmitter in the synaptic cleft or activity in the neuronal circuit. These types of mechanisms permit the nervous system to achieve complex modulations of information processing by modulating neurotransmission.
Inactivation of Neurotransmitters after Release
Following the release of neurotransmitter into the synaptic cleft and its binding with the postsynaptic membrane receptors, the remaining transmitter must be removed to prevent further excitatory or inhibitory signal transduction. This removal can be accomplished (a) by active reuptake of the substance back into the presynaptic terminal, (b) by enzymatic breakdown of the transmitter in the synaptic cleft, or (c) merely by diffusion of the neurotransmitter away from the region of the synapse or site of action (e.g., in the case of hormones that act on target cells distant from the synaptic terminals).
Neurotransmitters that are removed from the synaptic cleft by reuptake mechanisms include the biogenic amines (dopamine, norepinephrine, epinephrine, histamine, and serotonin). The reuptake mechanism is mediated by active transporters, which are transmembrane proteins that pump the neurotransmitter back across the presynaptic membrane.
An example of a neurotransmitter that is eliminated from the synaptic cleft by enzymatic action is ACh. The enzyme acetylcholinesterase (AChE), which is located in the synaptic cleft, breaks down ACh after it has acted on the postsynaptic membrane. In fact, special AChE stains (chemicals that bind to AChE) can be used to label AChE on muscle cells, thus revealing where motor neurons innervate the muscle.
To monitor the level of neurotransmitter in the synaptic cleft, presynaptic neurons have autoreceptors. These autoreceptors are located on the presynaptic terminal and bind with the released neurotransmitter, allowing the presynaptic neuron to regulate the synthesis and release of the transmitter.
Electrical Transmission

FIGURE 2.14 Electrical synapse between two neurons.
Electrical synapses are formed by gap junctions, places where multiple transmembrane proteins in the pre- and postsynaptic neurons connect to create pathways that connect the cytoplasms of the two neurons.
Some neurons communicate via electrical synapses. These synapses are very different from chemical synapses—in electrical synapses, no synaptic cleft separates the neurons. Instead, the neuronal membranes are touching at specializations called gap junctions, and the cytoplasms of the two neurons are essentially continuous. These gap junction channels create pores connecting the cytoplasms of the two neurons (Figure 2.14). As a result, the two neurons are isopotential (i.e., have the same electrical potential), meaning that electrical changes in one are reflected instantaneously in the other. Following the principles of electrotonic conduction, however, the passive currents that flow between the neurons when one of them is depolarized (or hyperpolarized) decrease and are therefore smaller in the postsynaptic neuron than in the presynaptic neuron. Under most circumstances, the communication is bidirectional; however, so-called rectifying synapses limit current flow in one direction, as is typical in chemical synapses.
Electrical synapses are useful when information must be conducted rapidly, such as in the escape reflex of some invertebrates. Groups of neurons with these synapses can activate muscles quickly to get the animal out of harm’s way. For example, the well-known tail flip reflex of crayfishes involves powerful rectifying electrical synapses. Electrical synapses are also useful when groups of neurons should operate synchronously, as with some hypothalamic neurosecretory neurons. Electrical synapses also have some limitations: They are much less plastic than chemical synapses, and they cannot amplify a signal (whereas an action potential that triggers a chemical synapse could cause a large release of neurotransmitter, thus amplifying the signal).
TAKE-HOME MESSAGES
The Role of Glial Cells
The other type of cell in the nervous system is the glial cell (also called neuroglial cell). There are roughly as many glial cells in the brain as there are neurons. Located throughout the nervous system, they may account for more than half of the brain’s volume. The term neuroglia means, literally, “nerve glue,” because anatomists in the 19th century believed that the main role of neuroglial cells in the nervous system was structural support. While glial cells do provide structural support, they also carry out other roles in the nervous system, such as helping to form the blood–brain barrier and aiding in the speed of information transfer. More recently, glial cells have revealed a bit of a surprise: They appear to have a previously unrecognized role in modulating neural activity.
The central nervous system has three main types of glial cells: astrocytes, microglial cells, and oligodendrocytes (Figure 2.15). Astrocytes are large glial cells with round or radially symmetrical forms; they surround neurons and are in close contact with the brain’s vasculature. An astrocyte makes contact with blood vessels at specializations called end feet, which permit the astrocyte to transport ions across the vascular wall. The astrocytes create a barrier, called the blood–brain barrier (BBB), between the tissues of the central nervous system and the blood. The BBB restricts the diffusion of microscopic objects (such as most bacteria) and large hydrophilic molecules in the blood from entering the neural tissue, but it allows the diffusion of small hydrophobic molecules such as oxygen, carbon dioxide, and hormones. For example, many drugs and certain neuroactive agents, such as dopamine and norepinephrine, when placed in the blood, cannot cross the BBB. Thus, it plays a vital role in protecting the central nervous system from blood-borne agents such as chemical compounds, as well as pathogens that might unduly affect neuronal activity.

FIGURE 2.15 Various types of glial cells in the mammalian central and peripheral nervous systems.
An astrocyte is shown with end feet attached to a blood vessel. Oligodendrocytes and Schwann cells produce myelin around the axons of neurons—oligodendrocytes in the central nervous system, and Schwann cells in the peripheral nervous system. A microglial cell is also shown.
Astrocytes are recognized for their supporting roles, so to speak, but recent evidence suggests that they have an active role in brain function. In vitro studies indicate that they respond to and release neurotransmitters and other neuroactive substances that affect neuronal activity and modulate synaptic strength. More recently, in vivo studies found that when astrocyte activity is blocked, neural activity increases. This finding supports the notion that neural activity is moderated by astrocyte activity (Schummers et al., 2008). It is hypothesized that astrocytes either directly or indirectly regulate the reuptake of neurotransmitters.
Microglial cells, which are small and irregularly shaped (Figure 2.15), come into play when tissue is damaged. They are phagocytes, literally devouring and removing damaged cells. Unlike many cells in the central nervous system, microglial cells can proliferate even in adults (as do other glial cells).
Glial cells are also the myelin formers in the nervous system. In the central nervous system, oligodendrocytes form myelin; in the peripheral nervous system, Schwann cells carry out this task (Figure 2.15). Both glial cell types create myelin by wrapping their cell membranes around the axon in a concentric manner during development and maturation. The cytoplasm in that portion of the glial cell is squeezed out, leaving primarily the lipid bilayer of the glial cell sheathing the membrane. Myelin is a good electrical insulator because the layers of cell membrane are composed of lipid bilayers, which are themselves poor electrical conductors.
TAKE-HOME MESSAGES
The Bigger Picture
Until now, we have been talking about only one or two neurons at a time. This approach is useful in understanding how neurons transmit information, but it fails to illuminate how the nervous system and the brain function. Neurons rarely work in isolation. Neural communication depends on patterns of connectivity in the nervous system, the neural “highways” that allow information to get from one place to another. Identifying these patterns of connectivity in the nervous system in order to map out the neural highways is tricky because most neurons are not wired together in simple, serial circuits. Instead, neurons are extensively connected in both serial and parallel circuits. A single cortical neuron is likely to be innervated by (i.e., receive inputs from) a large numbers of neurons: A typical cortical neuron has between 1,000 and 5,000 synapses, while a Purkinje neuron may have up to 200,000 synapses. The axons from these input neurons can originate in widely distributed regions. Thus, there is tremendous convergence in the nervous system. There is also divergence, in which a single neuron can project to multiple target neurons in different regions. Although most axons are short projections from neighboring cortical cells, some are quite long, originating in distant cortical regions. These may reach their target only after descending below the cortical sheath into the white matter, traveling through long fiber tracts, and then entering another region of cortex, subcortical nucleus, or spinal layer to synapse on another neuron. Thanks to this extensive interconnectivity, each neuron is only a few synapses away from any other given neuron, and each neuron makes a small contribution to overall function. Connections between two cortical regions are referred to as corticocortical connections, following the convention that the first part of the term identifies the source and the second part identifies the target. Inputs that originate in subcortical structures such as the thalamus would be referred to as thalamocortical connections; the reverse are corticothalamic, or more generally, corticofugal projections (projections extending from more central structures, like cortex, outward toward the periphery).
Groups of interconnected neurons that process specific kinds of information are referred to as neural circuits. Neural circuits have many different forms and purposes. Some are involved in reflexes, such as the “knee-jerk reflex”—a tap by your doctor on your patellar tendon at the knee sends a sensory signal to the spinal cord which stimulates motor neurons to fire action potentials leading to muscle contraction and the brief knee jerk. This is an example of a monosynaptic reflex arc, stimulation of which is used by all physicians to test the integrity of different parts of the nervous system. Other neural circuits throughout the nervous system perform other functions.
In general though, neural circuits share some basic features. They take in information (afferent inputs), they evaluate the input either at a synapse or within one or a group of neurons (local circuit neurons), and they convey the results to other neurons, muscles, or glands (efferent outputs).
One characteristic of some neural circuits is that they show plasticity. The patterns of activation within a neural circuit can change. This is what happens with learning and during development.
Neural circuits, in turn, can be combined to form neural systems. For example, the visual system is composed of many different neural circuits organized in both hierarchical and parallel processing streams to enable vision, and to provide outputs to cognitive and motor systems. Neural circuits involved in the visual system include such things as the retinogeniculostriate circuit that brings information from the eye to the visual cortex. Later in the book we will refer to visual areas, such as visual area V1, which is the striate (primary) visual cortex. Areas are intermediate between neural circuits and systems. That is, the visual system comprises neurons, neural circuits, and visual areas.
But before we can talk about neural circuits, systems, areas, or anything else about the brain for that matter, we need to get some neuroanatomy under our belts. Understanding anatomy is important for understanding function. So, next we present a tour of neuroanatomy, including a bit of function to put the brain anatomy into the context of cognitive neuroscience. For a brief discussion of celebral vasculature, see the box “How the Brain Works: Blood Supply and the Brain.”
Early in each of Chapters 4 through 14, there is a box called Anatomical Orientation, containing one or a few illustrations of the brain. This box highlights the anatomy that is relevant to the cognitive functions discussed in that chapter. The anatomy presented here and in the coming chapters will help you see how the structures of the brain are related to the functions of the mind.
Overview of Nervous System Structure
The nervous system is composed of the central nervous system (CNS), consisting of the brain and spinal cord, and the peripheral nervous system (PNS), consisting of the nerves (bundles of axons and glia) and ganglia (clumps of nerve cell bodies) outside of the CNS (Figure 2.16). The CNS can be thought of as the command-and-control center of the nervous system. The PNS represents a courier network that delivers sensory information to the CNS and carries the motor commands from the CNS to the muscles. These activities are accomplished through two systems, the somatic motor system that controls the voluntary muscles of the body and the autonomic motor system that controls visceral functions. Before we concentrate on the CNS, a word about the autonomic nervous system.

FIGURE 2.16 The peripheral and central nervous systems of the human body.
The nervous system is generally divided into two main parts. The central nervous system includes the brain and spinal cord. The peripheral nervous system, comprising the sensory and motor nerves and associated nerve cell ganglia (groups of neuronal cell bodies), is located outside the central nervous system.
The Autonomic Nervous System
The autonomic nervous system (also called the autonomic, or visceral, motor system) is involved in controlling the involuntary action of smooth muscles, the heart, and various glands. It has two subdivisions: the sympathetic and parasympathetic branches (Figure 2.17). The sympathetic system uses the neurotransmitter norepinephrine, and the parasympathetic system uses acetylcholine as its transmitter. The two systems frequently operate antagonistically. For example, activation of the sympathetic system increases heart rate, diverts blood from the digestive tract to the somatic musculature, and prepares the body for action (fight or flight) by stimulating the adrenal glands to release adrenaline. In contrast, activation of the parasympathetic system slows heart rate, stimulates digestion, and in general helps the body with functions germane to maintaining the body.
In the autonomic system, a great deal of specialization takes place that is beyond the scope of this chapter. Still, understanding that the autonomic system is involved in a variety of reflex and involuntary behaviors, mostly below the level of consciousness, is useful for interpreting information presented later in the book. In Chapter 10, on emotion, we will discuss arousal of the autonomic nervous system and how changes in a number of psychophysiological measures tap into emotion-related changes in the autonomic nervous system. For example, changes in skin conductance are related to sweat gland activity, and sweat glands are under the control of the autonomic nervous system.
In the rest of this chapter, we focus on the CNS in order to lay the groundwork for the studies of cognition that compose the rest of the book. But to talk about brain anatomy, we need some standard terminology that places parts of the brain in proper three-dimensional space. For that, please take a look at the box “Navigating the Brain.”

FIGURE 2.17 Organization of the autonomic nervous system, showing sympathetic and parasympathetic branches.
Please see the text for details.
The Central Nervous System

FIGURE 2.18 Organization of neurons in the CNS.
In the CNS, neurons can be organized in clumps called nuclei (top—not to be confused with the nucleus inside each neuron), which are most commonly found in subcortical and spinal structures, or sheets called layers (middle), which are most commonly found in the cortex. The cell bodies of glial cells are located in the white matter (e.g., oligodendrocytes), and in the cortex.
The CNS is made up of the delicate brain and spinal cord, each encased in its protective, bony shell and suspended in a sea of cerebrospinal fluid (CSF). Both the brain and the spinal cord are covered with three protective membranes—the meninges. The outer membrane is the thick dura mater; the middle is the arachnoid mater; and the inner and most delicate is the pia mater, which firmly adheres to the surface of the brain. The CSF occupies the subarachnoid space between the arachnoid membrane and the pia mater, as well as the brain ventricles, cisterns and sulci, and the central canal of the spinal cord (see “How the Brain Works: The Chambers of the Mind”).
In the CNS, neurons are bunched together in various ways (Figure 2.18). Two of the most common organizational clusters are in a nucleus or in a layer. A nucleus is a relatively compact arrangement of nerve cell bodies and their connections, ranging from hundreds to millions of neurons, with functionally similar inputs and outputs. They are located throughout both the brain and the spinal cord. The outer layer of the brain, the cerebral cortex, on the other hand, has billions of neurons. They are arranged in layers of thin sheets, folded across the surfaces of the cerebral hemispheres like a handkerchief. When we look at a slice of the brain, we see the cortex as a thin grayish layer overlaying the whitish interior. The gray matter is composed of neuronal cell bodies, and the white matter consists of axons and glial cells. Much like nerves in the PNS, these axons are grouped together in tracts that run in association tracts from one region to another within a hemisphere, or may cross into the other hemisphere in tracts called commissures. The largest of all the fiber tracts is the main commissure crossing between the hemispheres, the corpus callosum. Finally, there are projection tracts that run from the cerebral cortex to the deeper subcortical structures and the spinal cord.
TAKE-HOME MESSAGES
A Guided Tour of the Brain
When we see a brain, the cerebral cortex, the outer layer, is most prominent. But for the brain, the cerebral cortex is the frosting on the cake—it’s the last thing to develop from an evolutionary, as well as an embryological, point of view. Deep within, at the base of the brain, are structures that are found in most vertebrates and have evolved for hundreds of millions of years. These parts of the brain control our most basic survival functions, such as breathing, heart rate, and temperature. In contrast, the prefrontal cortex, which is found only in mammals, is evolutionarily the youngest part of our brain. Damage to the prefrontal cortex may not be immediately fatal, but it will likely affect such things as our ability to make decisions as well as other behaviors that we consider to be most advanced in humans. We begin our tour of the CNS with a brief look at the spinal cord.
The Spinal Cord
The spinal cord takes in sensory information from the body’s peripheral sensory receptors, relays it to the brain, and conducts the final motor signals from the brain to muscles. In addition, each level of the spinal cord has reflex pathways, such as the knee-jerk reflex mentioned earlier.
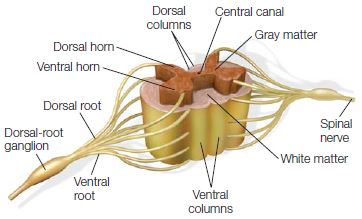
FIGURE 2.19 Gross anatomy of the spinal cord.
This cross-sectional and three-dimensional representation of the spinal cord shows the central butterfly-shaped gray matter, which contains neuronal cell bodies, and the surrounding white matter axon tracts, which convey information down the spinal cord from the brain to the peripheral neurons and up the spinal cord from peripheral receptors to the brain. The dorsal and ventral nerve roots are shown exiting and entering the cord; they fuse to form peripheral nerves. The cell bodies of peripheral sensory inputs reside in the dorsal-root ganglion and project their axons into the central nervous system via the dorsal root. The ventral horn of the spinal cord houses motor neurons that project their axons out the ventral roots to innervate peripheral muscles.
The spinal cord runs from the brainstem at about the first spinal vertebrae to its termination in the cauda equina (meaning “horse’s tail”). It is enclosed in the bony vertebral column—a stack of separate bones, the vertebrae, that extend from the base of the skull to the fused vertebrae at the coccyx (tailbone). The vertebral column is divided into sections: cervical, thoracic, lumbar, sacral, and coccygeal. The spinal cord is similarly divided (excluding the coccygeal region, since we no longer have tails) into 31 segments. Each segment has a right and a left spinal nerve that enters and exits from the vertebral column through openings called foramen. Each spinal nerve has both sensory and motor axons: one afferent neuron carries sensory input through the dorsal root into the spinal cord, and the other efferent neuron carries motor output through the ventral root away from it. In looking at a cross section of the spinal cord (Figure 2.19), we can see the peripheral region is made up of white matter tracts. The more centrally located gray matter, consisting of neuronal bodies, resembles a butterfly with two separate sections or horns: the dorsal horn and ventral horn. The ventral horn contains the large motor neurons that project to muscles. The dorsal horn contains sensory neurons and interneurons. The interneurons project to motor neurons on the same (ipsilateral) and opposite (contralateral) sides of the spinal cord to aid in the coordination of limb movements. The gray matter surrounds the central canal, which is an anatomical extension of the ventricles in the brain and contains cerebrospinal fluid.
THE COGNITIVE NEUROSCIENTIST’S TOOLKIT
Navigating the Brain
For anatomists, the head is merely an appendage to the body, so the terms that are used to describe the orientation of the head and its brain are in relation to the body. Confusion arises due to differences in how the head and body are arranged in animals that walk on four legs versus humans, who are upright. Let’s first picture the body of the cutest kind of dog, an Australian shepherd, looking off to the left of the page (Figure 1, top). The front end is the rostral end, meaning “nose.” The opposite end is the caudal end, the “tail.” Along his back is the dorsal surface, just like the dorsal fin is on the back of a shark. The bottom surface along the dog’s belly is the ventral surface. We can refer to the dog’s nervous system by using the same coordinates (Figure 1, bottom). The part of the brain toward the front is the rostral end (toward the frontal lobes); the posterior end is the caudal end (toward the occipital lobe). Along the top of his head is the dorsal surface, and the bottom surface of the brain is the ventral surface.
We humans are atypical animals because we stand upright and, therefore, tilt our heads forward in order to be parallel with the ground. Thus, the dorsal surface of the body and brain are now at right angles to each other (Figure 2). Luckily, we have a cerebral cortex that can understand this. In humans, we also use the terms superior and inferior to refer to the top and bottom of the brain, respectively.
Similarly, along with the terms rostral, which still means “toward the frontal pole,” and caudal, which still means “toward the occipital pole,” anterior and posterior are also used to refer to the front and back of the brain, respectively.

FIGURE 1 A dog brain in relation to the body.

FIGURE 2 Navigating the human brain.
When we consider the spinal cord, the coordinate systems align with the body axis. Thus, in the spinal cord, rostral means “toward the brain,” just as it does in the dog.
Throughout this book, pictures of brain slices usually will be in one of three planes (Figure 3). If we slice it from nose to tail, that is a sagittal section. When that slice is directly through the middle, it is a midsagittal or medial section. If it is off to the side, it is a lateral section. If sliced from top to bottom, separating the front of the brain from the back, we have made a coronal section. If we slice in a plane that separates dorsal from ventral, that is known as either an axial, transverse, or horizontal section.

FIGURE 3 Three orthogonal planes through the brain.
HOW THE BRAIN WORKS
The Chambers of the Mind
Scientists have understood for many decades that neurons in the brain are functional units, and that how they are interconnected yields specific circuits for the support of particular behaviors. Centuries ago, early anatomists, believing that the head contained the seat of behavior, examined the brain to see where the conscious self (soul, if you wish) was located. They found a likely candidate: Some chambers in the brain seemed to be empty (except for some fluid) and thus were possible containers for higher functions. These chambers are called ventricles (Figure 1). What is the function of these chambers within the brain?
The brain weighs a considerable amount but has little or no structural support; there is no skeletal system for the brain. To overcome this potential difficulty, the brain is immersed in a fluid called cerebrospinal fluid (CSF). This fluid allows the brain to float to help offset the pressure that would be present if the brain were merely sitting on the base of the skull. CSF also reduces shock to the brain and spinal cord during rapid accelerations or decelerations, such as when we fall or are struck on the head.
The ventricles inside the brain are continuous with the CSF surrounding the brain. The largest of these chambers are the lateral ventricles, which are connected to the third ventricle in the brain’s midline. The cerebral aqueduct joins the third to the fourth ventricle in the brainstem below the cerebellum. The CSF is produced in the lateral ventricles and in the third ventricle by the choroid plexus, an outpouching of blood vessels from the ventricular wall. Hence, CSF is similar to blood, being formed from an ultrafiltrate of blood plasma; essentially, CSF is a clear fluid containing proteins, glucose, and ions, especially potassium, sodium, and chloride. It slowly circulates from the lateral and third ventricles through the cerebral aqueduct to the fourth ventricle and on to the subarachnoid space surrounding the brain, to be reabsorbed by the arachnoid villi in the sagittal sinus (the large venous system located between the two hemispheres on the dorsal surface; not shown).

FIGURE 1 Ventricles of the human brain.
(left) Midsagital section showing the medial surface of the left hemisphere. (right) Transparent brain showing the ventricular system in 3D view.
|

|
|
FIGURE 2.20 Gross anatomy of a brain showing brain stem. |
|
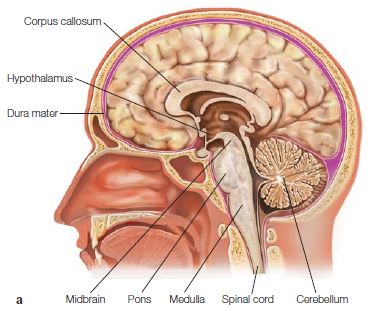
The Brainstem: Medulla, Pons, Cerebellum, and Midbrain
We usually think of the brainstem as having three main parts: the medulla (myelencephalon), the pons and cerebellum (metencephalon), and the midbrain (mesencephalon). These three sections form the central nervous system between the spinal cord and the diencephalon. Though the brainstem is rather small compared to the vast bulk of the forebrain (Figures 2.20 and 2.21), it plays a starring role in the brain. It contains groups of motor and sensory nuclei, nuclei of widespread modulatory neurotransmitter systems, and white matter tracts of ascending sensory information and descending motor signals.
Damage to the brainstem is life threatening, largely because brainstem nuclei control respiration and global states of consciousness such as sleep and wakefulness. The medulla, pons, and cerebellum make up the hindbrain, which we look at next.

FIGURE 2.21 Lateral view of the brainstem showing the thalamus, pons, medulla, midbrain, and spinal cord.
Anterior in the brain is at the top, and the spinal cord is toward the bottom in this left lateral view. The cerebellum is removed in this drawing.
Medulla The brainstem’s most caudal portion is the medulla, which is continuous with the spinal cord (Figure 2.21). The medulla is essential for life. It houses the cell bodies of many of the 12 cranial nerves, providing sensory and motor innervations to the face, neck, abdomen, and throat (including taste) as well as the motor nuclei that innervate the heart. The medulla controls vital functions such as respiration, heart rate, and arousal. All of the ascending somatosensory information entering from the spinal cord passes through the medulla via two bilateral nuclear groups, the gracile and cuneate nuclei. These projection systems continue through the brainstem to synapse in the thalamus en route to the somatosensory cortex. Another interesting feature of the medulla is that the corticospinal motor axons, tightly packed in a pyramid-shaped bundle (called the pyramidal tract), cross here to form the pyramidal decussation. Thus, the motor neurons originating in the right hemisphere cross to control muscles on the left side of the body, and vice versa. Functionally, the medulla is a relay station for sensory and motor information between the body and brain; it is the crossroads for most of the body’s motor fibers; it controls several autonomic functions, including the essential reflexes that determine respiration, heart rate, blood pressure, and digestive and vomiting responses.
Pons The pons, Latin for “bridge,” is so named because it is the main connection between the brain and the cerebellum. Sitting anterior to the medulla, the pons is made up of a vast system of fiber tracts interspersed with nuclei (Figure 2.21). Many of the cranial nerves synapse in the pons; these include the sensory and motor nuclei from the face and mouth and the visuomotor nuclei controlling some of the extraocular muscles. Thus, the pons is important for some eye movements as well as those of the face and mouth. In addition, some auditory information is channeled through another pontine structure, the superior olive. This level of the brainstem contains a large portion of the reticular formation that modulates arousal. Interestingly, the pons is also responsible for generating rapid eye movement (REM) sleep.
Cerebellum The cerebellum (literally, “small cerebrum” or “little brain”), which clings to the brainstem at the level of the pons, is home to most of the brain’s neurons (see Figures 2.20 and 2.22). Visually, the surface of the cerebellum appears to be covered with thinly spaced, parallel grooves; but in reality, it is a continuous layer of tightly folded neural tissue (like an accordion). It forms the roof of the fourth ventricle and sits on the cerebellar peduncles (meaning “feet”), which are massive input and output fiber tracts of the cerebellum (see Figure 2.21).

FIGURE 2.22 Gross anatomy of the cerebellum.
Anterior in the brain is at the top, and the spinal cord is toward the bottom (not shown). This dorsal view of the cerebellum shows the underlying deep nuclei in a see-through projection.
The cerebellum has several gross subdivisions, including the cerebellar cortex, four pairs of deep nuclei, and the internal white matter (Figure 2.22). In this way, the cerebellum resembles the forebrain’s cerebral hemispheres.
Most of the fibers arriving at the cerebellum project to the cerebellar cortex, conveying information about motor outputs and sensory inputs describing body position. Inputs from vestibular projections involved in balance, as well as auditory and visual inputs, also project to the cerebellum from the brainstem. The output from the cerebellum originates in the deep nuclei. Ascending outputs travel to the thalamus and then to the motor and premotor cortex. Other outputs project to nuclei of the brainstem, where they impinge on descending projections to the spinal cord.
The cerebellum is critical for maintaining posture, walking, and performing coordinated movements. It does not directly control movements; instead, it integrates information about the body, such as its size and speed, with motor commands. Then, it modifies motor outflow to effect smooth, coordinated movements. It is because of the cerebellum that Yo-Yo Ma can play the cello and the Harlem Globetrotters can dunk a ball with such panache. If your cerebellum is damaged, your movements will be uncoordinated and halting, and you may not be able to maintain balance. In Chapter 8, we look more closely at the cerebellum’s role in motor control. In the 1990s, it was discovered that the cerebellum is involved with more than motor functions. It has been implicated in aspects of cognitive processing including language, attention, learning, and mental imagery.

FIGURE 2.23 Anatomy of the midbrain.
The dorsal surface of the brainstem is shown with the cerebral cortex and cerebellum removed.
Midbrain The mesencephalon, or midbrain, lies superior to the pons and can be seen only in a medial view. It surrounds the cerebral aqueduct, which connects the third and fourth ventricles. Its dorsal portion consists of the tectum (meaning “roof”), and its ventral portion is the tegmentum (“covering”). Large fiber tracts course through the ventral regions from the forebrain to the spinal cord, cerebellum, and other parts of the brainstem. The midbrain also contains some of the cranial nerve ganglia and two other important structures: the superior and inferior colliculi (Figure 2.23). The superior colliculus plays a role in perceiving objects in the periphery and orienting our gaze directly toward them, bringing them into sharper view. The inferior colliculus is used for locating and orienting toward auditory stimuli. Another structure, the red nucleus, is involved in certain aspects of motor coordination. It helps a baby crawl or coordinates the swing of your arms as you walk. Much of the midbrain is occupied by the mesencephalic reticular formation, a rostral continuation of the pontine and medullary reticular formation.
TAKE-HOME MESSAGES
The Diencephalon: Thalamus and Hypothalamus
After leaving the brainstem, we arrive at the diencephalon, which is made up of the thalamus and hypothalamus. These subcortical structures are composed of groups of nuclei with interconnections to widespread brain areas.
Thalamus Almost smack dab in the center of the brain and perched on top of the brainstem (at the rostral end; see Figure 2.21), the thalamus is the larger of the diencephalon structures. The thalamus is divided into two parts—one in the right hemisphere and one in the left—that straddle the third ventricle. In most people, the two parts are connected by a bridge of gray matter called the massa intermedia (see Figure 2.23). Above the thalamus are the fornix and corpus callosum; beside it is the internal capsule, containing ascending and descending axons running between the cerebral cortex and the medulla and spinal cord.

FIGURE 2.24 The thalamus, showing inputs and outputs and major subdivisions.
The various subdivisions of the thalamus serve different sensory systems and participate in various cortical–subcortical circuits. The posterior portion of the thalamus (lower right) is cut away in cross section and separated from the rest of the thalamus to reveal the internal organization of the thalamic nuclei (upper left).
The thalamus has been referred to as the “gateway to the cortex” because, except for some olfactory inputs, all of the sensory modalities make synaptic relays in the thalamus before continuing to the primary cortical sensory receiving areas (Figure 2.24). The thalamus is involved in relaying primary sensory information. It also receives inputs from the basal ganglia, cerebellum, neocortex, and medial temporal lobe and sends projections back to these structures to create circuits involved in many different functions. It also relays most of the motor information that is on its way to the spinal cord. Thus, the thalamus, a veritable Grand Central Station of the brain, is considered a relay center where neurons from one part of the brain synapse on neurons that travel to another region. In the thalamus, information can be reorganized and shuttled, like in a train station switching yard, according to the connection patterns formed by the neurons.
The thalamus is divided into several nuclei that act as specific relays for incoming sensory information (Figure 2.24). The lateral geniculate nucleus receives information from the ganglion cells of the retina and sends axons to the primary visual cortex. Similarly, the medial geniculate nucleus receives information from the inner ear, via other brainstem nuclei in the ascending auditory pathway, and sends axons to the primary auditory cortex. Somatosensory information projects via the ventral posterior (medial and lateral) nuclei of the thalamus to the primary somatosensory cortex. Sensory relay nuclei of the thalamus not only project axons to the cortex but also receive heavy descending projections back from the same cortical area that they contact. Located at the posterior pole of the thalamus is the pulvinar nucleus, which is involved in attention and in integrative functions involving multiple cortical areas.
Hypothalamus The main link between the nervous system and the endocrine system is the hypothalamus, which is the main site for hormone production and control. Easily located, it lies on the floor of the third ventricle (see Figure 2.20a). The two bumps seen on the ventral surface of the brain, the mammillary bodies, belong to the small collection of nuclei and fiber tracks contained in the hypothalamus (Figure 2.25). It receives inputs from the limbic system structures and other brain areas. One of its jobs is to control circadian rhythms (light–dark cycles) with inputs from the mesencephalic reticular formation, amygdala, and the retina. Extending from the hypothalamus are major projections to the prefrontal cortex, amygdala, spinal cord, and pituitary gland. The pituitary gland is attached to the base of the hypothalamus.
The hypothalamus controls the functions necessary for maintaining the normal state of the body (homeostasis). It sends out signals that drive behavior to alleviate such feelings as thirst, hunger, and fatigue, and it controls body temperature and circadian cycles. You would not want to be in the broiling hot desert without your hypothalamus. It accomplishes much of this work through the endocrine system and via control of the pituitary gland.

FIGURE 2.25 Midsagittal view of the hypothalamus.
Various nuclear groups are shown diagrammatically. The hypothalamus is the floor of the third ventricle, and, as the name suggests, it sits below the thalamus. Anterior is to the left in this drawing.
The hypothalamus produces hormones, as well as factors that regulate hormone production in other parts of the brain. For example, hypothalamic neurons send axonal projections to the median eminence, an area bordering the pituitary gland. There it releases peptides (releasing factors) into the circulatory system of the anterior pituitary gland. These in turn trigger (or inhibit) the release of a variety of hormones from the anterior pituitary into the bloodstream, such as growth hormone, thyroid-stimulating hormone, adrenocorticotropic hormone, and the gonadotropic hormones.
Hypothalamic neurons in the anteromedial region, including the supraoptic nucleus and paraventricular nuclei, send axonal projections into the posterior pituitary gland. There they stimulate the gland to release the hormones vasopressin and oxytocin into the blood to regulate water retention in the kidneys, milk production, and uterine contractility, among other functions. Circulating peptide hormones in the bloodstream can also act on distant sites and influence a wide range of behaviors, from the fightor-flight response to maternal bonding. The hypothalamus can itself be stimulated by hormones circulating in the blood that were produced in other regions of the body.
TAKE-HOME MESSAGES
The Telencephalon: Limbic System, Basal Ganglia, and Cerebral Cortex
Toward the front of and evolutionarily newer than the diencephalon, the telencephalon develops into the cerebrum, which includes the cerebral cortex, the limbic system, and the basal ganglia. Compared to the diencephalon, the anatomy (and functions) of the forebrain above the thalamus are less straightforward. Instead of a rather linear stacking of structures, it forms a clump of structures found deep within the cerebral hemispheres nestled over and around the diencephalon. In the 17th century, Thomas Willis observed that the brainstem appeared to sport a cortical border encircling it and named it the cerebri limbus (in Latin, limbus means “border”). For better or for worse, in a move that began to tie the area with specific functioning, Paul Broca in 1878 renamed it the grand lobe limbique and suggested that it was a primary player in olfaction.
LimbicSystem The“classical” limbiclobe(Figure 2.26) is made up of the cingulate gyrus (a band of cerebral cortex that extends above the corpus callosum in the anterior–posterior direction and spans both the frontal and parietal lobes), the hypothalamus, anterior thalamic nuclei, and the hippocampus, an area located on the ventromedial aspect of the temporal lobe. In the 1930s James Papez (pronounced “payps”) first suggested the idea that these structures were organized into a system for emotional behavior, which led to the use of the term Papez circuit. It was named the limbic system by Paul MacLean in 1952 when he suggested the addition of more brain areas, such as the amygdala and prefrontal cortex. Note that the limbic system is neither anatomically nor functionally organized to the degree that other systems are in the brain. In fact, some researchers feel that the limbic system is sufficiently nebulous that the concept should be discarded or reevaluated. The classical limbic system, as noted earlier, has been extended to include the amygdala, a group of neurons anterior to the hippocampus, along with the orbitofrontal cortex and parts of the basal ganglia (see Figure 2.26). Sometimes the medial dorsal nucleus of the thalamus is also included. The organization and role of the limbic system are described in more detail in Chapter 10.
Basal Ganglia The basal ganglia are a collection of nuclei bilaterally located deep in the brain beneath the anterior portion of the lateral ventricles, near the thalamus (Figure 2.27). These subcortical nuclei, the caudate nucleus, putamen, globus pallidus, subthalmic nucleus, and substantia nigra, are extensively interconnected. The caudate nucleus together with the putamen is known as the striatum. The basal ganglia receive inputs from sensory and motor areas, and the striatum receives extensive feedback projections from the thalamus. A comprehensive understanding of how these deep brain nuclei function remains elusive. They are involved in a variety of crucial brain functions including action selection, action gating, motor preparation, timing, fatigue, and task switching (Cameron et al., 2009). Notably, the basal ganglia have many dopamine receptors. The dopamine signal appears to represent the error between predicted future reward and actual reward (Shultz et al., 1997), and plays a crucial role in motivation and learning. The basal ganglia may also play a big role in reward-based learning and goal-oriented behavior. One summary of basal ganglia function proposes that it combines an organism’s sensory and motor context with reward information and passes this integrated information to the motor and prefrontal cortex for a decision (Chakravarthy et al., 2009).

|

|
| a | b |
|
FIGURE 2.26 The human limbic system. |
|

a

b
FIGURE 2.27 Coronal and transparent views of the brain showing the basal ganglia.
(a) Cross sections through the brain at two anterior–posterior levels (as indicated), showing the basal ganglia. The inset shows a transparent brain with the basal ganglia in 3D in blue. (b) Corresponding high-resolution, structural MRI (4-tesla scanner) taken at approximately the same level as the more posterior drawing in (a). This image also shows the brainstem as well as the skull and scalp, which are not shown in (a).
TAKE-HOME MESSAGES
The Cerebral Cortex
The crowning glory of the cerebrum is its outermost tissue, the cerebral cortex. It is made up of large sheets of (mostly) layered neurons, draped and folded over the two symmetrical hemispheres like frosting on a cake. It sits over the top of the core structures that we have been discussing, including parts of the limbic system and basal ganglia, and surrounds the structures of the diencephalon. The term cortex means “bark,” as in tree bark, and in higher mammals and humans it contains many infoldings, or convolutions (Figure 2.28). The infoldings of the cortical sheet are called sulci (the crevices) and gyri (the crowns of the folded tissue that one observes when viewing the surface).
The folds of the human cortex serve several functions. First, they enable more cortical surface to be packed into the skull. If the human cortex were smoothed out to resemble that of the rat, for example, humans would need to have very large heads. The total surface area of the human cerebral cortex is about 2,200 to 2,400 cm2, but because of extensive folding, about two thirds of this area is confined within the depths of the sulci. Second, having a highly folded cortex brings neurons into closer three-dimensional relationships to one another, reducing axonal distance and hence neuronal conduction time between different areas. This savings occurs because the axons that make long-distance corticocortical connections run under the cortex through the white matter and do not follow the foldings of the cortical surface in their paths to distant cortical areas. Third, by folding, the cortex brings some nearby regions closer together; for example, the opposing layers of cortex in each gyrus are in closer linear proximity than they would be if the gyri were flattened.

|

|
| a | b |
|
FIGURE 2.28 The human cerebral cortex. |
|

FIGURE 2.29 Cerebral cortex and white matter tracts.
(a) Horizontal section through the cerebral hemispheres at the level indicated at upper left. White matter is composed of myelinated axons, and gray matter is composed primarily of neurons. This diagram shows that the gray matter on the surface of the cerebral hemispheres forms a continuous sheet that is heavily folded. (b) High-resolution structural MRI in a similar plane of section in a living human. This T2 image was obtained on a 4-tesla scanner (a high-magnetic-field scanner). Note that on T2 images, the white matter appears darker than the gray matter, but this is due to the imaging technique, not the actual appearance.
The cortex ranges from 1.5 to 4.5 mm in thickness, but in most regions it is approximately 3 mm thick. The cortex contains the cell bodies of neurons, their dendrites, and some of their axons. In addition, the cortex includes axons and axon terminals of neurons projecting to the cortex from other brain regions, such as the subcortical thalamus. The cortex also contains blood vessels. Because the cerebral cortex has such a high density of cell bodies, it appears grayish in relation to underlying regions that are composed primarily of the axons that connect the neurons of the cerebral cortex to other locations in the brain. These appear slightly paler or even white (Figure 2.29) because of their lipid sheaths (myelin). As described earlier, for this reason anatomists used the terms gray matter and white matter when referring to areas of cell bodies and axon tracts, respectively.
Dividing the Cortex Anatomically

FIGURE 2.30 The four lobes of the cerebral cortex.
This is a lateral view of the left hemisphere showing the four major lobes of the brain, and some of the major landmarks that separate them.
The cerebral hemispheres have four main divisions, or lobes, that are best seen in a lateral view: the frontal, parietal, temporal, and occipital lobes (Figure 2.30). These names are derived from names given to the overlying skull bones; for example, the temporal lobe lies underneath the temporal bone. The skull bones themselves are named for their locations. The temporal bone lies under the temple, where the passage of time can be observed first in the graying of hair. The word temporal derives from Latin “tempora,” meaning “time.”
The lobes can usually be distinguished from one another by prominent anatomical landmarks such as pronounced sulci. The central sulcus divides the frontal lobe from the parietal lobe, and the Sylvian (lateral) fissure separates the temporal lobe from the frontal and parietal lobes. The occipital lobe is demarcated from the parietal and temporal lobes by the parieto-occipital sulcus on the brain’s dorsal surface and the preoccipital notch located on the ventrolateral surface. The left and right cerebral hemispheres are separated by the interhemispheric fissure (also called the longitudinal fissure; see Figure 2.28b) that runs from the rostral to the caudal end of the forebrain.
Hidden from the lateral surface view are other parts of the cerebrum, not all of which are conveniently contained in the four lobes. For instance, the insula is located between the temporal and frontal lobe, and is, as its name implies, an island of folded cortex hidden deep in the lateral sulcus. The insula, which is surprisingly large, is divided into the larger anterior insula and smaller posterior insula.
Connections between the cerebral hemispheres are via axons from cortical neurons that travel through the corpus callosum, which, as previously mentioned, represents the largest white matter commissure in the nervous system. As we will discuss in Chapter 4, the corpus callosum carries out valuable integrative functions for the two hemispheres.
Dividing the Cortex Cytoarchitectonically
The cerebral cortex can be more finely divided, both anatomically and functionally. We will take a look at both.
Cytoarchitectonics uses the microanatomy of cells and their organization to subdivide the cortex (cyto– means “cell” and architectonics means “architecture”). Using histological analysis, tissue regions are defined in which the cellular architecture looks similar, and therefore might indicate areas of homogeneous function. This work began in earnest with Korbinian Brodmann at the beginning of the 20th century.
Brodmann identified approximately 52 regions of the cerebral cortex. These areas were categorized and numbered according to differences in cellular morphology and organization (Figure 2.31). Other anatomists further subdivided the cortex into almost 200 cytoarchitectonically defined areas. A combination of cytoarchitectonic and functional descriptions of the cortex is probably the most effective way of dividing the cerebral cortex into meaningful units. In the sections that follow, we use Brodmann’s numbering system and anatomical names to describe the cerebral cortex.

|

|
| a | b |

c |
FIGURE 2.31 Cytoarchitectonic subdivisions of the human cerebral cortex. |
The Brodmann system often seems unsystematic. Indeed, the numbering has more to do with the order in which Brodmann sampled a region than with any meaningful relation between areas. Nonetheless, in some regions the numbering system roughly corresponds with the relations between areas that carry out similar functions, such as vision—e.g., Brodmann areas 17, 18, and 19. Unfortunately, the nomenclature of the cortex (and indeed the nervous system) is not fully standardized. Hence, a region might be referred to by its Brodmann name, a cytoarchitectonic name, a gross anatomical name, or a functional name. For example, let’s consider the first area in the cortex to receive visual inputs from the thalamus—the primary sensory cortex for vision. The Brodmann name is area 17 (or Brodmann area 17; i.e., BA17), another cytoarchitectonic name is striate cortex (owing to the highly visible stripe of myelin in cross sections of this cortex, known as the Stria of Gennari), the gross anatomical name is calcarine cortex (the cortex surrounding the calcarine fissure in humans), and the functional name is primary visual cortex, which has been labeled area V1 (for “visual area 1”) based on studies of the visual systems of monkeys. We chose primary visual cortex as an example here, because all these different terms refer to the same cortical area. Unfortunately, for much of the cortex, this is not the case; that is, different nomenclatures often do not refer to precisely the same area with a one-to-one mapping. For example, BA18 of the visual system is not fully synonymous with V2 (for “visual area 2”).
Using different anatomical criteria, it is also possible to subdivide the cerebral cortex according to the general patterns of layering (Figure 2.32a, b). Ninety percent of cortex is composed of neocortex: cortex that contains six cortical layers or that passed through a developmental stage involving six cortical layers. Neocortex includes areas like primary sensory and motor cortex and association cortex (areas not obviously primary sensory or motor).
Mesocortex is a term for the so-called paralimbic region, which includes the cingulate gyrus, parahippocampal gyrus, insular cortex, and orbitofrontal cortex. Mesocortex is interposed between neocortex and allocortex and usually has six layers. Allocortex typically has only one to four layers of neurons and includes the hippocampal complex (sometimes referred to as archicortex) and primary olfactory cortex (sometimes referred to as paleocortex).

|

|
| a | b |

c |
FIGURE 2.32 Cerebral cortex, color-coded to show the regional differences in cortical layering that specify different types of cortex. |
In neocortex the cortical layers numbered 1–6 (or for the more classically minded users, I–VI) are sheets of neurons neatly stacked on top of each other. The neurons of each layer are typically similar within a layer, but different between layers. For instance, neocortical layer 4 is packed with stellate neurons, and layer 5 is predominantly pyramidal neurons (Figure 2.32c). The deeper layers, 5 and 6, mature earlier during gestation and project primarily to targets outside the cortex. Layer 4 is typically the input layer, receiving information from the thalamus as well as information from other, more distant cortical areas. Layer 5, on the other hand, is typically considered an output layer that sends information from the cortex back to the thalamus, facilitating feedback. The superficial layers mature last and primarily project to targets within the cortex. It has been suggested that the superficial layers and the connections they form within the cortex participate in the higher cognitive functions.
The neurons in any one sheet, while interwoven with the other neurons in the same layer, are also lined up with the neurons in the sheets above and below it, forming columns of neurons running perpendicular to the sheets. These columns are known as minicolumns or microcolumns. These columns are not just an anatomical nicety. The neurons within a column synapse with those from the layers above and below them, forming an elemental circuit, and appear to function as a unit. Neuronal columns are the fundamental processing unit within the cerebral cortex, and bundles of microcolumns assembled together, dubbed cortical columns, create functional units in the cortex.
Functional Divisions of the Cortex
The lobes of the cerebral cortex have a variety of functional roles in neural processing. Sometimes we get lucky, and the gross anatomical subdivisions of the cerebral cortex can be related fairly to specific functions, such as in the precentral gyrus where the primary motor cortex resides. More typically, however, cognitive brain systems are often composed of networks whose component parts are located in different lobes of the cortex. In addition, most functions in the brain—whether sensory, motor, or cognitive—rely on both cortical and subcortical components. Thus, it can be daunting to reveal relationships between cognitive functions and locations within the brain where they occur. The detailed functional anatomy of the brain will be revealed to you in the next twelve chapters. The rest of this section, however, provides a beginner’s guide to the functional anatomy of the cortex.
Motor Areas of the Frontal Lobe Among many other functions, the frontal lobe plays a major role in the planning and execution of movements. It has two main subdivisions: the prefrontal cortex and the motor cortex (Figure 2.33a). The motor cortex sits in front of the central sulcus, beginning in the depths of the sulcus and extending anteriorly. The primary motor cortex (M1) corresponds to BA4. It includes the anterior bank of the central sulcus and much of the precentral gyrus (the prefix pre- in neuroanatomy means “in front of”). Anterior to this area are two more main motor areas of cortex (within BA6; see Figure 2.31 for BA locations): the premotor cortex on the lateral surface of the hemisphere, and the supplementary motor cortex that lies dorsal to the premotor area and extends around to the hemisphere’s medial surface. These motor cortical areas contain motor neurons whose axons extend to the spinal cord and brainstem and synapse on motor neurons in the spinal cord. The output layer of primary motor cortex contains some of the most amazing neurons in the nervous system: the large pyramidal neurons known as Betz’s cells, named after Vladimir Aleksandrovich Betz, the Russian anatomist who described them in the 19th century. Betz’s cells are the largest neurons in the cerebral cortex. They reach 60 to 80 microns in diameter at the cell body, and some of them send axons several feet long down the spinal cord.

|

|
| a | b |
|
FIGURE 2.33 The human frontal cortex. |
|
Prefrontal Cortex The more anterior regions of the frontal lobe, the prefrontal cortex, take part in the more complex aspects of planning, organizing, and executing behavior—tasks that require the integration of information over time. Because of its facility with these tasks, the frontal lobe is often said to be the center of executive function. People with frontal lobe lesions often have difficulty reaching a goal. They may know the steps that are necessary to attain it, but they just can’t figure out how to put them together. Another problem associated with frontal lobe lesions is a lack of motivation to initiate action, to modulate it, or to stop it once it is happening. The main regions of the prefrontal cortex are the dorsolateral prefrontal cortex, the ventrolateral prefrontal cortex, the orbitofrontal cortex (Figure 2.33a), and the medial prefrontal regions, including the anterior cingulate cortex (Figure 2.33b).
Somatosensory Areas of the Parietal Lobe The parietal lobe receives sensory information from the outside world, sensory information from within the body, and information from memory, and integrates it. Parietal lobe lesions result in all sorts of odd deficits relating to sensation and spatial location: People think that parts of their body are not their own or parts of space don’t exist for them, or they may recognize objects only from certain viewpoints, or they can’t locate objects in space at all. Stimulating certain regions of the parietal lobe causes people to have “out of body” experiences (Blanke et al., 2002).

FIGURE 2.34 The somatosensory cortex, which is located in the postcentral gyrus.
Inputs from peripheral receptors project via the thalamus (shown in cross section) to the primary somatosensory cortex (S1). Secondary somatosensory cortex (S2) is also shown.
Sensory information about touch, pain, temperature sense, and limb proprioception (limb position) is received via receptor cells on the skin and converted to neuronal impulses that are conducted to the spinal cord and then to the somatosensory relays of the thalamus (Figure 2.34). From the thalamus, inputs travel to the primary somatosensory cortex (or S1), a portion of the parietal lobe immediately caudal to the central sulcus (see Figure 2.33a). The next stop is the secondary somatosensory cortex (S2), which is located ventrally to S1; S2 receives most of its input from S1. Together, these cortical regions are known as the somatosensory cortex.
Topographical Mapping The specific cortical regions of the somatosensory and motor cortices that process the sensations and motor control of specific parts of the body have been mapped out. The spatial relationships of the body are fairly well preserved in the map of neural representations draped across these cortices, by using a principle known as topography (see “How the Brain Works: Cortical Topography”). For example, within the somatosensory cortex, neurons that respond to touch of the index finger are adjacent to those that respond to touch of the middle finger, which are also next to neurons that respond to touch of the ring finger. Similarly, the hand area as a whole is adjacent to the lower arm area, which is near the upper arm, and so forth. This mapping of specific parts of the body to areas of the cortex is known as somatotopy, resulting in somatotopic maps in the cortical areas. It is interesting to ask why such maps exist, since there is no inherent necessity for the organization. Yet topographic maps are a common feature of the nervous system (see Chapter 5), perhaps reflecting the fact that neighboring body parts are frequently co-recruited, as when we’re gripping a ball or stroking a favorite pet.
HOW THE BRAIN WORKS
Cortical Topography
Early insights into human cortical organization were made possible by studies that involved direct stimulation of the cortex of humans undergoing brain surgery while they were awake. Because there are no pain receptors in the central nervous system, patients experience no discomfort from stimulation. Thus, stimulation can be applied even when they are awake and fully conscious, enabling researchers to gather the patient’s subjective experiences—a relative impossibility in animal studies. Wilder Penfield and Herbert Jasper (1954) at the Montreal Neurological Institute carried out such pioneering work in the 1940s. Taking advantage of the fact that the cortex is exposed during surgery, these surgeons removed damaged brain tissue and during the same procedure, systematically explored the effects of small levels of electrical current applied to the cortical surface.
In their studies, Penfield and his associates found a topographic correspondence between cortical regions and body surface with respect to somatosensory and motor processes. This correspondence is represented in Figure 1 by overlaying drawings of body parts on drawings of coronal sections of the motor and somatosensory cortex. These coronal sections are from the regions indicat ed by the color codes in the lateral view of the whole brain at the
top of the figure (only one hemisphere is shown here). The resulting map of the body surface on the cortex is sometimes called a homunculus, because it is an organized representation of the body across a given cortical area. Note that there is an indirect relation between the actual size of body parts and the cortical representation of the body’s parts. For example, areas within the motor homunculus that activate muscles in the fingers, mouth, and tongue are much larger than would be expected if the representation were proportional. The large drawings of the fingers and mouth indicate that large areas of cortex are involved in the fine coordination required when we manipulate objects or speak.
Is the representation of the homunculus in the figure correct? Recent evidence from brain-imaging studies using functional magnetic resonance imaging (fMRI; described in Chapter 3) suggests that it may not be. Ravi Menon and his colleagues (Servos et al., 1999) in Canada stimulated the foreheads and chins of healthy volunteers while their brains were being scanned. In contrast to the results of the electrical-stimulation studies, the researchers found that stimulating the forehead produced activity in a region that was below (inferior to) the region for activity related to chin stimulation—the reverse of the drawing in the figure based on the work of Penfield and his colleagues. If the latter pattern from neuroimaging turns out to be accurate, it will constitute a dramatic example of scientific revisionism.

FIGURE 1
Topographic correspondence between cortical regions and body surface with respect to somatosensory and motor processes.
Visual Processing Areas in the Occipital Lobe The business of the occipital lobes is vision. The primary visual cortex is where the cerebral cortex begins to process visual information. As mentioned earlier, this area is also known as striate cortex, V1 for visual area 1, or BA17. It receives visual information relayed from the lateral geniculate nucleus of the thalamus (Figure 2.35). In humans, the primary visual cortex is on the medial surface of the cerebral hemispheres, extending only slightly onto the posterior hemispheric pole. Thus, most of the primary visual cortex is effectively hidden from view, between the two hemispheres. The cortex in this area has six layers and begins the cortical coding of visual features like luminance, spatial frequency, orientation, and motion—features that we will take up in detail in Chapters 5 and 6.
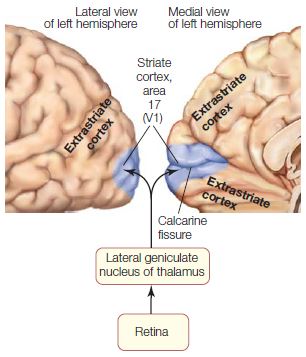
FIGURE 2.35 The visual cortex, which is located in the occipital lobe.
Brodmann area 17, also called the primary visual cortex, visual area 1 (V1), and striate cortex, is located at the occipital pole and extends onto the medial surface of the hemisphere, where it is largely buried within the calcarine fissure.
Visual information from the outside world is processed by multiple layers of cells in the retina and transmitted via the optic nerve to the lateral geniculate nucleus of the thalamus, and from there to V1—a pathway often referred to as the retinogeniculostriate, or primary visual pathway. The retina also sends projections to other subcortical brain regions by way of secondary projection systems. The superior colliculus of the midbrain is the main target of the secondary pathway and participates in visuomotor functions such as eye movements. In Chapter 7, we will review the role of the cortical and subcortical projection pathways in visual attention.
Surrounding the striate cortex is a large visual cortical region called the extrastriate (“outside the striate”) visual cortex (sometimes referred to as the prestriate cortex in monkeys, to signify that it is anatomically anterior to the striate cortex). The extrastriate cortex includes BA18 and BA19 and other areas.
Auditory Processing Areas in the Temporal Lobe The auditory cortex lies in the superior part of the temporal lobe in a region known as Heschl’s gyrus within the Sylvian fissure (Figure 2.36) and roughly corresponds with Brodmann areas 41 and 42. The auditory cortex has a tonotopic organization, meaning that the physical layout of the neurons is based on the frequency of sound. Neurons in the auditory cortex that respond best to low frequency are at one end of the cortex, and those that respond to high frequencies are at the other. The projection from the cochlea (the auditory sensory organ in the inner ear) proceeds through the subcortical relays to the medial geniculate of the thalamus and then to Heschl’s gyri, the primary auditory cortex (A1) in the supratemporal cortex. Surrounding and posterior to A1 is A2, the auditory association area. BA22, which surrounds the auditory cortex, aids in the perception of auditory inputs; when this area is stimulated, sensations of sound are produced in humans.

FIGURE 2.36 The human auditory cortex.
(a) Primary auditory cortex, which is located in the superior temporal lobe. The primary auditory cortex and surrounding association auditory areas contain representations of auditory stimuli and show a tonotopic organization. (b) This MRI shows areas of the superior temporal region in horizontal section that have been stimulated by tones of different frequencies (shown in red vs. blue) and show increased blood flow as a result of neuronal activity.
Association Cortex The portion of the neocortex that is neither sensory nor motor cortex has traditionally been termed the association cortex. These regions, which surround the identified sensory or motor cortical regions, contain cells that may be activated by more than one sensory modality. Association cortex receives and integrates inputs from many cortical areas; for example, inputs of the various qualities of a particular stimulus (e.g., pitch, loudness, timbre of a voice) are integrated with other sensory inputs, memory, attention, emotion, and so forth to produce our experience of the world. They are also the areas responsible for all of our high-end human abilities, such as language, abstract thinking, designing such things as a Maserati, and most important, vacation planning.
Each sense has a sensory association area. For example, though the primary visual cortex is necessary for normal vision, neither it nor the extrastriate cortex is the sole locus of visual perception. Regions of visual association cortex in the parietal and temporal lobes process information from the primary visual cortex about color, simple boundaries, and contours to enable people to recognize these features as a face, or a petunia, or that Maserati. Moreover, visual association cortex can be activated during mental imagery when we call up a visual memory even in the absence of visual stimulation. Or, in the case of the auditory system, the auditory association area is necessary to recognize sounds. If that area is damaged, a person can still hear sound but is unable to tell a dog’s bark from a piano concerto. As another example, the association areas of the parietal–temporal–occipital junction of the left hemisphere have a prominent role in language processing, whereas this region in the right hemisphere is implicated in attentional orienting (see Chapter 7). Thus, higher mental processes are the domain of the association cortical areas, in interaction with sensory and motor areas of cortex (Figure 2.37; “How the Brain Works: Billions and Billions”).
This wraps up our whirlwind tour of the brain, but leaves us with the question of how this complicated structure—the brain—is formed in the first place. We conclude this chapter with a brief look at brain development.
TAKE-HOME MESSAGES

FIGURE 2.37 Primary sensory and motor cortex and surrounding association cortex.
The blue regions show the primary cortical receiving areas of the ascending sensory pathways and the primary output region to the spinal cord. The secondary sensory and motor areas are colored pink. The remainder is considered association cortex.
HOW THE BRAIN WORKS
Billions and Billions: Brain Size, Complexity, and Human Cognition
In 2009, the big brain theory, the idea that humans were more intelligent and could credit all their high end abilities to the fact that they have a proportionately larger brain for their body than the other great apes, hit a wall. Although it had some major chinks in its armor already, for instance, the fact that Neanderthals had bigger brains than humans without possessing our scope of abilities, and that after split brain surgery the isolated left brain (with half the acreage) is just as intelligent as a whole brain, it still garnered quite a few fans. But then Suzana Herculano-Houzel (2009) and her coworkers stepped in using a new technique to more accurately count neuron numbers and found that the human brain is a proportionately scaled-up primate brain, no bigger than what you would expect for an ape of our size. It turns out that the human brain has on average 86 billion neurons, with 69 billion of them located in the cerebellum. The entire cortex, the area that we think is responsible for human thought and culture, has only 17 billion (19% of all the neutrons in the brain and similar to the percent found in other mammals), leaving only one billion for the entire rest of the brain. Not only that, but the visual and other sensory areas and the motor cortex have way more neurons than the frontal lobes (including the prefrontal cortex—that part of the human brain that is involved with all the high end abilities such as memory and planning, cognitive flexibility, abstract thinking, initiating appropriate behavior and inhibiting inappropriate behavior, learning rules, and picking out relevant information perceived through the senses). So what accounts for increased abilities?
Interestingly, the volume of the human cerebral cortex is 2.75 times larger than in chimpanzees, but has only 1.25 times more neurons (Shariff, 1953). One thing that neuroanatomists have discovered is that the dendritic tips of the front lobe neurons are more arborized: They are chock full of branches with the resulting possibility of increased neuronal connections. This suggests that it may be the connectivity patterns of the neurons themselves that is different.
Generally in the brain, the larger an area is, the better connected it is with more neurons, and more neurons connected to each other, but there is a limit. If our brains were fully connected, each neuron connected to every other one, our brains would have to be 20 kilometers in diameter (Clark & Sokoloff, 1999) and would require so much energy that all our time (and then some) would be spent eating. Big heads, indeed! With such distances for axons to travel across the brain, the processing speed would be slowed down, no doubt creating an uncoordinated body and rather dull witted person. So, as the primate brain evolved and the number of neurons increased, not every neuron connected to every other neuron. This resulted in an actual fall in the percent of connectedness. It appears that certain wiring “laws” apply to the evolutionary development of the large human brain (Striedter, 2005).

FIGURE 1 Variability of brain size and external topography.
Development of the Nervous System
Thus far, we have been discussing the neuroanatomy of the developed adult brain. In humans and many other species, the fetal brain is well developed and shows cortical layers, neuronal connectivity, and myelination; in short, it is already extremely complex, although by no means completely developed. To find out how this complex brain develops prenatally and to uncover the rules governing development, let’s examine the development of the nervous system and give special attention to the neocortex.
Overview of Gross Development
From a single fertilized egg, an organism made up of billions of cells with specialized functions will arise. This complexity clearly peaks in the nervous system. Fertilization is followed by a series of events leading to the formation of a multicellular blastula, which has already begun to specialize. The blastula contains three main cell lines, which after a few days form three layers: the ectoderm (outer layer) that will form the nervous system and the outer skin, lens of the eye, inner ear, and hair; the mesoderm (middle layer) that forms the skeletal system and voluntary muscle; and the endoderm (inner layer) that will form the gut and digestive organs. The early processes that go into forming the nervous system are called neurulation (Figure 2.38). During this stage, the ectodermal cells on the dorsal surface form the neural plate.
As the nervous system continues to develop, the cells at the lateral borders of the neural plate push upward. (Imagine joining the long sides of a rectangular piece of dough to form a tube.) This movement causes the more central cells of the neural plate to invaginate, or dip inward, to form the neural groove. As the groove deepens, the cells pushing up at the border of the neural fold region eventually meet and fuse, forming the neural tube that runs anteriorly and posteriorly along the embryo. The adjacent nonneural ectoderm then reunites to seal the neural tube within an ectodermal covering that surrounds the embryo.

FIGURE 2.38 Development of the vertebrate nervous system.
Cross sections through the blastula and embryo at various developmental stages during the first 21 days of life. Early in embryogenesis, the multicellular blastula (top) contains cells destined to form various body tissues. Migration and specialization of different cell lines leads to formation of the primitive nervous system around the neural groove and neural tube on the dorsal surface of the embryo. The brain is located at the anterior end of the embryo and is not shown in these more posterior sections, which are taken at the level of the spinal cord.
HOW THE BRAIN WORKS
Blood Supply and the Brain
Approximately 20% of the blood flowing from the heart is pumped to the brain. A constant flow of blood is necessary, because the brain has no way of storing glucose or extracting energy without oxygen. When the flow of oxygenated blood to the brain is disrupted for only a few minutes, unconsciousness and death can result. Two sets of arteries bring blood to the brain: the vertebral arteries, which supply blood to the caudal portion of the brain, and the internal carotid arteries, which supply blood to wider brain regions (Figure 1). Although the major arteries sometimes join together and then separate again, little mixing of blood occurs between the rostral and caudal arterial supplies or between the right and left sides of the rostral portion of the brain. As a safety measure, in the event of a blockage or ischemic attack, blood should be rerouted to reduce the probability of loss of blood supply; but in practice, this rerouting of the blood supply is relatively poor.
Blood flow in the brain is tightly coupled with metabolic demand of the local neurons. Hence, increases in neuronal activity lead to a coupled increase in regional cerebral blood flow. Increased blood flow is not primarily for increasing the delivery of oxygen and glucose to the active tissue, but rather to hasten removal of the resultant metabolic by-products of the increased neuronal activity. The precise mechanisms for altering blood flow, however, remain hotly debated. These local changes in blood flow permit regional cerebral blood flow to be used as a measure of local changes in neuronal activity, and serve as the basis for some types of functional neuroimaging. Particular examples are positron emission tomography, using techniques such as the 15O-water method, and functional magnetic resonance imaging, which is sensitive to changes in the concentration of oxygenated versus deoxygenated blood in the region of active tissue.

FIGURE 1 Blood supply and the brain.

FIGURE 2.39 Early stages of embryonic development in mammals.
(a) Developing embryo. The embryo goes through a series of folds, or flexures, during development. These alterations in the gross structure of the nervous system give rise to the compact organization of the adult brain and brainstem in which the cerebral cortex overlays the diencephalon and midbrain within the human skull. (b) There is significant similarity between the gross features of the developing fetuses of mammals, as shown by this comparison of a human fetus (top) and pig fetus (bottom).
At both ends of the neural tube are openings (the anterior and the posterior neuropores) that close on about the 23rd to 26th day of gestation. When the anterior neuropore is sealed, this cavity forms the primitive brain, consisting of three spaces, or ventricles. If the neuropores do not close correctly, neural tube defects such as anencephaly (absence of a major portion of the brain and skull) or spina bifida (some of the vertebrae are not formed) may result. From this stage on, the brain’s gross features are formed by growth and flexion (bending) of the neural tube’s anterior portions (Figure 2.39). The result is a cerebral cortex that envelops the subcortical and brainstem structures. The final three-dimensional relations of the brain’s structures are the product of continued cortical enlargement and folding. The posterior portion of the neural tube differentiates into a series of repeated segments that form the spinal cord.
In primates, almost all neurons are generated prenatally during the middle third of gestation. The entire adult pattern of gross and cellular neural anatomical features is present at birth, and there is little generation of neurons after birth (but see the section called “Birth of New Neurons Throughout Life,” later in this chapter). Although axonal myelination continues for some period postnatally (e.g., until adulthood in the human frontal lobe), the newborn has a well-developed cortex that includes the cortical layers and areas characterized in adults. For instance, BA17 (the primary visual cortex) can be distinguished from the motor cortex by cytoarchitectonic analysis of its neuronal makeup.
Neural Proliferation and Migration of Cortical Cells The neurons that form the brain arise from a layer of precursor cells in proliferative zones located adjacent to the ventricles of the developing brain. The cortical neurons arise from the subventricular zone, and those that form other parts of the brain arise from precursor cells in the ventricular zone. For this discussion, refer to Figure 2.40, which shows a cross section through the cortex and the precursor cell layers at various times during gestation. We will now concentrate on the cells that form the cortex. The precursor cells are undifferentiated cells from which all cortical cells, including neuronal subtypes and glial cells, arise through cell division and differentiation. For the first five to six weeks of gestation, the cells in the subventricular zone divide in a symmetrical fashion. The result is exponential growth in the number of precursor cells.
At the end of six weeks, when there is a stockpile of precursor cells, asymmetrical division begins. After every cell division, one of the two cells formed becomes a migratory cell destined to be part of another layer; the other cell remains in the subventricular zone, where it continues to divide asymmetrically. Later in gestation, the proportion of migratory cells increases until a laminar (i.e., layered) cortex made up of the migratory cells is formed. This cortex has a foundational epithelial layer that becomes the cell lining of the ventricles and is known as the ependymal cell layer.
The migratory cells travel outward from the subventricular zone by moving along peculiar cells known as radial glial cells, which stretch from the subventricular zone to the surface of the developing cortex. The work of radial glial cells does not end with development. These cells are transformed into astrocytes in the adult brain, helping to form part of the blood–brain barrier.
As the first migrating neurons approach the surface of the developing cortex—a point known as the cortical plate—they stop short of the surface. Neurons that migrate later pass beyond the termination point of the initial neurons and end up in more superficial positions—positions nearer the outer cortical surface. Thus, it is said that the cortex is built from the inside out, because the first neurons to migrate lie in the deepest cortical layers, whereas the last to migrate move farthest out toward the cortical surface.
Neuronal Determination and Differentiation The cortex is made up of many different types of neurons organized in a laminar fashion. Layer IV, for example, contains large pyramidal cells, layer III is populated primarily by stellate cells, and so on. You may be wondering how that population of virtually identical precursor cells gives rise to the variety of neurons and glial cells in the adult cortex. What determines the type of neuron that a migrating cell is fated to become? The answer lies in the timing of neurogenesis. Experimental manipulation of developing cells has shown that the differentiated cell type is not hardwired into the code of each developing neuron. Neurons that are experimentally prevented from migrating, by exposing them to high-energy X-rays, eventually form cell types and patterns of connectivity that would be expected from neurons that were created at the same gestational stage. Even though the thwarted neurons might remain in the ventricular zone, they display interconnections with other neurons that would be normal had they migrated to the cortical layers normally.
The timeline of cortical neurogenesis differs across cortical cytoarchitectonic areas, but the inside-out pattern is the same for all cortical areas. Because the timeline of cortical neurogenesis determines the ultimate pattern of cortical lamination, anything that affects the genesis of cortical neurons will lead to an ill-constructed cortex. A good example of how neuronal migration can be disrupted in humans is fetal alcohol syndrome. In cases of chronic maternal alcohol abuse, neuronal migration is severely disrupted and results in a disordered cortex, leading to a plethora of cognitive, emotional, and physical disabilities.
The Radial Unit Hypothesis We now have a picture of how cortical neurons are born and how they migrate radially from the ventricular zone toward the surface of the developing cortex. The neurons migrate along the radial glial cells that form a pathway for them. Because the radial glial highway is organized in a straight line from the ventricular zone to the cortical surface, there is a topographic relation between the precursor and proliferating neurons in the ventricular area and the cortical neurons that they yield in the adult. Hence, cells born next to each other in the ventricular zone end up near each other (in the plane perpendicular to the surface of cortex) in the cortex. In addition, cells derived from precursor cells distant from one another will ultimately be distant in the cortex.

FIGURE 2.40 Histogenesis of the cerebral cortex.
Cross-sectional views of developing cerebral cortex at early (left) and late (right) times during histogenesis. The mammalian cortex develops from the inside out as cells in the ventricular zone (VZ) divide, and some of the cells migrate to the appropriate layer in the cortex. Radial glial cells form a superhighway along which the migrating cells travel en route to the cortex. CO = cortex; CP = cortical plate; EL = ependymal layer; IZ = intermediate zone; ML = molecular layer; MZ = marginal zone; SP = subplate; SZ = subventricular zone; WM = white matter.

FIGURE 2.41 Radial unit hypothesis.
Radial glial cells in the ventricular zone project their processes in an orderly map through the various cortical layers, thus maintaining the organizational structure specified in the ventricular layer. (E = Embryonic day.)
According to this concept, termed the radial unit hypothesis by neuroscientist Pasko Rakic (1995) of Yale University, the columnar organization in the adult cortex is derived during development from cells that divide in the ventricular region (Figure 2.41). The cortical column is thus a principal unit of organization that has functional consequences and a developmental history. The radial unit hypothesis also provides a method for the evolutionary expansion of cortical size: Each unit is not enlarged; instead, the number of units increases. The radial unit and the cortical columns that arise from these groupings have functional and anatomical consequences in the adult. For example, the intracortical interconnectivity of local neurons appears to be well suited to the sizes of cortical columns, which vary in adults from about 100 μm to 1 μm on a side, depending on the species and cortical area.
Birth of New Neurons Throughout Life
One principle about the human brain that, until recently, dominated in the neuroscience community, is the idea that the adult brain produces no new neurons (Figure 2.42). This view has been held despite a variety of claims of neurogenesis in the brain in histological studies dating as far back as the time of Ramón y Cajal. Recent studies using an array of modern neuroanatomical techniques have challenged this belief.

FIGURE 2.42 This cartoon exposes the commonly held belief that once we lose neurons, they can never be replaced.
© Bryan Reading / www.cartoonstock.com.
Neurogenesis in adult mammals has now been well established in two brain regions: the hippocampus and the olfactory bulb. Neurogenesis in the hippocampus is particularly noteworthy because it plays a key role in learning and memory (see Chapter 9). In rodents, studies have shown that stem cells in a region of the hippocampus known as the dentate gyrus produce new neurons in the adult, and these can migrate into regions of the hippocampus where similar neurons are already functioning. It is important to know that these new neurons can form dendrites and send out axons along pathways expected of neurons in this region of the hippocampus, and they can also show signs of normal synaptic activity. These findings are particularly interesting because the number of new neurons correlates positively with learning or enriched experience (more social contact or challenges in the physical environment) and negatively with stress (e.g., living in an overcrowded environment). Moreover, the number of newborn neurons is related to hippocampal-dependent memory (Shors, 2004).
Other investigators have found that these new neurons become integrated into functional networks of neurons and participate in behavioral and cognitive functions in the same way that those generated during development do (Ramirez-Amaya et al., 2006). Future work will be required to establish whether adult neurogenesis occurs more broadly in the mammalian brain or is restricted to the olfactory bulb and hippocampus.
What about the adult human brain? Does neurogenesis also occur in mature humans? In a fascinating line of research, a team of scientists from California and Sweden (Eriksson et al., 1998) explored this question in a group of terminally ill cancer patients. As part of a diagnostic procedure related to their treatment, the patients were given BrdU, a synthetic form of thymidine used as a label to identify neurogenesis. The purpose was to assess the extent to which the tumors in the cancer patients were proliferating; tumor cells that were dividing would also take up BrdU, and this label could be used to quantify the progress of the disease.

|
|

|

|

|

|
FIGURE 2.43 Newly born neurons in adult human.
(a) The hippocampus of the adult human brain, stained for a neuronal marker (NeuN). (b) The dentate gyrus granule cell layer (GCL) in a NeuN-stained section. (c) Bromodeoxyuridine (BrdU)-labeled nuclei (arrows) in the granule cell layer of the dentate gyrus. (d) BrdU-labeled cells (arrow) in the granule cell layer of the dentate gyrus. (e) BrdU-stained cells (arrows) adjacent to the ependymal lining in the subventricular zone (SZ) of the human caudate nucleus. These neurons have elongated nuclei resembling the migrating cells that typically are found in the rat subventricular zone. (f) BrdU-stained cells (arrows) with round to elongated nuclei in the subventricular zone of the human caudate nucleus. The horizontal black bars are scale bars representing 50 μm.
FIGURE 2.44 The birth of new neurons in the dentate gyrus of the adult human (a–h) compared to those in the adult rat (i–l).
New neurons show simultaneous labeling for different stains. (a) A neuron is labeled for NeuN, a neuronal marker. (b) The same cell is labeled with BrdU, indicating that it is newly born (full arrow). (Note that the lone arrowheads in (a) through (d) are pointing to neurons that are fluorescing red or green, owing to nonspecific staining; i.e., these are not newly born neurons). (c) This same cell is not stained by glial fibrillary acidic protein (GFAP), indicating that it is not an astrocyte. (d) The three stained sections are merged. The image shows that a BrdU-labeled cell could specifically coexpress NeuN without expressing GFAP. Confocal microscopy permits examination of the coexpression of NeuN and BrdU in the neuron by focusing the image above (e, f) and below (g, h) the level of the section shown in panel (d). Note that red blood cells and endothelial cells, present in several small blood vessels, show nonspecific staining, as indicated by the asterisks in (e) through (h). Panels (i) through (l) show the similarity of the BrdU-labeled neurons in rat dentate gyrus. Note: The scale bar in (a) is 25 μm, and the scale is the same for panels (a) through (h). The scale bar in panel (i) is also 25 μm and is the scale for (i) through (l), but the magnification for (i) through (l) is higher than for (a) through (h).
Neurons undergoing mitotic division during neurogenesis in these patients also took up the BrdU, which could be observed in postmortem histological examinations of their brains. The postmortem tissue was immunostained to identify neuron-specific cell surface markers. The scientists found cells labeled with BrdU in the subventricular zone of the caudate nucleus and in the granular cell layer of the dentate gyrus of the hippocampus (Figure 2.43). By staining the tissue to identify neuronal markers, the researchers showed that the BrdU-labeled cells were neurons (Figure 2.44). These findings demonstrate that new neurons are produced in the adult human brain, and that our brains renew themselves throughout life to an extent not previously thought possible.
These exciting results hold great promise for the future of neuroscience. Research is under way to investigate the functionality of new neurons in the adult brain and to determine whether or not such neuronal growth can be facilitated in order to ameliorate brain damage or the effects of diseases such as Alzheimer’s.
The Baby Brain: Ready to Rock ’n’ Roll?
A host of behavioral changes takes place during the first months and years of life. What accompanying neurobiological changes enable these developments? Even if we assume that neuronal proliferation continues, we know that at birth the human brain has a fairly full complement of neurons, and these are organized to form a human nervous system that is normal, even if not complete in all details. What details are incomplete, and what is known about the time course of the maturation of the brain?
Although the brain nearly quadruples in size from birth to adulthood, it is not because of an increase in neuron number. A substantial amount of that growth comes from synaptogenesis (the formation of synapses) and the growth of dendritic trees. Synapses in the brain begin to form long before birth—prior to week 27 in humans (counting from conception)—but they do not reach peak density until after birth, during the first 15 months of life. Synaptogenesis is more pronounced early in the deeper cortical layers and occurs later in more superficial layers, following the pattern of neurogenesis described earlier. At roughly the same time that synaptogenesis is occurring, neurons of the brain are increasing the size of their dendritic arborizations, extending their axons, and undergoing myelination. Synaptogenesis is followed by synapse elimination (sometimes called pruning), which continues for more than a decade. Synapse elimination is a means by which the nervous system fine-tunes neural connectivity, presumably eliminating the interconnections between neurons that are redundant, unused, or do not remain functional. An example comes from primary visual cortex (BA17): Initially, there is overlap between the projections of the two eyes onto neurons in BA17. After synapse elimination, the cortical inputs from the two eyes within BA17 are nearly completely segregated. The axon terminals relaying information from each eye form a series of equally spaced patches (called ocular dominance columns), and each patch receives inputs from predominantly one eye.
One of the central hypotheses about the process of human synaptogenesis and synapse elimination is that the time course of these events differs in different cortical regions. The data suggest that in humans, synaptogenesis and synapse elimination peak earlier in sensory (and motor) cortex than in association cortex. By contrast, in the brain development of other primates, synaptogenesis and pruning appear to occur at the same rates across different cortical regions. Differences in methodology, however, must be resolved before these interspecies variations will be wholly accepted. Nonetheless, compelling evidence suggests that different regions of the human brain reach maturity at different times.
The increase in brain volume that occurs postnatally is also a result of both myelination and the proliferation of glial cells. White matter volume increases linearly with age across cortical regions (Giedd et al., 1999). In contrast, gray matter volume increases nonlinearly, showing a preadolescent increase followed by a postadolescent decrease. In addition, the time course of gray matter increase and decrease are not the same across different cortical regions. In general, these data support the idea that postnatal developmental changes in the human cerebral cortex may not occur with the same time course across all cortical regions (see also Shaw et al., 2006).
TAKE-HOME MESSAGES
Summary
In terms of evolution, the oldest parts of the brain, which make up the brain stem structures, control our most basic survival functions, such as breathing, heart rate, and temperature. The more rostral structures evolved more recently and mediate more complex behaviors. The most rostral and youngest structure is the prefrontal cortex and is found only in mammals.
In the brain and the rest of the nervous system, nerve cells (neurons) provide the mechanism for information processing. Neurons can receive and process sensory inputs, plan and organize motor acts, and enable human thought. At rest, the neuronal membrane has properties that allow some materials (primarily ions) dissolved in intracellular and extracellular fluids to pass through more easily than others. In addition, active transport processes pump ions across the membrane to separate different species of ions, thereby setting the stage for differences in electrical potential inside and outside the neuron. These electrical differences are a form of energy that can be used to generate electrical currents that, via action potentials, can travel great distances down axons away from the neuron’s cell body. When the action potential reaches an axon terminal, it prompts the release of chemicals at a specialized region, the synapse, where the neuron contacts another neuron, muscle, or gland.
These chemicals (neurotransmitters) diffuse across the synaptic cleft between the neurons and contact receptor molecules in the next (postsynaptic) neuron. This chemical
transmission of signal leads to the generation of currents in the postsynaptic neuron and the continuation of the signal through the system of neurons that make up a neuronal circuit. Ion channels are the specialized mediators of neuronal membrane potential. They are large transmembrane proteins that create pores through the membrane. Transmembrane proteins also form receptors on postsynaptic neurons. These are the receptors that bind with neurotransmitters, leading to changes in the membrane potential. Neurotransmitters come in a large variety of forms. Small-molecule transmitters include amino acids, biogenic amines, and substances like ACh; large-molecule transmitters are the neuropeptides.
Neuronal circuits are organized to form highly specific interconnections between groups of neurons in subdivisions of the central nervous system. The functions might be localized within discrete regions that contain a few or many subdivisions, identifiable either anatomically or functionally, but usually by a combination of both approaches. Brain areas are also interconnected to form higher level circuits or systems that are involved in complex behaviors such as motor control, visual perception, or cognitive processes such as memory, language, and attention. Neurodevelopment begins at an early stage in fetal growth and continues through birth and adolescence. New research also suggests that new neurons and new synapses form throughout life, allowing, at least in part, for cortical plasticity.
Key Terms
action potential (p. 30)
amygdala (p. 47)
association cortex (p. 56)
autonomic nervous system (p. 38)
axon (p. 26)
axon collateral (p. 26)
axon hillock (p. 30)
basal ganglia (p. 47)
blood–brain barrier (BBB) (p. 36)
brainstem (p. 43)
central nervous system (CNS) (p. 37)
central sulcus (p. 51)
cerebellum (p. 44)
cerebral cortex (p. 38)
commissure (p. 39)
corpus callosum (p. 39)
cytoarchitectonics (p. 51)
dendrite (p. 25)
depolarize (p. 31)
dura mater (p. 38)
electrical gradient (p. 29)
electrotonic conduction (p. 30)
equilibrium potential (p. 31)
frontal lobe (p. 50)
glial cell (p. 35)
gray matter (p. 39)
gyrus (p. 49)
hippocampus (p. 47)
hyperpolarization (p. 31)
hypothalamus (p. 45)
insula (p. 51)
ion channel (p. 28)
ion pump (p. 28)
layer (p. 38)
limbic system (p. 47)
medulla (p. 43)
midbrain (p. 44)
myelin (p. 26)
neocortex (p. 52)
neural circuit (p. 37)
neural system (p. 37)
neuron (p. 24)
neurotransmitter (p. 33)
node of Ranvier (p. 26)
nucleus (p. 38)
occipital lobe (p. 50)
parietal lobe (p. 50)
peripheral nervous system (PNS) (p. 37)
permeability (p. 29)
pituitary gland (p. 46)
pons (p. 44)
postsynaptic (p. 27)
prefrontal cortex (p. 54)
presynaptic (p. 27)
refractory period (p. 31)
resting membrane potential (p. 27)
saltatory conduction (p. 32)
soma (p. 25)
somatotopy (p. 56)
spike-triggering zone (p. 30)
spine (p. 26)
sulcus (p. 49)
Sylvian (lateral) fissure (p. 51)
synapse (p. 26)
synapse elimination (p. 67)
synaptic cleft (p. 32)
synaptogenesis (p. 67)
temporal lobe (p. 50)
thalamus (p. 45)
threshold (p. 31)
topography (p. 54)
tract (p. 39)
vesicle (p. 32)
voltage-gated ion channel (p. 30)
white matter (p. 39)
Thought Questions
Suggested Reading
Aimone, J. B., Deng, W., & Gage, F. H. (2010). Adult neurogenesis: Integrating theories and separating functions. Trends in Cognitive Sciences, 14(7), 325–337. Epub 2010 May 12.
Bullock, T. H., Bennett, M. V., Johnston, D., Josephson, R., Marder, E., & Fields, R. D. (2005). The neuron doctrine, redux. Science 310, 791. doi:10.1126/science.1114394
Haeusser, M. (2000). The Hodgkin–Huxley theory of the action potential. Nature Reviews Neuroscience, 3, 1165.
Mesulam, M.-M. (2000). Behavioral neuroanatomy: Large-scale networks, association cortex, frontal syndromes, the limbic system, and hemispheric specialization. In Shaw, P., Greenstein, D., Lerch, J., Clasen, L., Lenroot, R., Gogtay, N., et al. (2006). Intellectual ability and cortical development in children and adolescents. Nature, 440, 676–679.
Shepherd, G. M. (1988). Neurobiology (2nd ed.). New York: Oxford University Press.
Shors, T. J. (2004). Memory traces of trace memories: Neurogenesis, synaptogenesis and awareness. Trends in Neurosciences, 27, 250–256.
Streidter, G. (2005). Principles of brain evolution, pps. 217–53. Sunderland, MA: Sinawer.