Chapter 6
Standardized Mental Test Schedules: Their Uses and Abuses
Introduction
A large number of mental test schedules have been used over the years, which range in complexity from the 10-item Hodgkinson Mental Test, which takes only moments to complete, to the much more complex Dementia Rating Scale (DRS), which takes 30 minutes or more to administer. For practical purposes, however, such tests can be divided into two broad groups: (i) the brief schedules that can easily be used in the clinic, or at the bedside, and do not require specialized equipment or training; and (ii) the more elaborate scales, which are used largely, at least at present, in research studies, and require the purchase of test materials and some training in their administration. The Addenbrooke’s Cognitive Examination (ACE) was developed in an attempt to bridge this divide and to provide a test with greater sensitivity to early cognitive decline than the Mini-Mental State Examination (MMSE) and which could also differentiate between different brain diseases. Chapter 7 describes in detail the latest version of the ACE with instructions on administration and scoring plus suggestions for supplementary testing in selected cases.
The remainder of this chapter covers possible alternative cognitive screening instruments. Because of the plethora of potential tests, I have chosen to describe two of the most commonly used brief assessment schedules: the MMSE, and the Montreal Cognitive Assessment (MoCA); plus two longer tests, which are widely used in dementia research: the Mattis DRS and the Cambridge Cognitive Examination–Revised (CAMCOG-R). Finally I have also included a description of the Alzheimer’s Disease Assessment Scale– Cognitive subscale (ADAS-Cog) since it has been used widely in drug evaluation studies.
All schedules have limitations and are open to abuses, but this applies particularly to the shorter tests. They are undoubtedly useful for screening large populations, since they have good inter-rater reliability and fairly well-established normative data; but the results must be interpreted with caution when applied to individual patients and they are of very limited application in a Memory or Cognitive Disorder Clinic. A number of general points deserve consideration before each test is described.
All the schedules sample a number of different areas of cognitive ability (for example, attention/concentration, memory, language, or visuospatial abilities); hence, failure can be due to various combinations of cognitive impairment. A low score may reflect appalling performance in one domain only, or slight impairment across all the domains evaluated. As a practical example, consider a score of 27 out of 30 on the MMSE. This could be due to a loss of all three recall points in the memory subset, or a loss of one point from each of three of the five subsets: orientation, attention, memory (registration and recall), language, or visuospatial. Both patterns produce the same total score, but the former is clearly much more significant. This illustrates how essential it is to consider the profile of performance on these tests, and not just the overall total score.
It should be emphasized that all the schedules were developed with a view to quantifying the cognitive failings in elderly subjects with dementia or delirium, or both. They may, with certain provisos, be reliable measures in these situations; but this does not mean that they can be applied generally to patients with all types of cognitive impairment, both focal and general, acute and chronic. Of particular note is their insensitivity to circumscribed cognitive deficits. This is exemplified in patients with extensive right hemisphere lesions, who may have major visuospatial and perceptual deficits, but score almost perfectly on any of the mental test schedules under consideration (except the ACE-III). They are also notoriously insensitive to frontal lobe disorders, and patients with disabling and profound deficits in ‘executive’ and social functions typically perform normally, even on the more extensive screening batteries.
It should also be realized that the normative values and ‘cut-off ’ levels generally applied in these tests veer towards specificity, rather than sensitivity, in dementia. A score below the cut-off of 24 on the MMSE (in the absence of features of delirium) is a fairly good marker of dementia. However, many patients with early Alzheimer’s disease (AD) score above this cut-off, particularly if they are young and of superior background intellectual ability.
This brings up the important question of considering background demographic features, which are likely to affect performance. Age, education, and socioeconomic status are the most important variables. Ethnic group and first language should also be considered. These factors are additive, so that the lower limit of normal for an elderly person with only a few years of education is radically different to that of a young, highly educated professional. These points will be discussed further in relation to the test schedules under consideration.
Mini-Mental State Examination (MMSE)
The MMSE, designed by Folstein and colleagues from Baltimore, United States, in the 1970s, is the most widely used and studied screening measure of cognitive impairment. It has the advantages of brevity, ease of administration, and high inter-rater reliability. It can be easily incorporated into routine clinical practice, and provides a good rough-and-ready screening test for dementia and delirium. It is also of practical value in monitoring progression in these disorders. It is not useful, however, for the detection of mild cognitive impairment (MCI), focal deficits (amnesia, aphasia, visuospatial disorders, etc.), and is insensitive to frontal lobe disorders.
A score of less than 24 was initially suggested for distinguishing between impaired and normal subjects, respectively, with a reasonably high degree of specificity and sensitivity. However, these values were derived from screening elderly hospitalized patients with delirium or fairly advanced degrees of dementia, not outpatients with mild disease. Mild cases with early, but clinically definite, AD score above this level. It has also been clearly established that the MMSE is quite vulnerable to the effects of age, education, and socioeconomic status. The following age-related cut-offs have been proposed:
40s: 29/30
50s: 28/30
60s: 28/30
70s: 28/30
80s: 26/30
Further adjustments are required for educational level, especially in the older age-groups. For subjects aged over 70 years who left school before the age of 15 (i.e. those with less than 10 years of education), a score of up to 3 less than the age-related score listed is acceptable as normal.
There are also difficulties with scoring the attentional subtest. The authors of the MMSE originally suggested that the spelling of the word WORLD backwards should be given to those unable to perform serial subtraction. However, this instruction is rather loose, and leads to confusion. We have tended to administer serial sevens, and if any errors occur we give subjects the task of spelling WORLD backwards. The score is then taken as the best performance on either of these two. Other authors have opted to give either the serial sevens or the WORLD backward test to each subject.
The subtests most useful in detecting early AD are the recall of the three items, followed by orientation and drawing, although we have shown that the MMSE is far less sensitive than the ACE for screening in a memory clinic setting. The language tests are the least sensitive component of the MMSE. In Huntington’s disease and other forms of subcortical dementia, such as progressive supranuclear palsy (PSP), the attentional subtests are those most vulnerable to disease, but again, the MMSE lacks sensitivity. The MMSE is susceptible to ‘floor effects’ in severely demented cases. That is to say, once patients reach a fairly advanced stage of disease they tend to score very few points, and beyond this, progression cannot be assessed.
The MMSE was for many years used free of charge and was widely available. It has, however, been copyrighted and published by Psychological Assessment Resources, Inc. (PAR) which has perhaps affected its popularity and contributed to the rising popularity of the MoCA
Montreal Cognitive Assessment (MoCA)
See Table 6.1.
Table 6.1 The Montreal Cognitive Assessment (MoCA)
| Test domain | Score |
|---|---|
| Visuospatial/executive function | |
| Short alternating (letters and numbers) trails test | __/ 1 |
| Copy a cube | __/ 1 |
| Draw clock | __/ 3 |
| Naming | |
| Name lion, rhinoceros and camel | __/ 3 |
| Memory | |
| Subject is read a list of 5 words (face, velvet, church, daisy, red) | |
| List is read twice even if first trial is successful NOT SCORED | |
| Then after 5 minutes subject is asked to recall words without cueing | __/ 5 |
| Attention | |
| Digit span 5 digit span forwards and 3 backwards | __/ 2 |
| Patient is read a list of letters and must tap each letter A | __/ 1 |
| Serial subtraction of 7s starting at 100 | __/ 3 |
| Language | |
| Repetition of two phrases | __/ 2 |
| Fluency: asked to name words beginning with F | __/ 1 |
| Abstraction | |
| Similarity between banana–orange, train–bicycle, watch–ruler | __/ 2 |
| Orientation in time | |
| Date, month, year, day, place and city | __/ 6 |
| TOTAL SCORE |
|
Copyright © Z. Nasreddine MD. Reproduced with permission. Copies are available at www.mocatest.org.
The Montreal Cognitive Assessment (MoCA) was created in 1996 by Dr Ziad Nasreddine in Montreal, Canada. The MoCA test is a one-page, 30-point test administered in approximately 10 minutes. The test and administration instructions are freely accessible for clinicians at http://www.mocatest.org. The test is available in 55 languages or dialects. There are alternate forms designed for use in longitudinal settings. There is also a Basic form to test illiterate subjects or subjects with lower education levels.
The original MoCA test validation study showed it to be a promising tool for detecting MCI and early AD compared with the MMSE. Using a cut-off of <26, the sensitivity and specificity for detecting MCI were 90% and 87% respectively, compared with 18% and 100% respectively for the MMSE. The sensitivity and specificity of the MoCA for detecting early AD were 100% and 87% respectively, compared with 78% and 100% respectively for the MMSE. Normal controls had an average age of 72.84 years and average education of 13.33 years. Subsequent work in other settings is less promising, though generally superior to the MMSE. It has since been applied to many neurological and psychiatric disorders.
Mattis Dementia Rating Scale (DRS)
Originally designed by Mattis for use in a prospective study of dementia, the DRS assesses a fairly wide range of cognitive abilities, and contains a sufficient number of less-demanding items such that valid and reliable information can be obtained in more severely demented subjects. Its principal use is in research, particularly that involving longitudinal studies of demented patients or the comparison of patients with different pathologies.
It is easy to administer and score. The first four sections—attention (37 points), initiation (37 points), construction (6 points), and conceptualization (39 points)—are graded in difficulty, and contain screening tests at the beginning. If these are passed, the remainder of the section need not be administered. The final memory section (25 points) is given to all subjects. Approximately 20–40 minutes are required to administer it to demented patients, depending upon their level of impairment. Test–retest reliability is excellent and it has good construct validity compared to formal neuropsychological evaluation as the ‘gold standard’.
Normal elderly subjects perform well on the DRS. The mean total score in early studies ranged from 137 to 140 out of a total of 144 points. Our own early experience, together with that of the San Diego Alzheimer’s disease research group, suggested a cut-off of 132 for separating impaired from normal subjects. Since the publication of the first edition of this book, a number of studies have been published showing the predicted effects of age, education, and socioeconomic status. For instance, a study from Brazil suggested that a cut-off score of 122 produced around 90% sensitivity and specificity for the diagnosis of dementia, but age and schooling level had a significant effect on the scores. A similar study from North America recommended that a cut-off of 123 was required in a broader population to produce acceptable sensitivity and specificity values.
The DRS has been most widely used in patients with AD. It is more sensitive to early disease than the brief scales discussed earlier, and shows less marked floor effects. It may also be helpful in distinguishing between different dementing illnesses. The memory section is most sensitive to AD whereas patients with Huntington’s disease are most impaired on the initiation subtest. We have recently compared performance on the DRS and ACE of large groups of patients with AD, PSP, corticobasal degeneration (CBD), and multiple system atrophy (MSA). Each disorder produced a distinctive profile which was apparent on both the ACE and the DRS. Fig. 6.1 shows the proportion of cases demonstrating impairment on each subtest. It can be seen that the ACE was more sensitive overall, MSA patients showed least impairment, while those with CBD showed global deficits compared with the much more selective profiles demonstrated in AD and PSP.
It should also be noted that the DRS provides a broad test of the distributed cognitive functions (attention, memory, and abstraction/conceptualization), but includes virtually no assessment of localized functions, especially language. This is of relevance when attempting to screen for semantic dementia and progressive non-fluent aphasia.
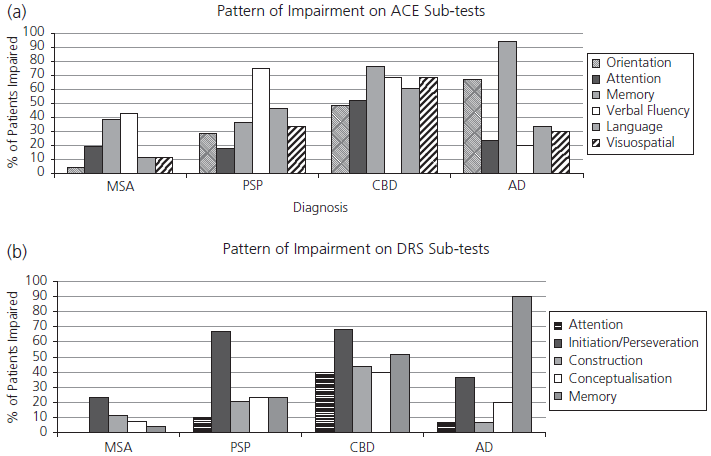
Fig. 6.1 Proportion of patients with multiple system atrophy (MSA), progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and Alzheimer’s disease (AD) showing impairment on subtests of (a) Addenbrooke’s Cognitive Examination (ACE) and (b) Dementia Rating Scale (DRS).
Cambridge Cognitive Examination—Revised (CAMCOG-R)
See Table 6.2.
Table 6.2 Cambridge Cognitive examination: CAMCOG
| CAMCOG-R subtests | Scores |
|---|---|
| Orientation | |
| Time—day, date, month, year, season | |
| Place—county, town, street, floor, place | Total __/10 |
| Language | |
| Comprehension | |
| Expression: naming of objects and pictures, definitions | |
| Verbal fluency (animals) | |
| Repetition of ‘no ifs, ands, or buts’ | |
| Reading comprehension | |
| Total __/30 | |
| Memory | |
| Registration and recall of three items from MMSE | |
| Recall and recognition of pictures | |
| Remote and recent information retrieval | |
| Total __/27 | |
| Attention and calculation | |
| Counting backwards and serial 7s | |
| Total __/9 | |
| Praxis | |
| Copying and drawing (pentagons, spiral, house, clock) | |
| Actions to command | |
| Writing—spontaneous and to dictation | |
| Total __/12 | |
| Abstract thinking | |
| Similarities | |
| Total __/8 | |
| Perception | |
| Visual recognition, unusual views and famous people naming | |
| Total __/9 | |
|
|
|
| Additional tasks in CAMCOG-R | |
| Ideational fluency | |
| Visual reasoning | |
| Total __/14 | |
| Overall grand score __/119 |
Reproduced from CAMDEX-R: The Revised Cambridge Examination for Mental Disorders of the Elderly, 2nd Edition, 1999, Cambridge university Press.
The CAMCOG forms part of a standardized psychiatric assessment schedule, CAMDEX (Cambridge Examination for Mental Disorders of the Elderly), devised by Roth and colleagues and published by Cambridge University Press (1988). CAMDEX was designed specifically for use in elderly people with the diagnosis of dementia and was later revised as CAMDEX-R (1998). It includes a structured psychiatric interview with the patient; a relative or other informant interview; a brief physical examination; and a neuropsychological test battery, CAMCOG-R.
CAMCOG-R assesses a wider range of cognitive functions, both distributed (attention, memory, abstraction) and localized (language, praxis, etc.), than any other standardized schedule. It incorporates both the MMSE and the Information–Memory–Concentration Test within the battery. The CAMCOG-R also includes two additional tests of executive function (ideational fluency—uses of a bottle—and a visual reasoning test similar to Raven’s matrices). The average administration time is 20–40 minutes, depending on the degree of impairment. The maximum overall score is 105. A cut-off of 80 was found to discriminate between demented and normal subjects, respectively, on the original validation studies with a high degree of specificity and sensitivity. Further work has shown that, in keeping with other mental test schedules, the normal range varies considerably with age, and age-appropriate normative values are now available. For instance, in a large community sample the 10th centile score was shown to fall from 85 for subjects aged 60–69 years to 65 for those aged 85–89 years.
CAMCOG has been used extensively in community-based studies of dementia around Europe, and is the best-validated and normed of the longer mental test batteries. The CAMCOG-R was used in a multinational harmonization project (EURO-HARPID) and has been translated for use around Europe. The value of either CAMCOG or CAMCOG-R in distinguishing between patients with various types of dementia is less well established, but recent work has shown that it is sensitive to cognitive dysfunction in Parkinson’s disease and post-stroke dementia. Evidence is also emerging to suggest that it can reveal distinct profiles in AD and dementia with Lewy bodies (DLB).
In comparison with the DRS, CAMCOG is likely to be more sensitive to mild degrees of dementia, and should be better at detecting patients with predominantly language or visuospatial dysfunction as the DRS contains no language tests and only a limited assessment of drawing ability. A disadvantage of CAMCOG is the relative lack of very easy items, so that it is unlikely to be as valuable as the DRS for monitoring progress in patients with moderately severe dementia.
Alzheimer’s Disease Assessment Scale (ADAS)
The ADAS-Cognitive subscale (ADAS-Cog) was the primary cognitive outcome measure in the first clinical trials of drugs for the treatment of dementia and has subsequently become one of the two primary outcome measures required by the US Food and Drug Administration authority for the licensing of new drugs. Numerous translations are available. It was designed by Rosen and her colleagues to test four cognitive domains: orientation (8 points), memory (27 points), language (25 points), and praxis (10 points). It has a mixture of objective assessments, such as word-list learning and naming, together with observer-rated assessments of language and praxis.
The ADAS score is based upon the number of errors ranging from 0 to 70 with the highest scores indicating the greatest impairment. It takes 30–35 minutes to administer. As with other similar tests, age and education have a significant effect on ADAS-Cog performance. Inter-rater reliability is good. It has been used extensively in drug trials but we have limited experience in Cambridge. It has very doubtful sensitivity to other dementia syndromes and its usefulness in screening for MCI is doubtful.