Chapter 7
The Addenbrooke’s Cognitive Examination: Revised and Supplementary Test Suggestions
Introduction
This chapter describes the use of the revised version of the Addenbrooke’s Cognitive Examination: ACE-III. The original test was developed in the Cambridge memory clinic in the 1990s and was shown to be sensitive to early Alzheimer’s disease (AD) and to differentiate AD from frontotemporal dementia (FTD). In addition it was shown to be useful in the separation of organic brain disease from psychiatric states, and in the detection of cognitive dysfunction associated with the parkinsonian syndromes of progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and multiple system atrophy (MSA) (for references to the ACE see ‘Selected Further Reading’ at the end of this book). The main weaknesses were the imbalance across domains (especially the limited range of visuospatial/perceptual tasks), the insensitivity of the naming component, and the difficulty in translation of certain components. Another goal in developing the ACE-R was to have a version with clearer cognitive subtest scores. The ACE-R went through multiple prototypes before arriving at the final version.
The ACE and the ACE-R incorporated the Mini-Mental State Examination (MMSE) which was included at the time that the MMSE was freely available. It subsequently came under copyright and was published by Psychological Assessment Resources, Inc. (PAR). This necessitated changes to the ACE-R to remove MMSE-specific items and resulting in the ACE-III was shown to be highly equivalent to the ACE-R with a correlation of 0.99 between the two versions of the ACE.
This chapter describes the ACE-III (Fig. 7.1) together with scoring criteria and normative data followed by suggestions for ‘add-on’ bedside tasks that test areas not well covered by the ACE-III.
There is also now an iPad version of the ACE-III which is freely available via https://www.neura.edu.au/research-clinic/frontier/research/downloads/ as well as the mini-ACE which is a reduced 30-item version of the ACE-III.
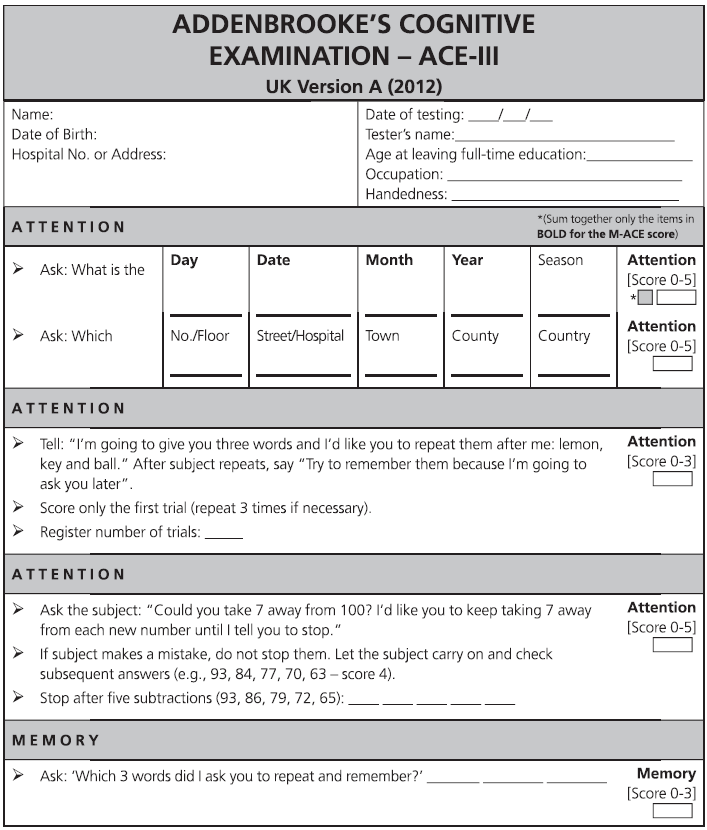
Fig. 7.1 ACE-III English version and scoring instructions.
Copyright © John Hodges.

Fig. 7.1 (continued)
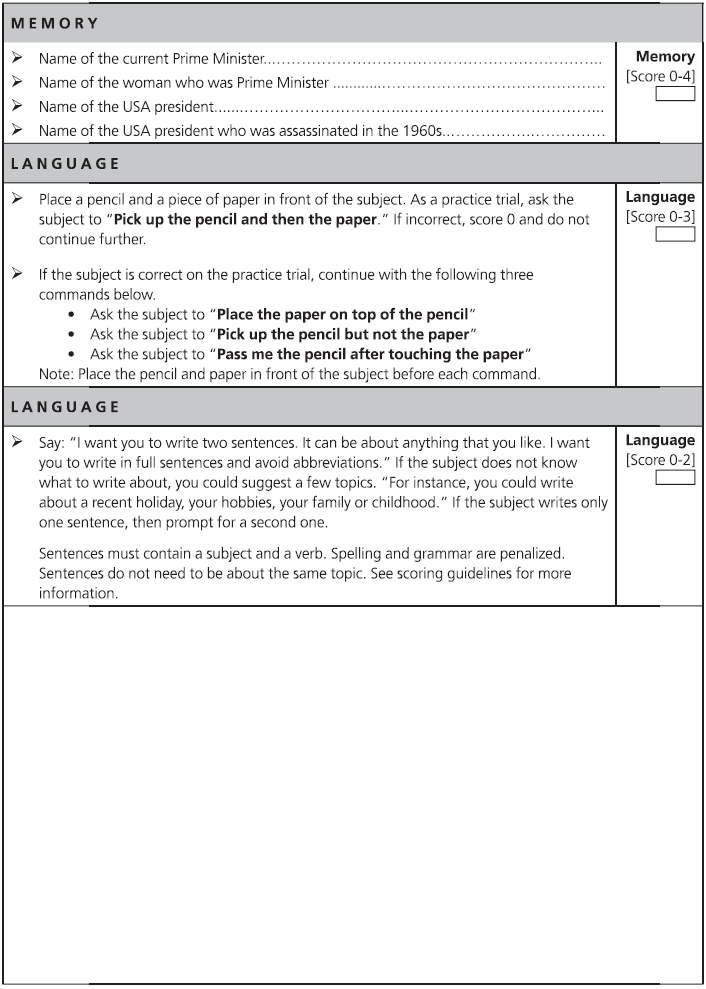
Fig. 7.1 (continued)
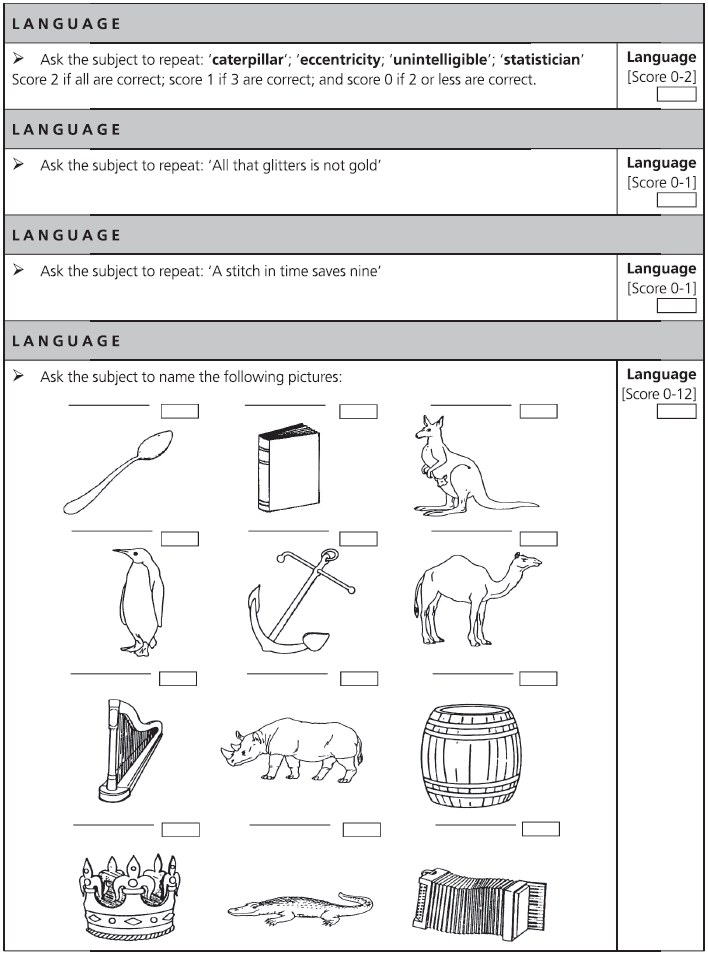
Fig. 7.1 (continued)
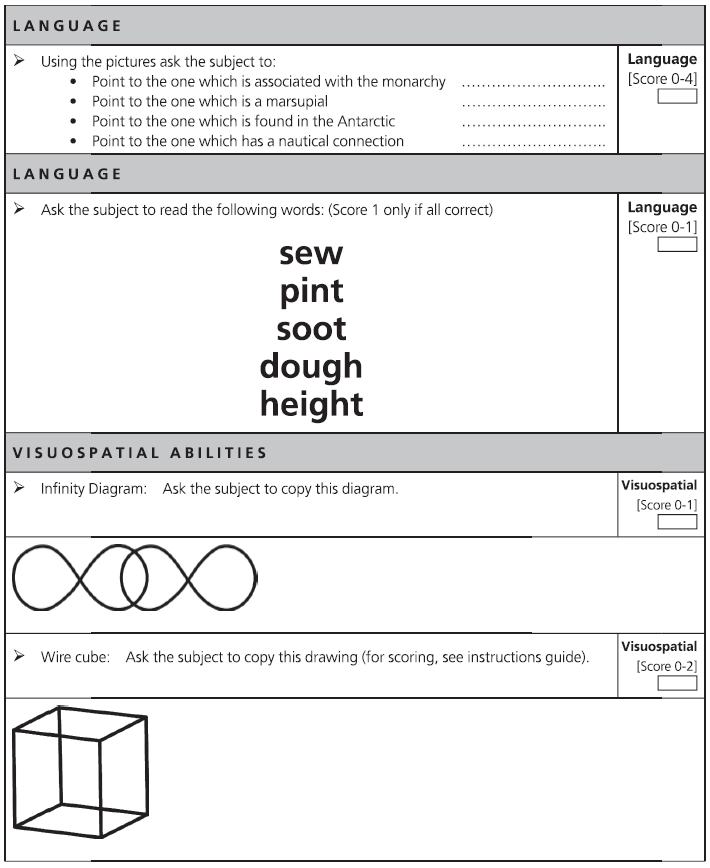
Fig. 7.1 (continued)
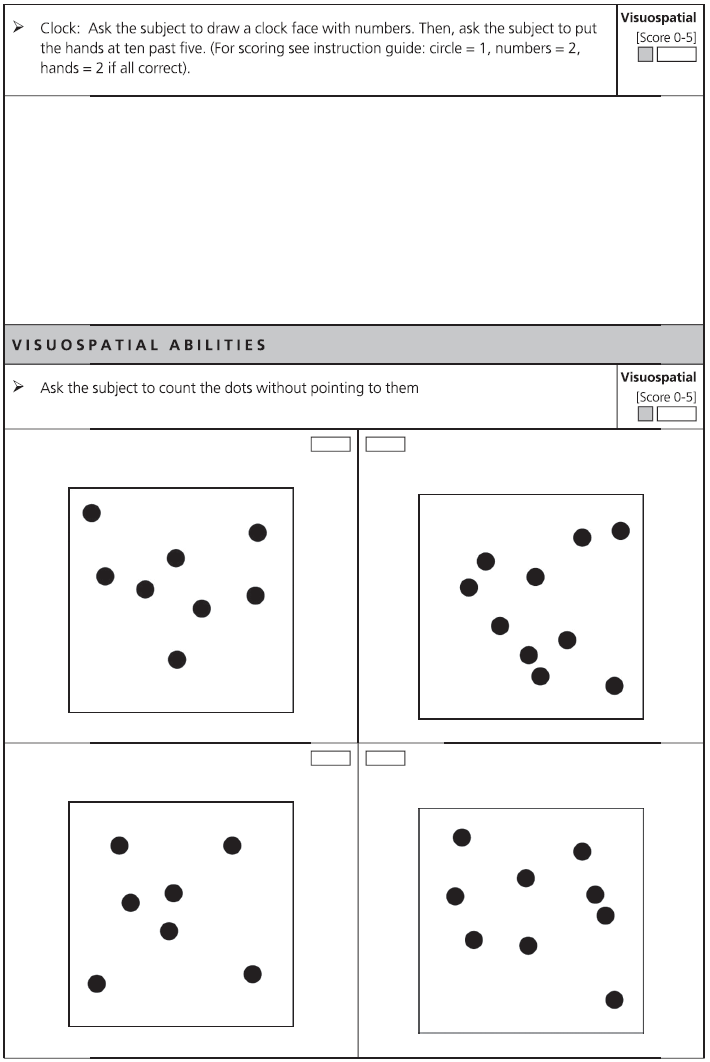
Fig. 7.1 (continued)
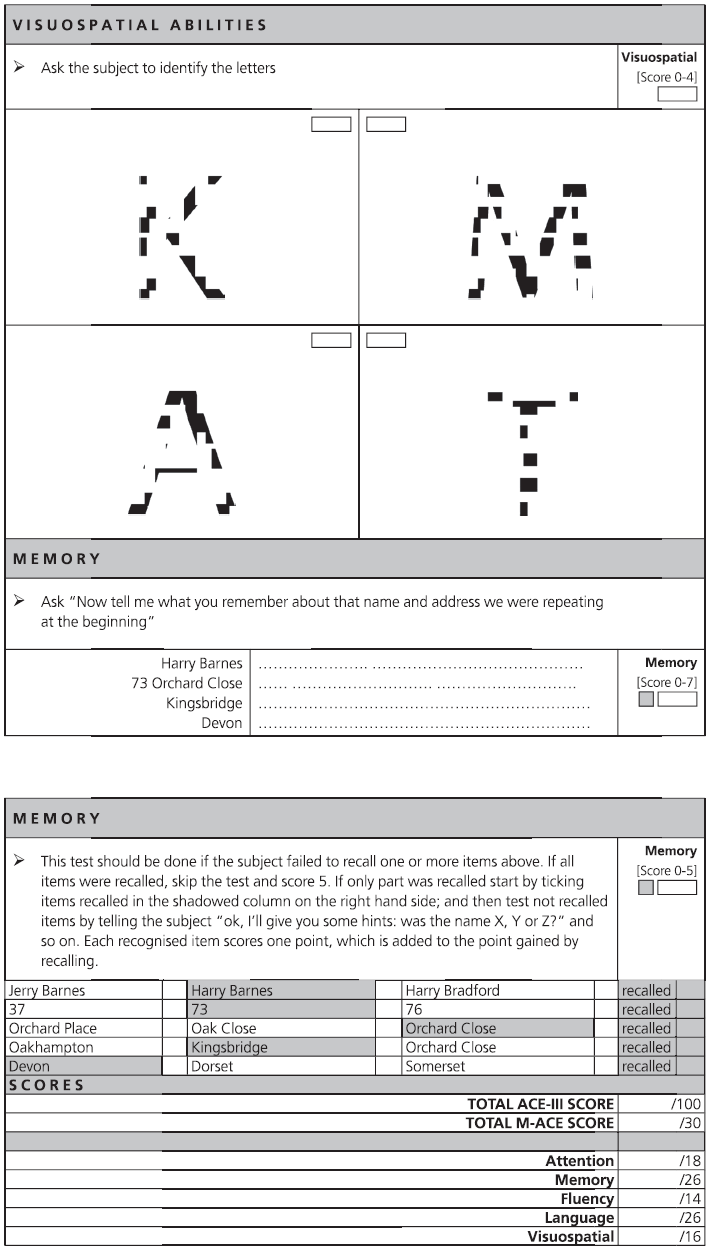
Fig. 7.1 (continued)
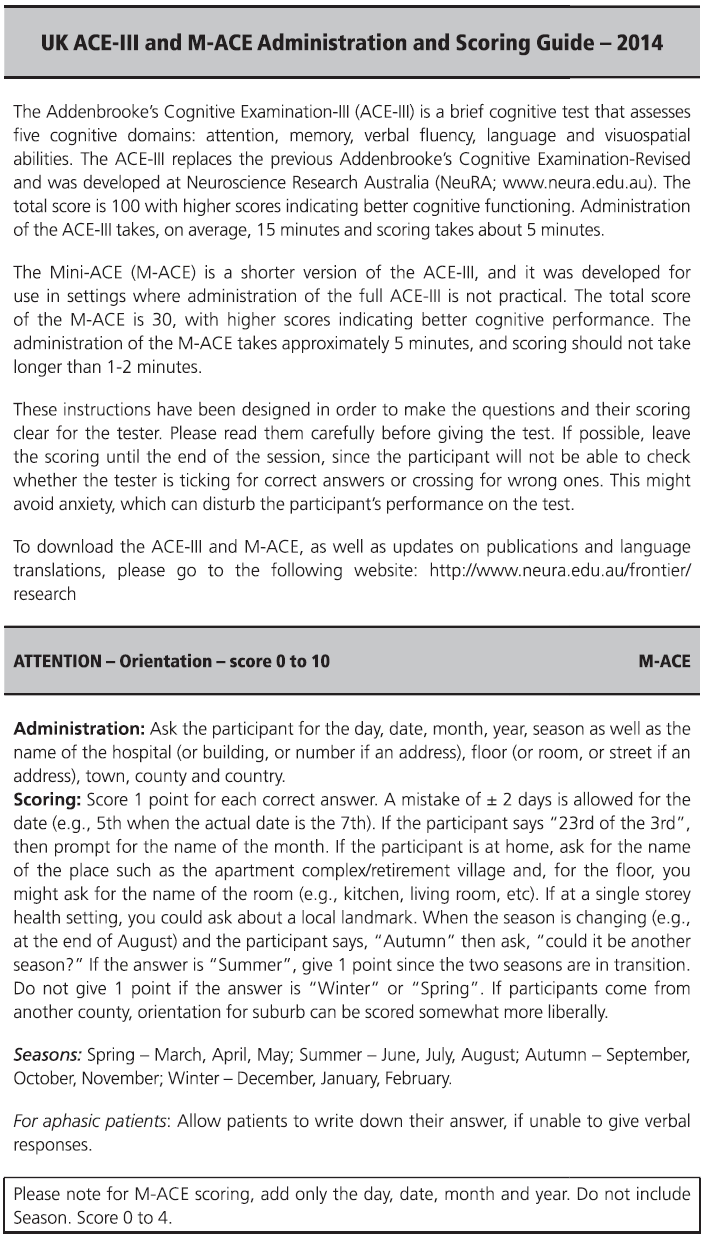
Fig. 7.1 (continued)
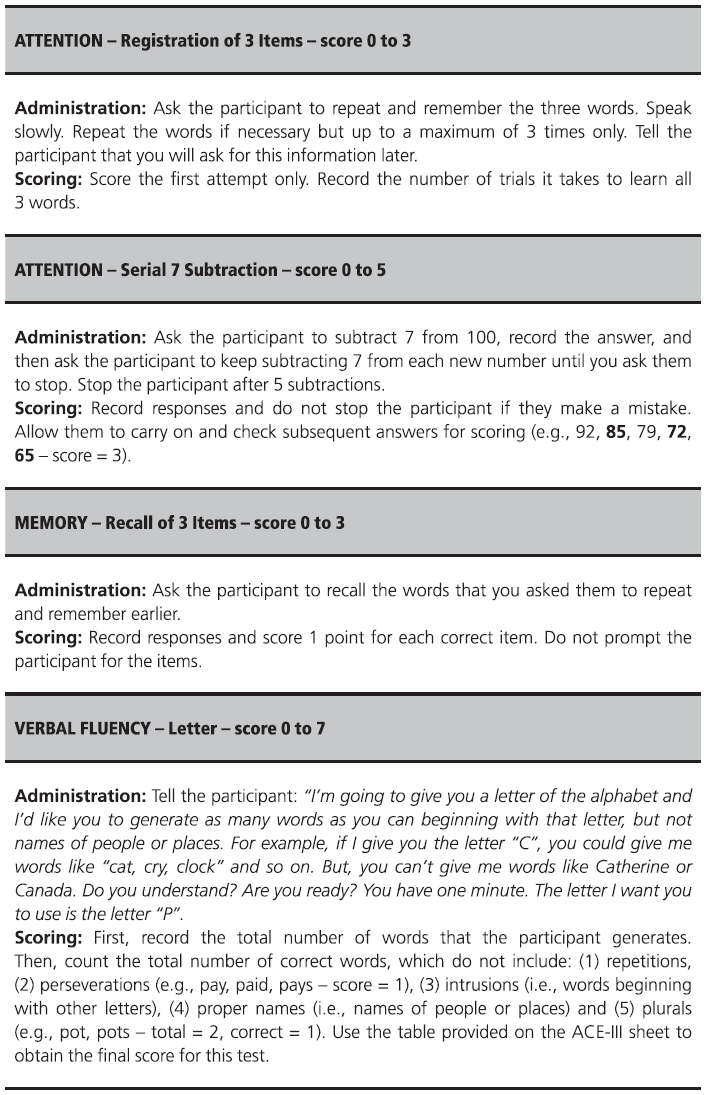
Fig. 7.1 (continued)

Fig. 7.1 (continued)
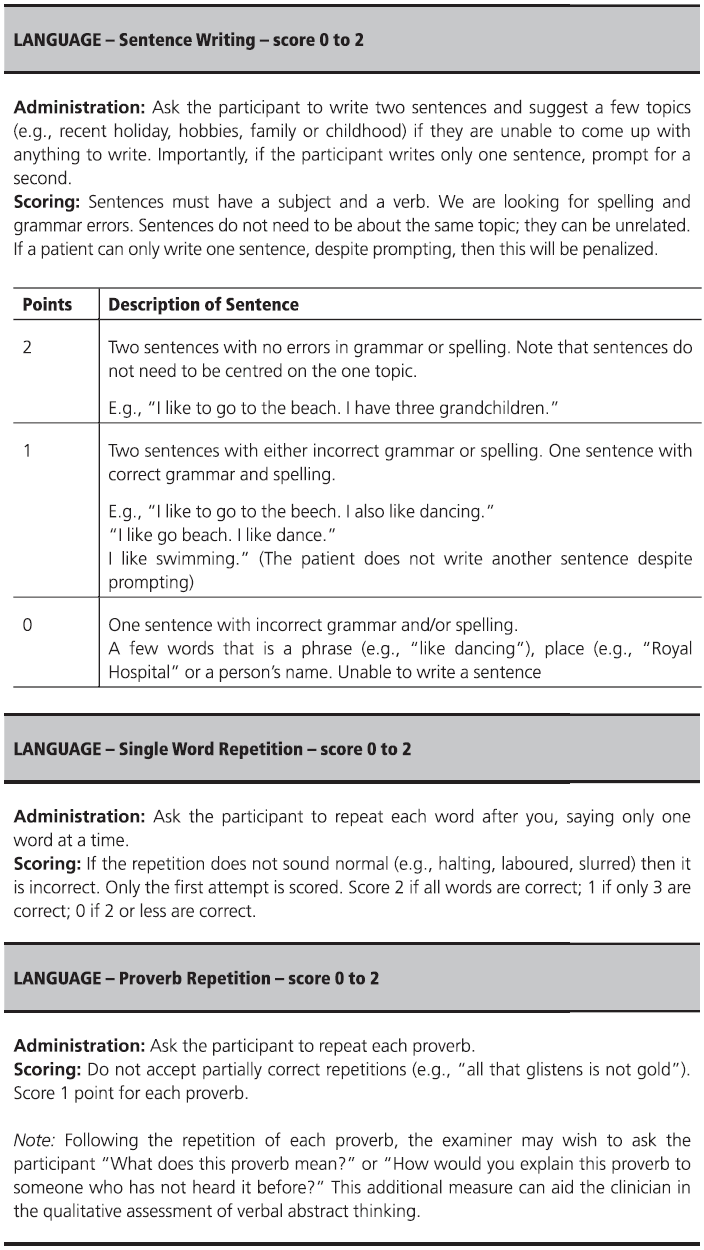
Fig. 7.1 (continued)
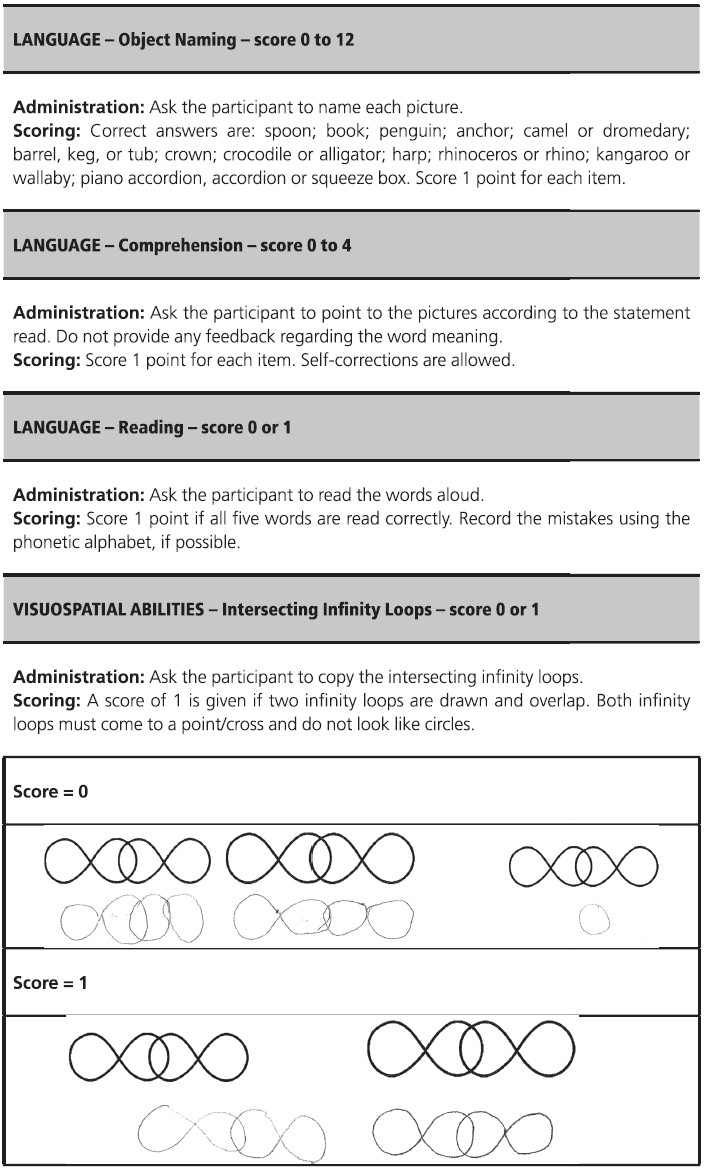
Fig. 7.1 (continued)
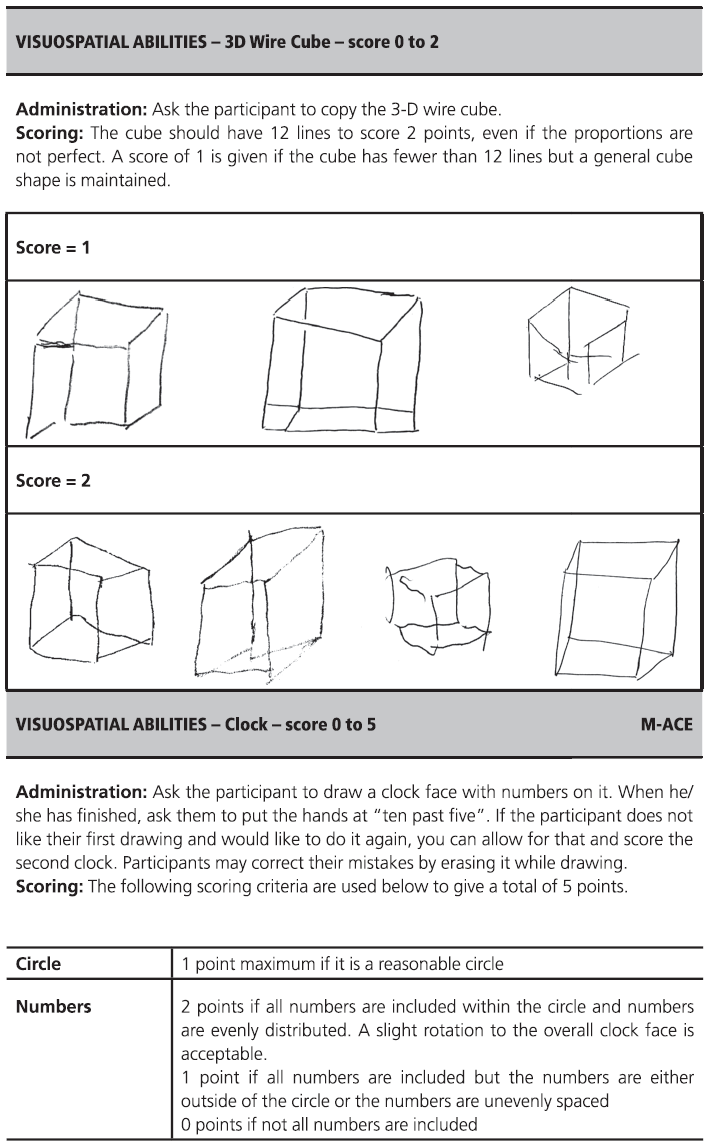
Fig. 7.1 (continued)
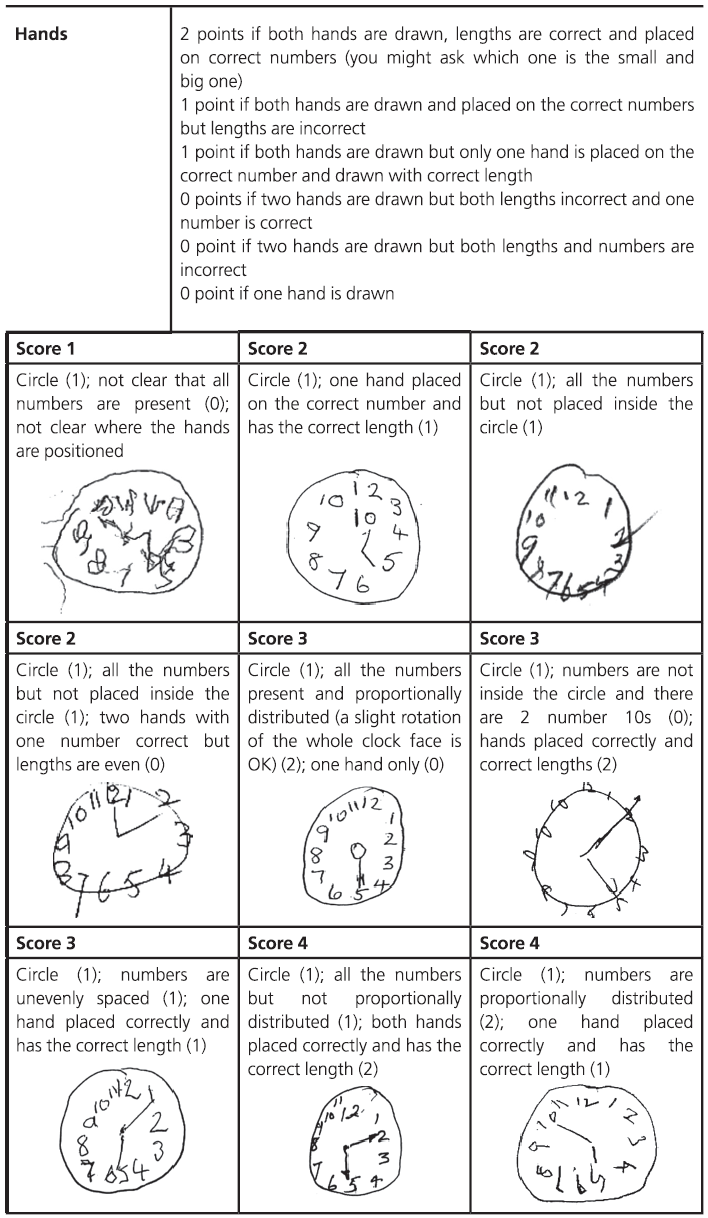
Fig. 7.1 (continued)
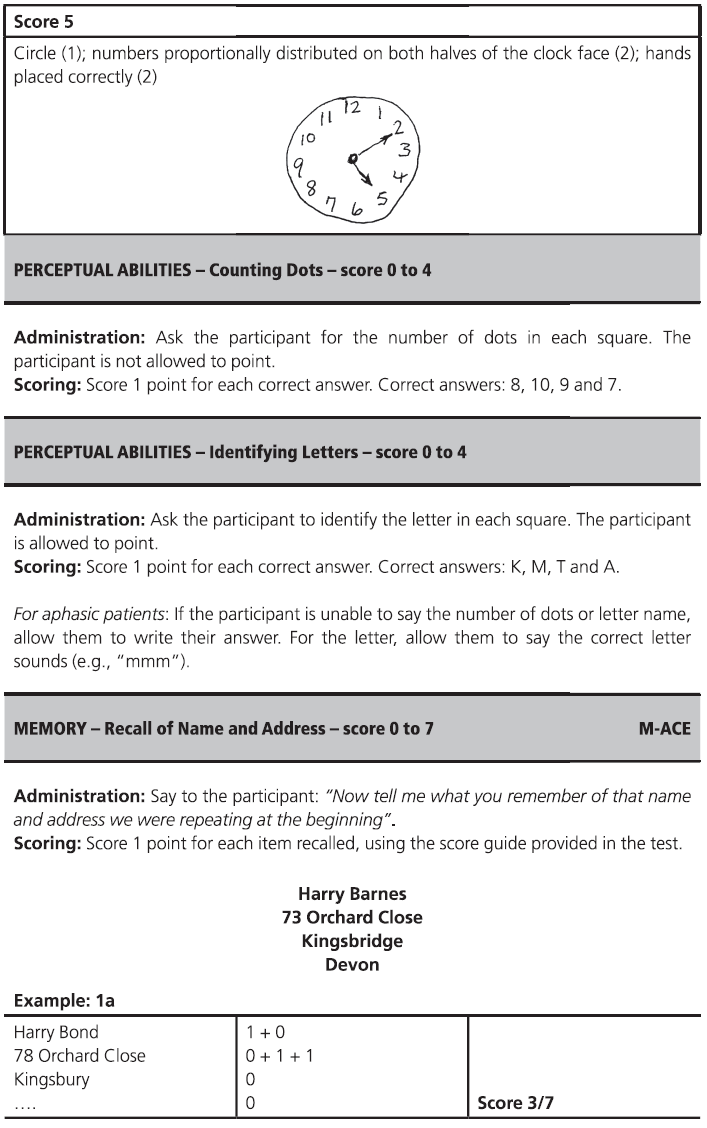
Fig. 7.1 (continued)
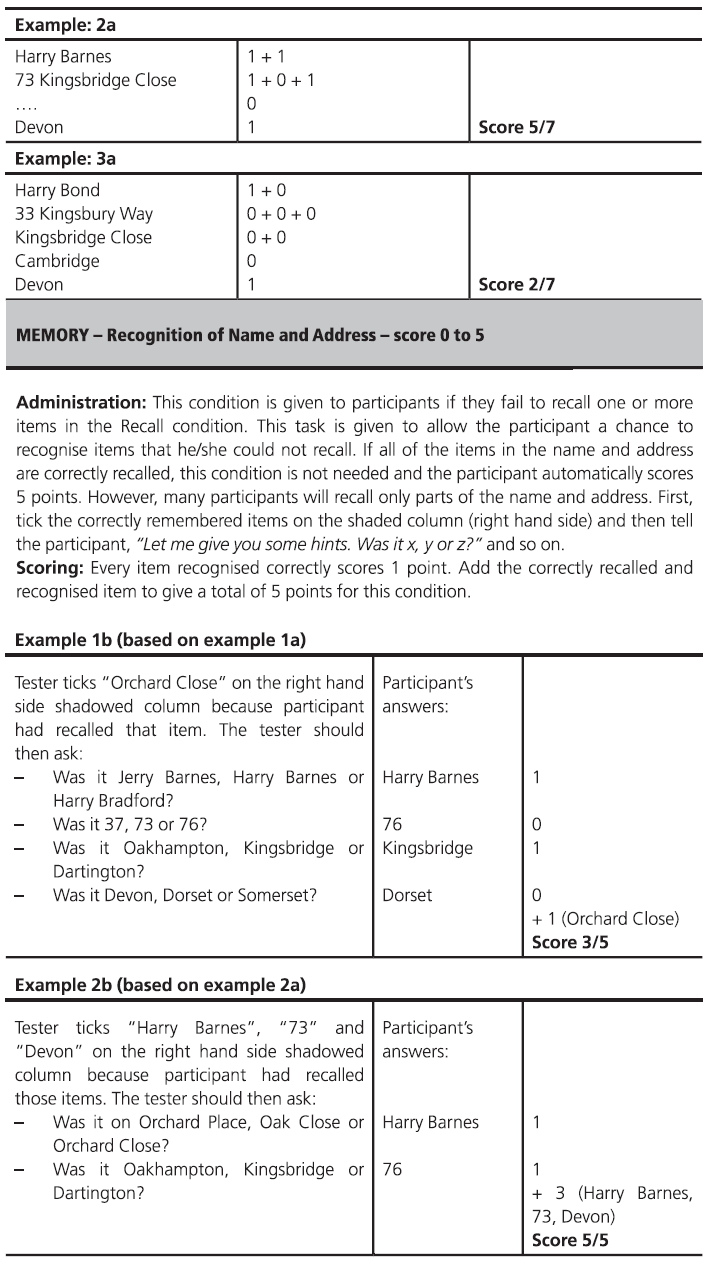
Fig. 7.1 (continued)
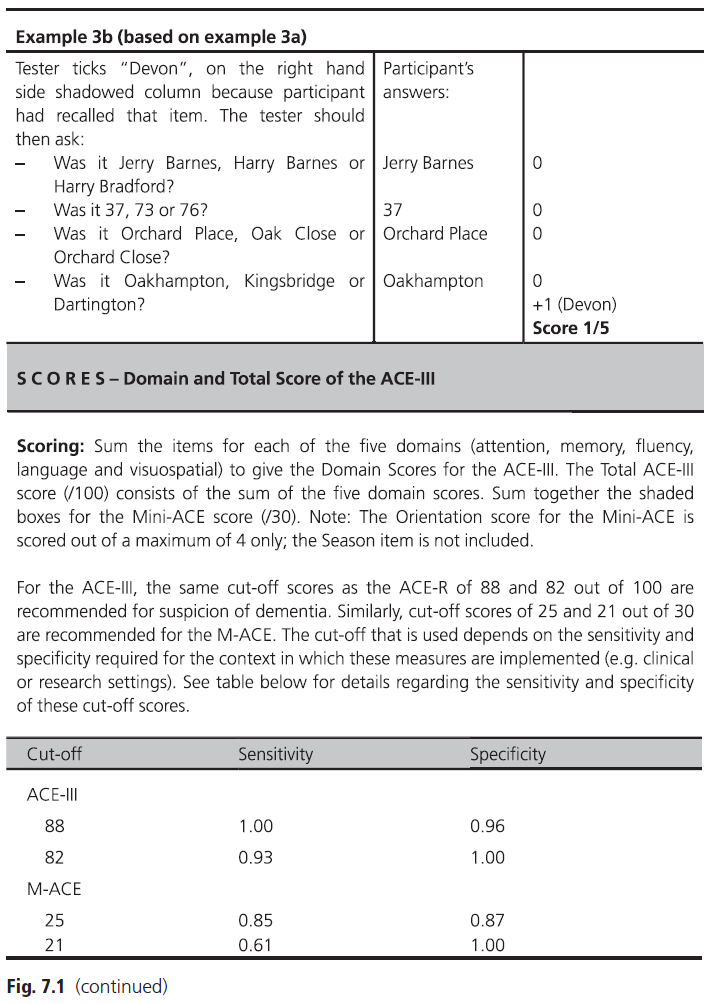
Fig. 7.1 (continued)
Normative Data
For the original version of the ACE we derived normative data from 127 controls and recommended the use of two cut-offs (<88 and <82) with high sensitivity and high specificity, respectively. We expected that the revised version might perform differently but we were very pleased to find almost identical results when it was administered to 63 normal volunteer subjects along with 178 clinic attenders (142 with dementia). Again, a cut-off of 88 produced high sensitivity (0.94) but lower specificity (0.89) whereas a cut-off of 82 resulted in lower sensitivity (0.84) but great specificity (1.00). In other words, patients with a score of <88 are at high risk of having an organic brain disease, but at this high cut-off you will generate false positives. Conversely, at the lower cut-off (<82) you will almost certainly detect all dementia patients but you will miss cases with early AD (false negatives). Table 7.1 shows the full sensitivity, specificity, and positive predictive values (PPV) at different base rates of dementia and Table 7.2 shows cut-off for the various subscores. In order to tackle the ‘grey zone’ between 88 and 82, we devised likelihood ratios for probability of dementia. Table 7.3 illustrates that as the ACE-R cut-off falls from 88 to 82, the likelihood ratio rate rises progressively from 8.4 to 100, which means that a score of 82 is 100 times more likely to come from a patient with dementia than one without.
One of the aims of the old ACE was to differentiate AD from FTD. After various attempts we produced a ratio score (VLOM ratio) that reflects the relative balance of cognitive dysfunction in these two disorders:
VLOM ratio = [verbal fluency and language]/ [orientation and memory].
Table 7.1 Sensitivity and specificity of different ACE-R (and the MMSE) cut-off scores for diagnosing dementia, with corresponding positive predictive values (PPV) at different rates of dementia prevalence
| PPV at different prevalence rates | ||||||
| ACE-R cut-off | Sensitivity | Specificity | 5% | 10% | 20% | 40% |
| 88 | 0.94 | 0.89 | 0.31 (1.0) | 0.48 | 0.68 | 0.85 (1.0) |
| 82 | 0.84 | 1.00 | 1.0 (0.96) | 1.0 | 1.0 | 1.0 (0.90) |
Copyright © John Hodges.
Table 7.2 Lower limit of normal (cut-off scores) for total ACE-R and subscores according to age, showing control mean minus two standard deviations
| Age range (years) | Education (years) | Total ACE-R score | Attention/ orientation | Memory | Fluency | Language | Visuospatial |
|---|---|---|---|---|---|---|---|
| 50–59 | 12.7 | 86 | 17 | 18 | 9 | 24 | 15 |
| 60–69 | 12.9 | 85 | 17 | 19 | 8 | 21 | 14 |
| 70–75 | 12.1 | 84 | 16 | 17 | 9 | 22 | 14 |
Copyright © John Hodges.
Table 7.3 Likelihood ratios for probability of dementia at various ACE-R cut-off scores
| ACE-R score | Likelihood ratio of dementia |
|---|---|
| 88 | 8.4 |
| 87 | 11.5 |
| 86 | 14.2 |
| 85 | 18.9 |
| 84 | 27.6 |
| 83 | 52.5 |
| 82 | 100 |
Copyright © John Hodges.
Using both the old and the revised ACE, a VLOM ratio of >3.2 was found to be optimal in differentiating AD from FTD (74% sensitivity and 85% specificity), whereas a ratio of <2.2 was highly suggestive of FTD (sensitivity 58% and specificity 95%). Scores between 2.2 and 3.2 were poorly predictive of diagnosis. A number of other groups have reported similar findings and that VLOM is a generally useful adjunct to diagnosis.
In a small validation study of the ACE-III involving a group of 61 patients with mixed syndromes dementia and 25 controls scores on the ACE-R and ACE-III were extremely close in all groups suggesting that the cut-offs used for the ACE-R are applicable to the ACE-III.
Additional Material for Particular Cases
Although we consider the ACE-III to be a good general screening instrument, especially in the context of a memory or cognitive disorders clinic, it is often necessary to supplement the instrument with other tasks targeted towards suspected cognitive dysfunction. A full description of cognitive evaluation is presented in Chapter 5. The tasks described in the following sections are ‘selected highlights’ which have been chosen because either the ACE-III is deficient in this domain (e.g. frontal executive function, praxis, face recognition, etc.) or further tests are frequently required to clarify the nature of the deficit (e.g. aphasia or agnosia).
Remote memory
To assess remote memory we typically ask patients about recent news events, which should be tailored towards the patient’s interests and cultural setting. In the UK, the following are helpful in guiding assessment:
Recent sporting events: Olympic Games, Football World Cup, Test cricket series, etc.
Royal family news.
General elections.
Political scandals and resignations.
Disasters: 2004 Indian Ocean tsunami, 9/11 Twin Towers, Brighton bombing.
Wars: Iraq, Afghanistan, Gulf War, Falklands, etc.
Frontal executive function
As described earlier in this book, it is notoriously difficult to assess executive function at the bedside. The verbal fluency component of the ACE-III is the most sensitive part but it is often necessary to supplement the ACE-III in patients with suspected frontal pathology.
1. Abstraction: proverb interpretation
‘A rolling stone gathers no moss.’
‘Too many cooks spoil the broth.’
‘Still waters run deep.’
‘A bird in the hand is worth two in the bush.’
2. Similarities
In what way are the following the same?
‘An apple and a banana.’
‘A coat and a dress.’
‘A table and a chair.’
‘A poem and a statue.’
‘Praise and punishment.’
3. Go–no-go
The patient places one hand on the table and is asked to raise one finger in response to a single tap, but to hold still in response to two taps. The examiner taps the undersurface of the table using a random sequence of single and double taps.
4. Motor sequencing
Luria three-step: demonstrate hand sequence (fist–edge–palm) five times, and then ask subject to repeat sequence.
Alternating hand movements: demonstrate sequence five times (start position: one hand has fingers extended and the other has clenched fist, then positions alternate), and then ask subject to copy.
5. Cognitive estimates test
This very helpful task is described in the Appendix.
Language
1. Speech production
In patients whose principal complaint is difficulty with speech or language and for those in whom the ACE-III reveals such problems, it is important to evaluate language more thoroughly. It is very helpful to ask the patients to describe a complex scene such as that shown in Fig. 7.2. Particular note should be made of speech rate, dysarthria, phonological or semantic errors, word-finding pauses, and grammatical errors.
2. Repeat and define
The following is a useful list of multisyllabic words that can be used to test repetition and comprehension. Patients with phonological processing deficits have difficulty with repetition but can convey the meaning when asked to define these words, whereas those with semantic impairments show the opposite pattern: perfect repetition but poor, or absent, understanding often with vague superordinate responses such as ‘Is it a flower/animal?’.

Fig. 7.2 The seaside scene from the Queen Square Screening Test for Cognitive Deficits: an example of a complex interactive scene for use in eliciting spontaneous language.
Reprinted by permission of Professor Elizabeth Warrington.
Caterpillar
Antelope
Chrysanthemum
Stethoscope
Encyclopaedia.
3. Comprehension of grammar/syntax
This can be tested using an array of three objects (e.g. pen, keys, and watch) placed in front of the patient who is then asked to obey a sequence of commands of increasing syntactic complexity.
‘Put the pen on the watch.’
‘Touch the watch with the pen.’
‘Touch the keys and then the pen.’
‘Touch the pen before touching the keys.’
‘Touch the pen but not the keys.’
‘Put the pen between the watch and the keys.’
‘You pick up the watch and give me the pen.’
A list of regular, exception, and non-words that can be used to screen for types of dyslexia are shown in Table 5.4 in Chapter 5.
Calculation
Testing basic arithmetic skills should be done in patients with suspected left angular gyrus lesions and is useful in the differentiation of semantic dementia (where calculation is invariably spared) from AD or CBD (in which these abilities are compromised from an early stage). Suggestions are given in Chapter 5.
Praxis
Tests for ideomotor or ideational apraxia are not included in the ACE-III but are important additions in the context of patients with progressive motor syndromes associated with cognitive dysfunction such as PSP and CBD as well as patients with focal left hemisphere pathology.
1. Buccofacial: first to command and then after imitation of examiner
Blow out a match.
Lick lips.
Cough.
Sip through a straw.
2. Limb
Copy of meaningless hand gestures as demonstrated by examiner using right and left hands (see Fig. 5.2 in Chapter 5).
Mime use of objects (combing hair, brushing teeth, using scissors, hammering): first to command then after imitation of examiner.
Symbolic gestures (waving, saluting, beckoning): to command and then after imitation of examiner.
Neglect phenomena
In addition to the drawings in the ACE-R it is useful to screen for suspected neglect using the following tasks:
Double-headed daisy: ask the patient to copy the figure (Fig. 7.3).
Visual search: using an array of As and Bs unevenly distributed across a sheet of A4, ask the patient to cross out all the As.
Line bisection: ask the patient to mark the half-way point on an array of lines of varying length with a cross (✗).
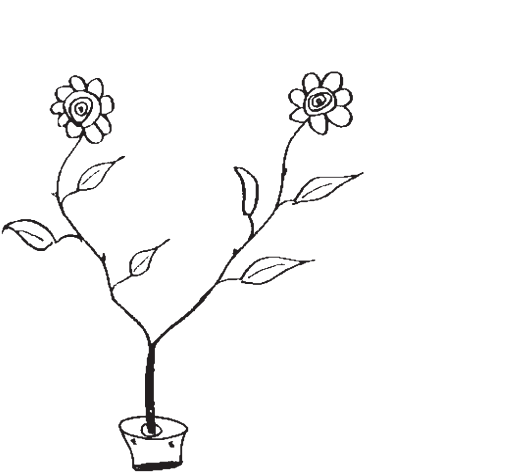
Fig. 7.3 Double-headed daisy in a pot.
Table 7.4 Profile of performance on tests of picture naming, picture description, generation of knowledge, and naming from description from the same target names in patients with perceptual, semantic, and anomic difficulties
| Perceptual | Semantic | Anomic | |
|---|---|---|---|
| Picture naming | ✗ | ✗ | ✗ |
| Describe | ✗ | ✗ | ✓ |
| Knowledge from name | ✓ | ✗ | ✓ |
| Name from verbal description | ✗ | ✗ |
✗ = impaired, ✓ = intact.
Complex visuoperceptual abilities and prosopagnosia
Performance on the naming component of the ACE-III should reveal evidence of apperceptive or associative agnosia but the following checklist should help tease apart various causes of object mis-recognition and naming (see Table 7.4 for further details).
1. Object agnosia
Naming of picture/object (note type of error).
Description of physical appearance of visually presented pictures or objects.
Verbal knowledge about the visually presented stimulus (picture/object).
Naming from verbal description.
2. Prosopagnosia
If a disorder of face recognition is suspected, use pictures of famous people and test the following as described more fully in Chapter 5 (see ‘Prosopagnosia’ in the ‘Right Hemisphere Functions’ section):
Face description: ability to describe age, gender, and emotion.
Face identification: can they correctly identify the person (e.g. ‘the Tory woman Prime Minister’).
Face naming: (‘Margaret Thatcher’).